Gender-differences in prevalence and outcome of ischemic stroke and promoting factors of atrial thrombi
K.K. and M.G. contributed equally and should both be considered as first authors.
- DOI
- 10.1016/j.artres.2018.05.004How to use a DOI?
- Keywords
- Ischemic stroke; Sex; LAA; Left atrial appendix; Gender; Atrial fibrillation; Mortality; Stroke
- Abstract
Background: Ischemic stroke is an important cause of death and disability. However, data about gender-differences in stroke are controversial.
Methods: In the nationwide sample, male and female inpatients were selected by screening for ischemic stroke by ICD-Code(I63) and compared. In a second study, we performed a retrospective analysis of patients who underwent transesophageal echocardiography (TEE) and screened for gender specific associations between clinical and echocardiographic parameters and atrial thrombi formation.
Results: Males had a higher incidence of ischemic stroke than females (372 vs. 340 per 100,000 citizens) with a substantial age-depending increase. Percentage of stroke patients with atrial fibrillation/flutter (AF, 34.2% vs. 26.5%) and the case-fatality rate (9.4% vs. 7.1%) were higher in females. AF seems to aggravate stroke events. In the retrospective study, 227 patients were enrolled (87 females (38.3%)). Females were older (IQR 72.0 (72.0–79.0) vs. 66.5 (57.3–76.8) years, P = 0.013), showed smaller right atrial (RA) area and slower blood flow velocity in left atrial appendage (LAA) (41.2 (29.2–58.5) vs. 50.0 (34.3–67.1) cm*sec−1, P = 0.038). Promoting factors of atrial thrombi in both genders were lower blood-flow velocity in LAA, larger LAA diameters, higher CHA2DS2-VASc-score and heart failure. AF, larger atrial septal-lateral diameters and areas were associated with atrial thrombi especially in males.
Conclusions: Our study demonstrated gender-specific differences in ischemic stroke. Incidence of ischemic stroke was higher in males than in females increasing exponentially with growing age in both genders. Females had a higher case-fatality rate presumably due to higher rate of AF. Promoting factors of atrial thrombi differ especially regarding atrial volumes and blood flow velocity in the LAA.
- Copyright
- © 2018 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Ischemic stroke is an important cause of death and disability worldwide in men and women.1–8 While its incidence in low- and middle-income countries has doubled during the last 40 years, it declined in the high-income countries by approximately one third.2,9 Overall, the incidence of stroke rises exponentially with increasing age.1,7,10–13 However, data about gender-differences in stroke and its outcomes are conflicting.8
Sex-specific differences in cardiovascular diseases (CVD) are well recognized.14–19 In general, male sex is connected with both an increased risk of both developing CVD as well as fatal outcome.20 While some studies reported a higher proportion of female stroke patients,21 other studies showed a higher incidence of ischemic stroke in males.1,16 Although women and men share most of the cardiovascular risk factors and comorbidities, the importance of these factors and comorbidities in stroke patients of both sexes are different.8,22 In accordance with other manifestations of cardiovascular disease, women are older at the ischemic stroke event23,24 and female and male stroke patients differ significantly in presentation of symptoms and outcomes.8,22 In females, imaging diagnostic procedures and carotid surgery are less frequent and some studies additionally report on a more restricted use of thrombolytic treatment as well as a less favourable outcome.22,25–28 The mortality risk increases significantly with growing age.27
Cardio-embolic stroke events account for about one fifth of ischemic strokes29 and patients with atrial fibrillation have a 3- to 5-fold elevated relative risk for occurrence of stroke in comparison to individuals without.3,30 Thromboembolic strokes due to atrial fibrillation are frequently devastating, leading to severe impairment or death in the majority of patients.29,31,32 The left atrial appendage (LAA) harbors approximately 90% of intra-cardiac thrombi in atrial fibrillation.13
Thus, the objectives of this study were to elucidate gender-specific differences in ischemic stroke in the nationwide sample and to identify gender-differences in atrial thrombi development.
Methods and patients
Two separate patient cohorts were analyzed
- 1.
German nationwide cohort with analysis for predictors of death in ischemic stroke patients
The German nationwide in-patient statistics (Diagnosis related groups (DRG statistics)) of the year 2015 was used for this first analysis. The information includes treatment data from all inpatients processed according to the DRG system. In Germany, diagnoses of inpatients are coded according to ICD-10-GM (International Classification of Diseases, 10th Revision with German Modification). DRG-coded diagnoses data are gathered at the Federal Statistical Office in Germany (Statistisches Bundesamt, DEStatis). In the year 2015, overall 19.2 million in-patient files from 1956 hospitals were registered. For this analysis, mortality data of in-patients diagnosed for ischemic stroke (ICD code I63) with and without additionally coded atrial fibrillation/flutter (AF) (ICD code I48) stratified for gender were obtained from the Federal Statistical Office of Germany (Statistisches Bundesamt, DEStatis, source: DRG-Statistik, Sonderauswertung des Statistischen Bundesamtes). Since this study part did not involve direct access by the investigators to data on individual patients but only to summary results provided by the Research Data Center, approval by an ethics committee and informed patient consent were not required according to German law.
- 2.
Single center cohort at the University Medical Center Mainz for the analysis regarding the correlation between echocardiographic parameters and clinical features on the one hand and atrial thrombi as the source of cardio-embolic stroke on the other hand
Patients who underwent a transthoracic echocardiography (TEE) at the Center of Cardiology of the University Medical Center Mainz (Mainz, Germany) were included in this retrospective analysis. This cohort included consecutive patients, aged ≥18 years, who were examined in the echocardiography department of the Center of Cardiology (accredited echocardiography institution by the European Society of Cardiology) by TEE between January and March 2013. Patients were stratified by gender in the two subgroups of female and male patients.
We assessed patients’ anthropometric and clinical characteristics, comorbidities and echocardiographic parameters. The CHA2DS2-VASc-score (Congestive heart failure, Arterial Hypertension, Age ≥75 years, Diabetes mellitus, prior ischemic Stroke, TIA or thromboembolism, Vascular disease, Age 65–74 years, female sex (but female sex is only a risk factor if other risk factors are also present))33 was calculated for all patients. Echocardiographic measurements were obtained by evaluation of two- and three-dimensional TTE and TEE loops stored on the clinic’s server in DICOM-standard and assessment via Philips Xcelera® and Qlab® software (trademark by Philips® healthcare). All echocardiographic analyses were performed and confirmed by at least two experienced echocardiographers. The echocardiographic measurements comprised evaluation of thrombogenic material, which was defined as the presence of solid thrombi or relevant spontaneous echo contrast (SEC) in the LAA, blood flow velocity in LAA detected by Pulsed-waved (PW)Doppler as well as the spatial dimensions of the LAA in kind of width (septal-lateral diameter obtained in short axis view/45° angulation) and length (aperture-apex) of the LAA in TEE (Fig. 1). Confirmation or exclusion of atrial thrombi or SEC was performed in all recorded angulations and three-dimensional reconstructions, if available.
