IL-17 producing CD4+CD45RO+ T-cells in atherosclerosis express GITR molecule
These authors contributed equally in this manuscript.
- DOI
- 10.1016/j.artres.2017.12.004How to use a DOI?
- Keywords
- Atherosclerosis; Immunopathology; T cell; Inflammation
- Abstract
Background: Atherosclerosis (AS) is a chronic inflammatory disease of vessel walls associated with infiltration of immune cells which their function is controlled by different co-stimulatory and co-inhibitory receptors. We investigated the expression of co-inhibitory molecules on the memory and effector T-cells in patients with Atherosclerosis.
Methods: Patients included 9 hypertensive, dyslipidemic, non-diabetic, non-smoker individuals with the diagnosis of coronary artery disease and controls were 8 normotensive, normolipemic, non-diabetic, non-smoker individuals with normal coronary angiography/insignificant coronary artery disease. PBMCs were separated from the blood and memory T-cell subsets as well as the expression of Glucocorticoid-induced tumor necrosis factor receptor (GITR), Programmed Death-1 (PD-1), IL-17A and IFN-γ were quantified by flowcytometry.
Results: CD4+CD45RO+ memory T-cells and CD4+CD45RO− effector T-cells in patients expressed the highest level of GITR molecule. The IL-17 producing memory CD4+CD45RO+ T-cells were enriched in GITR molecule in the patients group (P = 0.03). The increased population of GITR+effector CD4+CD45RO− T-cells in patients, however, did not produce IL-17 (P = 0.03). PD-1 expression on memory T-cells of the patients was higher than the controls and was concomitant with the lack of IFN-γ expression (P = 0.05). IFN-γ production by effector T-cells was only seen in the PD-1− population in both groups.
Conclusions: We provide data on the expression of GITR molecule on IL-17 producing memory T-cells in patients with CAD. A population of memory T-cells, which expressed PD-1 and were not producing IFN-γ, also increased in patients’ blood. These data suggest the modified phenotype/function of T-cell subsets in the atherosclerotic inflammation.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Activated T-cells play a key function in the defense and protection of the organisms in the hostile living environment. However, inflammatory responses associated with T-cell activation are known to be involved in the pathogenesis of several diseases including atherosclerosis.1
Various studies have shown the persistence of different subsets of CD4+ T-cells in the atherosclerotic plaque, where they play pro-atherogenic and/or anti-atherogenic roles during the progression of atherosclerosis. In this regard, Th1 and Th17 cells exert pro-atherogenic actions whereas Treg cells play athero-protective roles.2
T-cell function is controlled by different co-stimulatory and co-inhibitory receptors.3 The balance between these receptors is critical for the prevention of inflammatory and autoimmune diseases.4,5 Co-inhibitory T-cell receptors such as Program Death-1 (PD-1) and Cytotoxic T Lymphocyte Antigen-4 (CTLA-4), exert their inhibitory actions by recruitment of protein phosphatases to block activation signaling, up-regulation of proteins that prevent autoimmune reactions and down-regulation of the ligands of co-stimulatory receptors.6
PD-1 (CD279) belongs to the CD28 family of proteins and is expressed on the peripheral CD4+ and CD8+ T-cells, B cells, T regulatory (Treg) and Natural Killer T (NKT) cells.7 PD-1 ligand 1, PD-L1 (B7-H1), is expressed on T-cells, B cells, mast cells, monocytes, epithelial and vascular endothelial cells (ECs) while PD-L2 (B7-DC) is mainly expressed on innate immune cells such as macrophages and dendritic cells.8 PD-1 ligation on Tregs leads to inhibition of cell proliferation, decreased TNF-α and IFN-γ secretion and reduced activity of PI3K signaling pathway.9 In healthy individuals, resting T-cells express low levels of PD-1, which upon their engagement, the initial events in T-cell activation are blocked.10 Increased expression of PD-1 after repeated antigen exposure leads to reduced effector function of exhausted T-cells, as well.11
Other than strict co-inhibitory or co-stimulatory functions, there are molecules in the immune system which exert either functions depending on the cellular milieu. A member of TNF receptor superfamily; i.e. Glucocorticoid-induced tumor necrosis factor receptor (GITR) is known to differentially function depending on the cell on which it is expressed. In general, GITR is expressed on activated CD4+ and CD8+ T-cells, B cells, Natural Killer (NK) cells, and macrophages.12 Because GITR