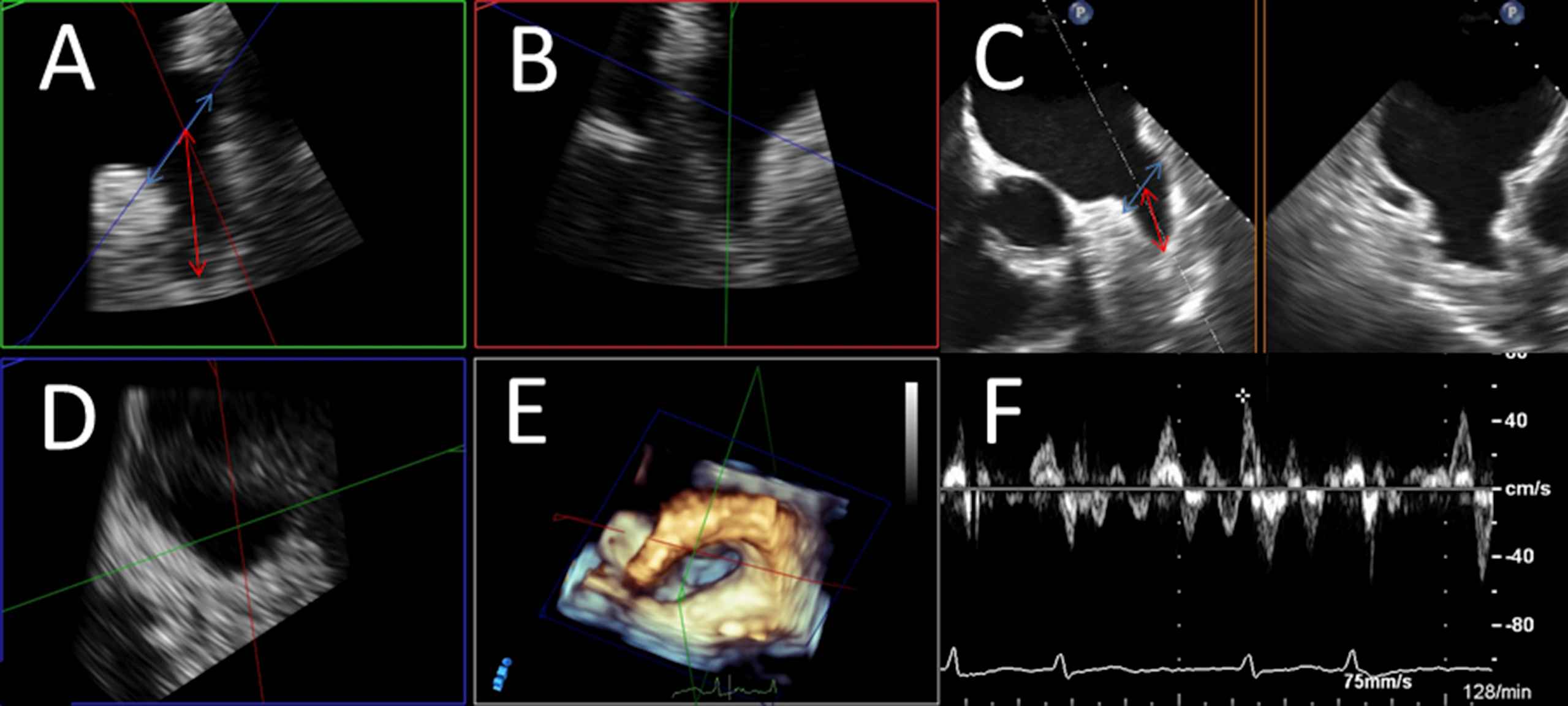
Assessment of LAA diameters and LAA velocity. LAA expansions were measured by determining orifice width and LAA depth. LAA orifice width (marked as red arrow) was estimated by measuring septo-lateral diameter in short axis view (SAX) as an approximated plane from the left coronary height (septal) to 1–2 cm below the highest point of the LUPV (left upper pulmonary vein)-limbus (“rim”/lateral); LAA depth (marked as blue arrow) was determined as distance from orifice plane to the deepest point of the LAA. If available, measurements were performed by multi-planar reconstruction of three-dimensional echo loops (Panels A, B, D: Panel A shows a reconstructed plane resembling SAX-view in left upper quadrant, Panel B another reconstructed longitudinal plane orthogonal on Panel A, Panel D the reconstructed “orifice” of the LAA). Panel C demonstrates measurements in “conventional” 2-dimensional TEE at about 45° resembling SAX-view. Panel E shows spatial relations in the LA in a three-dimensional overview in “non-surgical” orientation. Panel F shows the determination of blood velocity in the LAA via PW-doppler in a patient with present atrial fibrillation (obviously shown by PW-curve demonstrating rapid and irregular LAA contractions).
Left ventricle ejection fraction (LVEF), left and right septal-lateral and longitudinal diameters as well as atrial areas and systolic pulmonary artery pressure were measured, if a TTE was available (from the same hospital visit). Ventricular dimensions, LVEF and atrial areas were determined in apical 4 chamber view using Simpson’s method according to the EACVI- and ASE-recommendations on cardiac chamber quantification.34 Proposed systolic artery pressure (sPAP) was estimated from Doppler regurgitation velocity on tricuspid regurgitation (Fig. 1).
Since the study involved only an anonymous, retrospective analysis of diagnostic standard data, ethics approval was not required according to German laws.
Statistics
First, we calculated the incidence of ischemic stroke for the German male and female citizens overall and stratified by age-groups in the year 2015. The proportion of ischemic stroke patients with additionally diagnosed AF and the relative mortality rate in female and male ischemic stroke patients was computerized stratified by age groups. Moreover, we calculated the mortality rate in all ischemic stroke patients, patients with and without additional AF in females relative to males 2015 in Germany stratified by age groups. The mortality rate of the males was taken as reference group and equal mortality rate in both genders was equated with 1.
In the second mono-centric retrospective study cohort, we compared male and female patients who underwent a TEE due to different reasons.
Descriptive statistics for the relevant baseline comparison of both genders are provided with mean ± standard deviation, median and interquartile range (IQR), depending on Gaussian or skewed distribution, or absolute numbers and corresponding percentages. Continuous variables found not to follow a normal distribution, when tested with the modified Kolmogorov–Smirnov test (Lilliefors test), were compared using the Wilcoxon-Whitney U test. Normal distributed continuous variables were compared using the Students’ T-Test and categorical variables with Fisher’s exact or Chi2 test, as appropriate.
We calculated univariate logistic regression models to examine the associations between thrombogenic material and parameters such as age, sex, body height, weight, body-mass-index, echocardiographic measurements and comorbidities (inclusively AF), respectively.
The parameters CHA2DS2Vasc-score, LAA longitudinal diameter, left and right atrial area, which were respectively associated with thrombogenic material, were entered in a receiver operating characteristics (ROC) analysis and a cut-off value for these parameters were calculated.
The software SPSS® (version 22.0; SPSS Inc., Chicago, Illinois) was used for the majority of computerised analysis. Only P values of <0.05 (two-sided) were considered to be statistically significant.
Results
Analysis of age-dependence and the predictors of death in ischemic stroke patients
The nationwide sample comprised 150,595 male and 141,801 female inpatients with ischemic stroke in Germany 2015. The incidence of ischemic stroke was 372 per 100,000 individuals in males and 340 per 100,000 individuals in females. Total numbers as well as the incidences of ischemic stroke events increased with growing age in both genders (Figs. 2 and 3). Also the proportion of stroke patients with diagnosed AF inclined exponentially with growing age in males and females (Fig. 4A). Interestingly, the percentage of female stroke patients with diagnosed AF was distinctly higher than in male patients (34.2% vs. 26.5%).
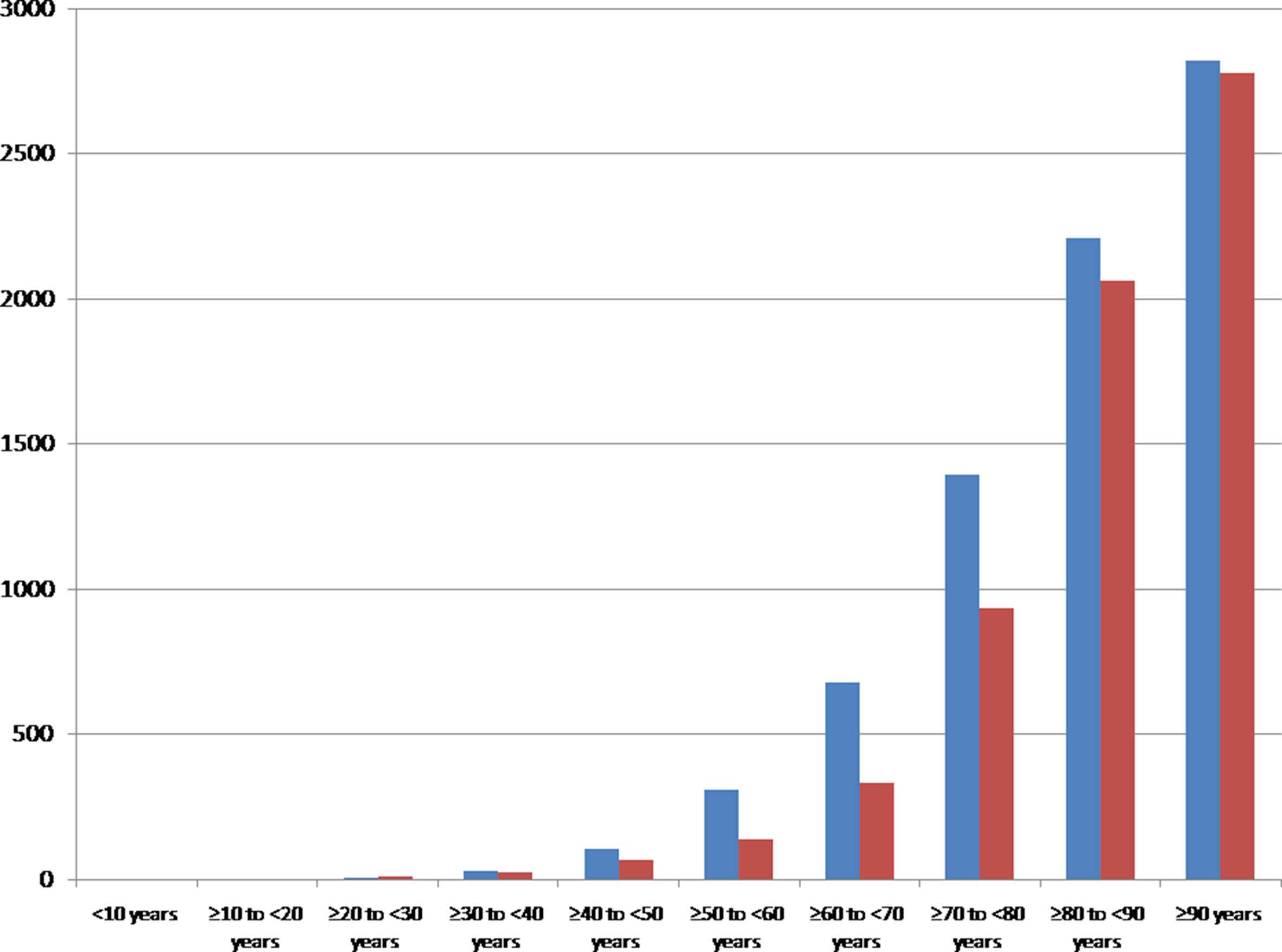
Incidence of ischemic strokes (ICD-code I63) in women (red bars) and men (blue bars) 2015 in Germany stratified by age groups. (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
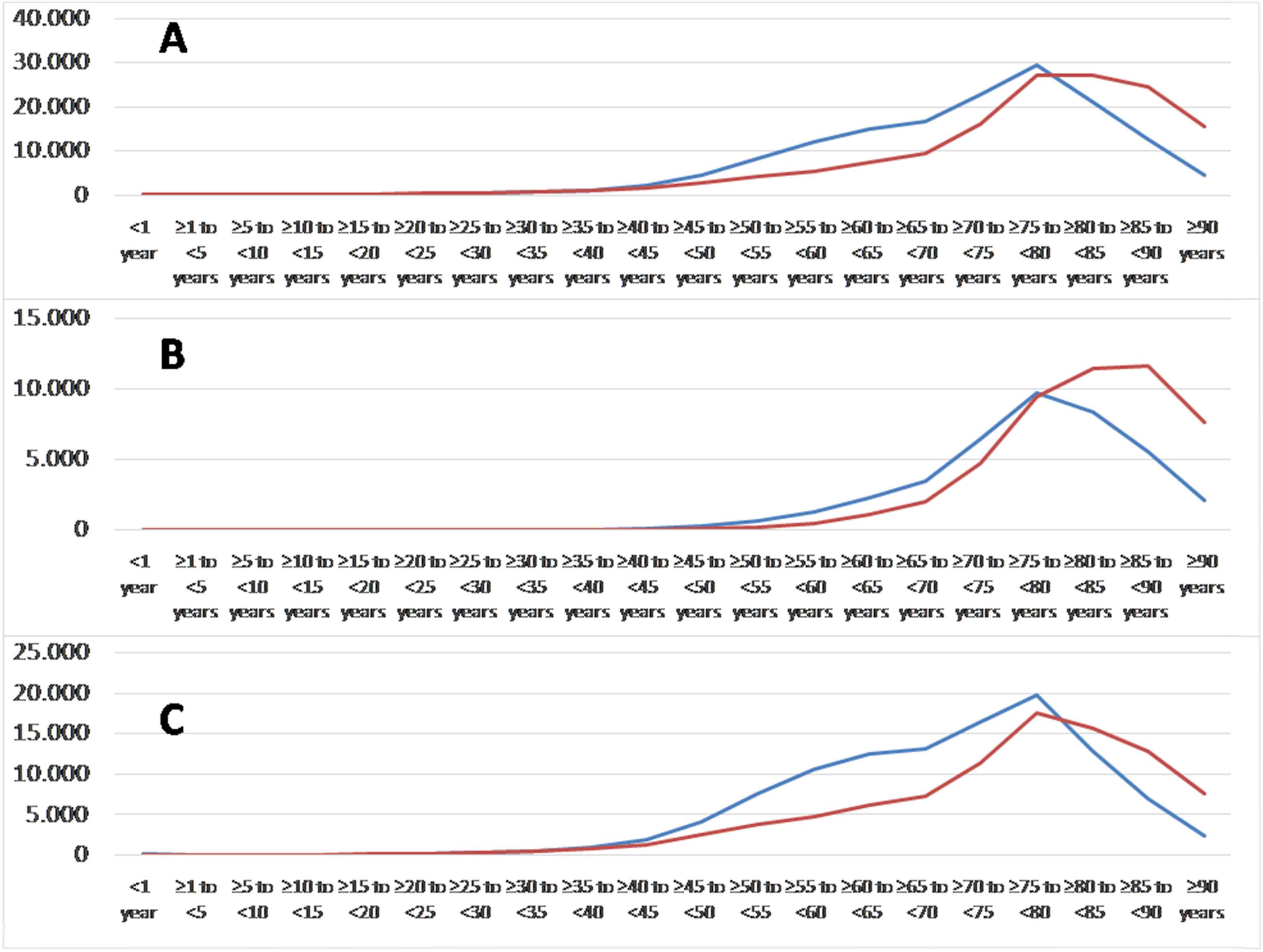
Total numbers of ischemic strokes (ICD-code I63) in women (red lines) and men (blue lines) 2015 in Germany stratified by age groups: overall (A), with (B) and without additional atrial fibrillation (C). (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
In total, 10,715 male and 13,336 female patients with ischemic stroke died in-hospital. Therefore, the case-fatality rate was relevantly higher in females than in males (9.4% vs. 7.1%). The relative mortality rate increased in both genders with growing age (Fig. 4B).
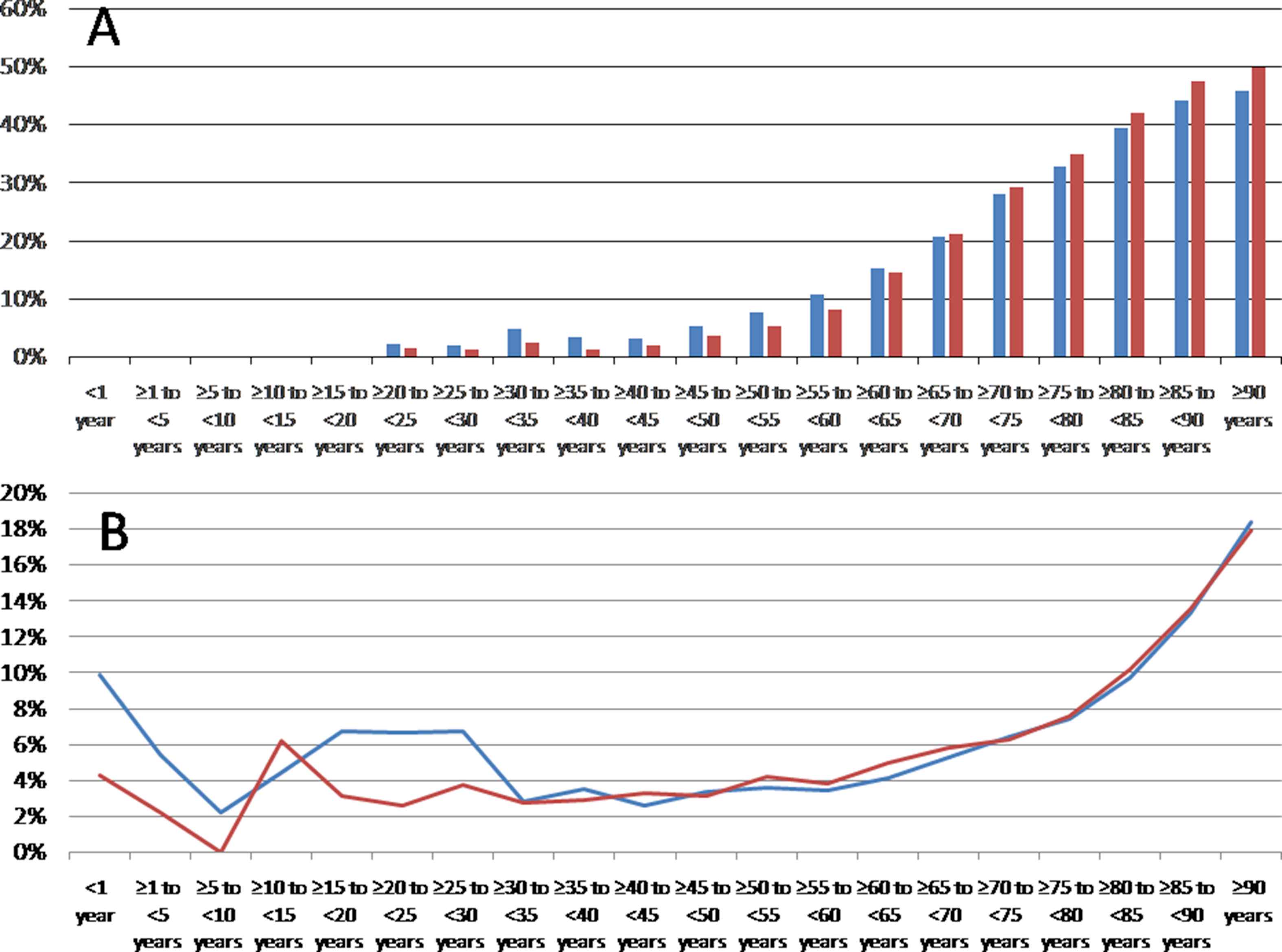
(A) Proportion of ischemic stroke patients diagnosed with atrial fibrillation in men (blue bars) and women (red bars) 2015 in Germany stratified by age groups. (B) Relative mortality rate in female (red line) and male (blue line) ischemic stroke patients 2015 in Germany (percentage of in-hospital mortality for the different age groups). (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
AF seems to aggravate stroke events especially after the 75th life-year significantly (Fig. 5).
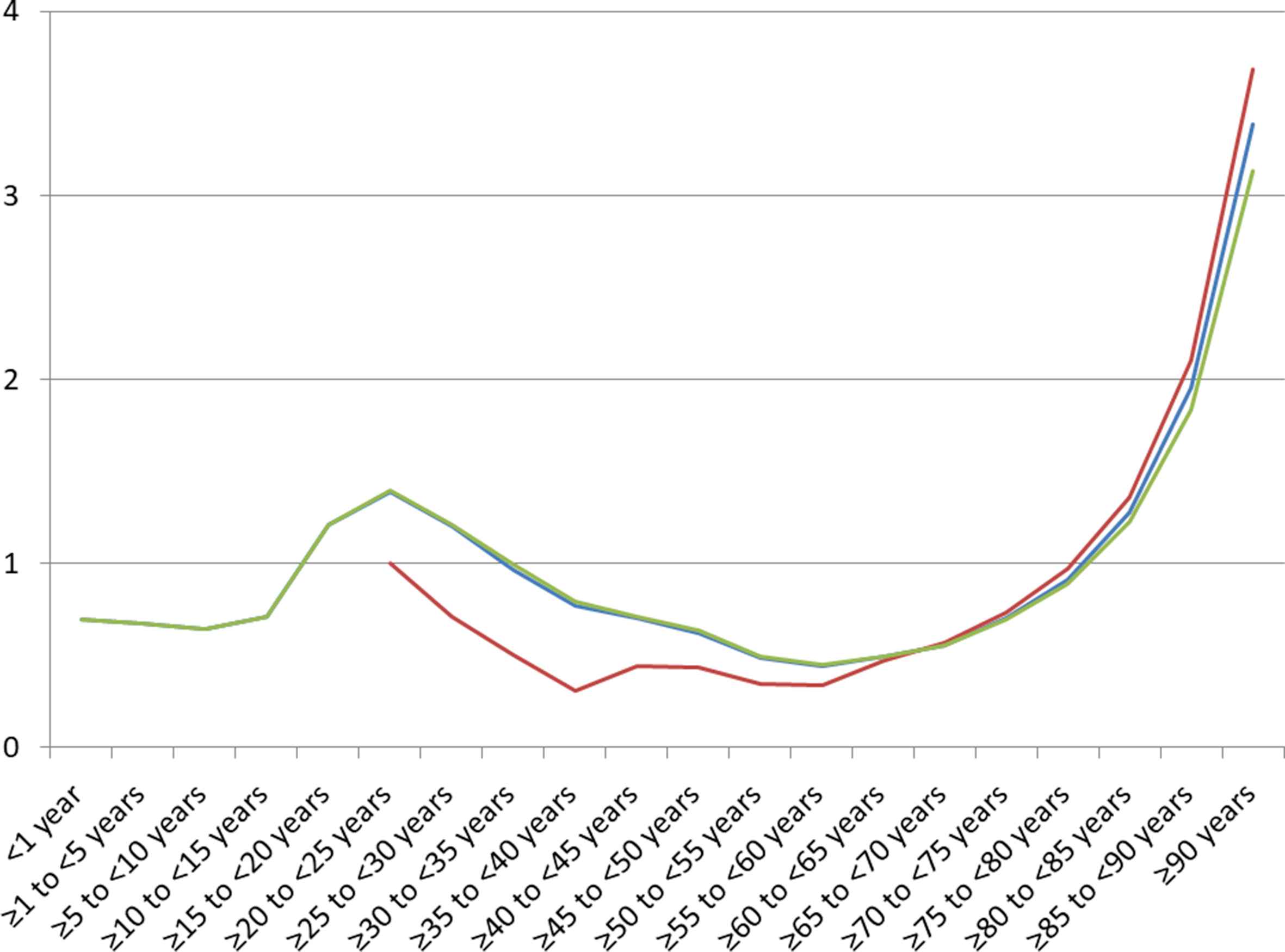
Mortality rate in all ischemic stroke patients (blue line), patients with additional atrial fibrillation (red line) and without additional atrial fibrillation (green line) in females relative to males 2015 in Germany stratified by age groups. Equal mortality rate in both genders is equated with 1. Values > 1 represent higher mortality rate in women. (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
Correlation between echocardiographic parameters, clinical features and atrial thrombi potentially induce ischemic stroke
In total, 227 consecutive patients who underwent TEE for various reasons were enrolled for this analysis. Among these patients 140 were of male and 87 of female gender. The medical indications for TEE comprised i) evaluation regarding intra-cardiac thrombogenic material before planned cardioversion in 58.2% of the females and 65.2 of the males, ii) further evaluation of valve disorders (including suspected endocarditis) in 32.2% of the women and 32.9% of the men, as well as iii) other reasons in 9.6% of the female and 1.9% of the male patients.
The clinical characteristics and echocardiographic parameters of the patients stratified for gender were presented in Table 1. Briefly, females were older at the examination date (72.0 (IQR 72.0–79.0) vs. 66.5 (57.3–76.8) years, P = 0.013), and as expected, less tall (1.64 ± 0.08 vs. 1.77 ± 0.08 m, P < 0.001) and weight less (69.0 (62.0–80.0) vs. 84.0 (73.0–95.0) kg, P < 0.001) than their male counterparts, while the BMI was similar between both subgroups.
| Variable | Females (n = 87) | Males (n = 140) | P-value |
|---|---|---|---|
| Age (years) | 72.0 (72.0–79.0) | 66.5 (57.3–76.8) | 0.013 |
| Body height (m) | 1.64 ± 0.08 | 1.77 ± 0.08 | <0.001 |
| Body weight (kg) | 69.0 (62.0–80.0) | 84.0 (73.0–95.0) | <0.001 |
| BMI (kg/m2) | 26.3 (22.9–28.7) | 26.5 (24.1–29.4) | 0.546 |
| Comorbidities | |||
| Heart failure | 16 (18.4%) | 35 (25.0%) | 0.246 |
| Arterial hypertension | 65 (74.7%) | 93 (66.4%) | 0.187 |
| Coronary artery disease | 23 (26.4%) | 49 (35.0%) | 0.178 |
| Diabetes mellitus | 20 (23.0%) | 23 (16.4%) | 0.220 |
| History of stroke or TIA | 10 (11.5%) | 18 (12.9%) | 0.761 |
| History of myocardial infarction | 8 (9.2%) | 14 (10.0%) | 1.000 |
| Peripheral artery disease | 12 (13.8%) | 19 (13.6%) | 0.761 |
| History of venous thromboembolism | 12 (13.8%) | 7 (5.0%) | 0.026 |
| History of pulmonary embolism | 5 (5.7%) | 5 (3.6%) | 0.512 |
| History of deep venous thrombosis | 12 (13.8%) | 6 (4.3%) | 0.020 |
| Atrial fibrillation | 48 (55.8%) | 67 (50.4%) | 0.431 |
| CHA2DS2-VASc-Score (points) | 4 (3–5) | 2 (1–4) | 0.102 |
| Echocardiographic parameters | |||
| Thrombus or spontaneous echo contrast | 9 (11.7%) | 7 (6.3%) | 0.197 |
| Thrombus without spontaneous echo contrast | 4 (5.2%) | 0 (0.0%) | 0.026 |
| Left ventricle ejection fraction | 55.0% (50.0–55.0) | 55.0% (37.5–55.0) | 0.103 |
| Left atrial longitudinal diameter (cm) | 6.2 (5.5–6.9) | 5.9 (5.3–6.6) | 0.223 |
| Left atrial septal-lateral diameter (cm) | 4.2 (3.9–4.8) | 4.3 (3.8–4.7) | 0.787 |
| Left atrial area (cm2) | 22.2 ± 6.6 | 21.6 ± 6.8 | 0.613 |
| Right atrial septal-lateral diameter (cm) | 3.8 ± 0.9 | 4.1 ± 0.8 | 0.642 |
| Right atrial longitudinal diameter (cm) | 5.3 (4.8–5.9) | 5.4 (4.9–6.1) | 0.190 |
| Right atrial area (cm2) | 15.8 (12.7–19.8) | 18.1 (15.0–22.9) | 0.024 |
| Systolic pulmonary artery pressure (mmHg) | 39.1 ± 9.3 | 38.9 ± 11.5 | 0.907 |
| Blood flow velocity in the LAA detected by Pulsed-waved Doppler (cm*sec−1) | 41.2 (29.2–58.5) | 50.0 (34.3–67.1) | 0.038 |
| LAA width (septal-lateral diameter) (cm) | 1.8 (1.2–2.1) | 1.7 (1.4–2.0) | 0.455 |
| LAA longitudinal diameter from aperture to apex (cm) | 3.3 ± 0.8 | 3.3 ± 0.7 | 0.512 |
Abbreviations: LAA indicates left atrial appendage; BMI – Body mass index; TIA – transient ischemic attack.
P values of <0.05 (two-sided) were considered to be statistically significant.
Clinical characteristics and echocardiographic parameters in female and male patients.
Regarding comorbidities, females showed higher frequency of prior deep venous thrombosis (DVT, 12 (13.8%) vs. 6 (4.3%), P = 0.020) in their medical history, but not pulmonary embolism (PE, P = 0.512). While thrombogenic material (solid thrombus material and spontaneous echo contrast) in the LAA was similar often identified in both genders (P = 0.197), solid thrombi were more often identified in females (5.2% vs. 0%, P = 0.026).
Echocardiographic measures revealed a larger right atrial (RA) area in males, whereas all LA parameters, inclusively the LAA measurements were not significantly larger in men compared to women. Interestingly, the blood flow velocity in the LAA detected by Pulsed-waved Doppler was higher in male than in female patients (50.0 (34.3–67.1) vs. 41.2 (29.2–58.5) cm*sec−1, P = 0.038).
Promoting factors of atrial thrombi (solid thrombus or SEC) in both genders were a lower blood flow velocity in the LAA, a larger LAA diameter, a higher CHA2DS2-VASc-score and the comorbidity of heart failure (Table 2). Additionally, larger left and right atrial septal-lateral diameters and areas were also associated with atrial thrombogenic material in male patients, but not in females.
| Variable | Females (n = 87) | Males (n = 140) | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Age (per year) | 1.054 (0.979–1.135) | 0.161 | 1.064 (0.981–1.154) | 0.136 |
| Blood flow velocity in the LAA detected by Pulsed-waved Doppler (cm*sec−1) | 0.904 (0.837–0.976) | 0.010 | 0.953 (0.913–1.001) | 0.053 |
| LAA longitudinal diameter (cm) | 2.587 (1.070–6.253) | 0.035 | 8.477 (1.849–38.861) | 0.006 |
| LAA septal-lateral diameter (cm) | 4.028 (0.999–16.250) | 0.50 | 2.464 (0.910–6.673) | 0.076 |
| Left ventricle ejection fraction (%) | 1.017 (0.933–1.110) | 0.699 | 0.955 (0.894–1.019) | 0.165 |
| Right atrial septal-lateral diameter (cm) | 0.903 (0.247–3.297) | 0.878 | 3.484 (1.091–11.128) | 0.035 |
| Right atrial longitudinal diameter (cm) | 1.001 (0.682–1.467) | 0.997 | 1.012 (0.857–1.195) | 0.889 |
| Right atrial area (cm2) | 1.112 (0.895–1.380) | 0.339 | 1.200 (1.043–1.380) | 0.011 |
| Left atrial septal-lateral diameter (cm) | 0.638 (0.121–3.362) | 0.596 | 4.728 (1.360–16.432) | 0.015 |
| Left atrial longitudinal diameter (cm) | 0.851 (0.383–1.891) | 0.691 | 2.801 (0.974–8.060) | 0.056 |
| Left atrial area (cm2) | 0.979 (0.800–1.198) | 0.835 | 1.245 (1.049–1.478) | 0.012 |
| Systolic pulmonary artery pressure (mmHg) | 0.989 (0.886–1.104) | 0.842 | 1.004 (0.921–1.095) | 0.923 |
| BMI (kg/m2) | 1.028 (0.924–1.144) | 0.610 | 0.909 (0.725–1.139) | 0.407 |
| Presence of Atrial Fibrillation | 3.014 (0.583–15.587) | 0.188 | Regression was not calculable, therefore Chi–Quadrat test was used for calculation | 0.016 |
| History of venous thromboembolism | 0.725 (0.082–6.442) | 0.773 | Regression was not calculable, therefore Chi–Quadrat test was used for calculation | 1.000 |
| Heart failure | 4.640 (1.060–20.305) | 0.042 | 4.754 (0.992–22.775) | 0.051 |
| Arterial Hypertension | 1.357 (0.258–7.126) | 0.718 | 3.000 (0.348–25.898) | 0.318 |
| Coronary artery disease | 3.750 (0.902–15.588) | 0.069 | 0.800 (0.148–4.332) | 0.796 |
| Diabetes mellitus | 2.600 (0.623–10.856) | 0.190 | 5.308 (1.066–26.438) | 0.042 |
| History of stroke or TIA | 2.490 (0.430–14.405) | 0.308 | 3.418 (0.591–19.764) | 0.170 |
| Peripheral artery disease | 3.750 (0.780–18.025) | 0.099 | 2.831 (0.497–16.122) | 0.241 |
| History of myocardial infarction | 1.292 (0.137–12.150) | 0.823 | 3.418 (0.591–19.764) | 0.170 |
| CHA2DS2-VASc-Score (points) | 1.629 (1.072–2.477) | 0.022 | 1.701 (1.091–2.652) | 0.019 |
Abbreviations: LAA indicates left atrial appendage; BMI – Body mass index; TIA – transient ischemic attack.
P values of <0.05 (two-sided) were considered to be statistically significant.
Univariate regression analyses for the associations between several parameters and intra-cardiac thrombus formation (comprising solid thrombi and spontaneous echo contrast).
The ROC analyses confirmed the regression results. The best diagnostic performance of the parameters was observed for LA area in prediction of atrial thrombogenic material with an AUC of >0.8 in males and for LAA longitudinal diameter with an AUC of >0.7 in females (Table 3, Fig. 6), whereas the AUC values for the CHA2DS2-VASc-scores of women and men were similar.
| Parameter | Gender | P-value | Area under the curve (95% CI) | Calculated cut-off value | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|---|---|---|
| CHA2DS2Vasc-score (points) | Female | 0.039 | 0.712 (0.542–0.883) | 5.5 points | 0.444 | 0.853 | 0.286 | 0.921 |
| Male | 0.049 | 0.722 (0.545–0.900) | 4.5 points | 0.571 | 0.095 | 0.040 | 0.769 | |
| LAA longitudinal diameter (cm) | Female | 0.040 | 0.713 (0.535–0.891) | 3.90 cm | 0.556 | 0.817 | 0.313 | 0.925 |
| Male | 0.005 | 0.817 (0.654–0.981) | 3.95 cm | 0.714 | 0.859 | 0.263 | 0.977 | |
| Right atrial area (cm2) | Female | 0.567 | 0.600 (0.217–0.983) | 18.95 cm2 | 0.667 | 0.650 | 0.125 | 0.963 |
| Male | 0.028 | 0.795 (0.566–1.000) | 22.50 cm2 | 0.800 | 0.772 | 0.211 | 0.981 | |
| Left atrial area (cm2) | Female | 0.965 | 0.493 (0.157–0.830) | 23.15 cm2 | 0.500 | 0.730 | 0.167 | 0.931 |
| Male | 0.011 | 0.842 (0.715–0.970) | 24.10 cm2 | 1.000 | 0.667 | 0.185 | 1.000 |
Abbreviations: LAA indicates left atrial appendage.
P values of <0.05 (two-sided) were considered to be statistically significant.
Prognostic performance for prediction of thrombus formation (comprising solid thrombus and spontaneous echo contrast).
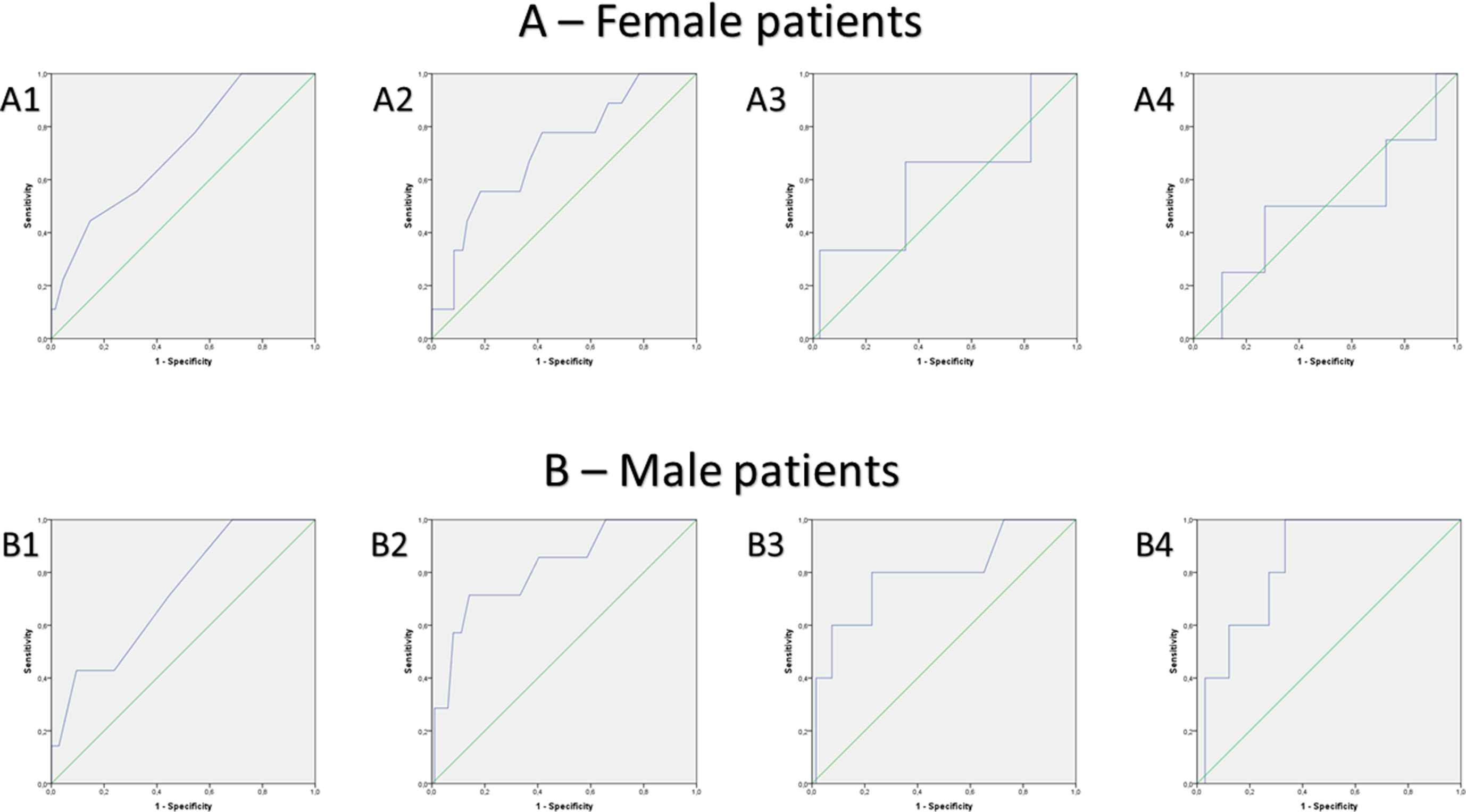
ROC curves for prediction of thrombus formation (comprising solid thrombus and spontaneous echo contrast) by several parameters in female (A) and male (B) patients: A1 and B1: CHA2DS2Vasc-score, A2 and B2: Left atrial appendage longitudinal diameter, A3 and B3: Right atrial area, A4 and B4: Left atrial area.
Discussion
Ischemic stroke is a major cause of death and disability in both genders1–8 and especially the subgroup of cardio-embolic strokes due to AF is often accompanied by a poor outcome with severe impairment or death.29,31,32 Study results about gender-specific differences and aspects of ischemic stroke are inconsistent.8,35 Thus, the objectives of this study were to elucidate gender-specific differences in ischemic stroke in the German nationwide sample and to identify gender-specific differences in atrial thrombi development.
The main findings of our study can be summarized as follows: I) the incidence of ischemic stroke was higher in male than in female citizens of Germany and II) it inclined dramatically with age in both genders. III) AF as additional diagnosis was more frequent in female stroke patients. IV) The case-fatality rate during in-hospital course was distinctly higher in females compared to males and V) the mortality rate increased in both genders with age. VI) Regarding cardio-embolic stroke, lower blood flow velocity in the LAA, larger LAA diameter, higher CHA2DS2-VASc-score and heart failure promoted atrial thrombi in both genders, while VII) AF, diabetes, larger left and right atrial septal-lateral diameters and areas were associated with atrial thrombogenic material especially in males.
Incidence of ischemic stroke
In accordance with the literature, the incidence of ischemic stroke was higher in male than in female citizens in our study.16,35 Incidence in men was in the nationwide sample of Germany 9.4% higher than in women. The higher incidence in males could be observed for all age-decades as described before.35 In addition our study confirmed, that the incidence of ischemic stroke inclined relevantly with growing age in both genders.1,7,10–13,27 Traditional risk factors for ischemic stroke comprise arterial hypertension, type II diabetes, hyperlipidemia, AF, smoking, overweight/obesity and other entities of metabolic syndrome as well as coronary artery disease.36 In general these cardiovascular risk factors are present at a later age in women than in men, explaining a higher age in female stroke patients compared to males.36,37 Diabetes mellitus, previous myocardial infarction and peripheral artery disease were more often found in male stroke patients, whereas women more frequently suffered from arterial hypertension and obesity.38 AF and smoking were equally frequent in both genders.38 The use of oral contraceptives, higher number of pregnancies and peri-as well as the post-menopausal periods might account for the increased stroke risk.36
Case-fatality rate after ischemic stroke
Our study results demonstrated a higher case-fatality rate in women compared to men after ischemic stroke events, which is in accordance with most reported studies.26,35 The in-hospital mortality for women was 1.2-fold higher related to men. The most suggestive explanation for this higher in-hospital mortality rate is a higher burden of ischemic stroke in later life of females compared to males (Fig. 3), while the age-dependent mortality rate inclined parallel in both genders (Fig. 4B). Beside this effect on the in-hospital mortality, studies about stroke survivors showed in addition a more favourable outcome in men with better rehabilitation results.26,36,37
Although current guidelines recommend equal diagnostic and treatment strategies for stroke prevention in both genders, previously published studies have reported gender-differences.26,36 A lower rate of imaging diagnostic procedures, carotid surgery antiplatelet agent treatment and - in some studies - additionally thrombolytic treatment is reported.22,25–28,36 These factors may contribute to a less favourable outcome in females.
Cardio-embolic stroke and promoting factors of atrial thrombi
Interestingly, AF was more frequently detected in female stroke patients compared to males in the German nationwide sample. This may be attributed to a later peak in life of ischemic stroke in women compared to men (Fig. 3), as the incidence of AF inclines with growing age in both genders.39 Also Fang et al.40 reported a higher AF-related thromboembolism leading to ischemic strokes in women compared to men.40 In general, patients with AF have a 3- to 5-fold elevated relative risk for occurrence of stroke in comparison to individuals without.3,30 Cardio-embolic stroke events account for about one fifth of the ischemic stroke events.29 Thromboembolic strokes due to AF are often devastating, resulting in severe impairment or death in the majority of patients.29,31,32
For cardio-embolic stroke, the left atrial appendage (LAA) plays the most important role due to the fact that LAA harbors approximately 90% of intra-cardiac thrombi in AF.13 Supporting previously published study results, lower blood flow velocity in the LAA41,42 as well as larger LAA dimensions43 were accompanied with atrial thrombi development. As expected, a higher CHA2DS2-VASc-score and presence of heart failure both promoted atrial thrombi in both genders.
Surprisingly, AF, diabetes, larger left and right atrial septal-lateral diameters and areas were associated with atrial thrombi in male patients, but not in females, which might be driven by the gender-difference in solid thrombi in our study. However, these results have to be interpreted with great caution, particularly in the view of fact that our single-center study cohort sample is only of medium size. It is well established, that female gender is one of the risk factors (in co-prevalence with other cofactors) for higher thromboembolic burden in AF patients and therefore was included in the CHA2DS2-VASc-Score.33 However, some gender-differences in cardio-embolic stroke are well recorded such as the fact that underlying valvular disease is more common in female, whereas more men with this condition suffer from coronary artery disease.44 In general, AF, recent myocardial infarction, the presence of mechanical prosthetic valves, dilated myocardiopathy and mitral rheumatic stenosis are conditions connected with a highly elevated risk for a cardio-embolic stroke.45
But beyond thrombi development in AF, a growing body of evidence indicates that additionally the LA size has been determined to be a predictor of stroke and death in the general population and an increased LA volume is predictive for the onset of first stroke in elderly individuals who are in sinus rhythm and do not have a history of ischemic neurologic events, AF, or valvular heart disease.46 Therefore, it might be hypothetically, whether a larger LA volume47 and larger LAA sizes48 per se may attribute to atrial thrombi development in men in absence of AF, especially in combination with a decreased blood flow velocity. This may one possible explanation regarding the gender-differences in atrial thrombi development.
Conclusions
Our study demonstrated gender-specific differences in ischemic stroke. Incidence of ischemic stroke was higher in males than in females increasing exponentially with growing age in both genders. Females had a higher case-fatality rate presumably due to higher rate of AF. Promoting factors of atrial thrombi differ especially regarding atrial volumes and blood flow velocity in the LAA.
Limitations
Our study has some limitations. The data of the nationwide sample from Federal Statistical Office of Germany (Statistisches Bundesamt, DEStatis) for this analysis are pooled/aggregated data. Therefore, we can only present a descriptive data analysis, without statistical testing for difference with P-values and without adjustment for age and other cofactors such as comorbidities (e.g. cardiovascular disease). Furthermore, only patients treated in-hospital were included. In addition, antithrombotic regimes was not reported, thus its influence remains unclear.
The central limitations of the second study-part are the single-center design and the retrospective data assessment of a medium sized patient cohort. Follow-up examinations are missing. In addition, only a small number of solid thrombi could be detected in the patient cohort, not allowing for statistically significant evidence. Thus, we decided to analyse solid thrombi and SEC as equivalent for atrial thrombi.13 The occurrence of SEC was assessed by at least two experienced echocardiographers.
Despite of these limitations, we were able to identify important gender-specific differences in ischemic stroke.
Notes
Funding
This study was supported by the
Potential conflicts of interests
None.
Acknowledgements
We thank the Federal Statistical Office of Germany (Statistisches Bundesamt, DEStatis) for providing the data/results and the kind permission to publish these data/results.
Appendix A
Supplementary data
Supplementary data related to this article can be found at
References
Cite this article
TY - JOUR AU - Karsten Keller AU - Martin Geyer AU - Thomas Münzel AU - Mir Abolfazl Ostad PY - 2018 DA - 2018/05/26 TI - Gender-differences in prevalence and outcome of ischemic stroke and promoting factors of atrial thrombi JO - Artery Research SP - 68 EP - 78 VL - 22 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2018.05.004 DO - 10.1016/j.artres.2018.05.004 ID - Keller2018 ER -
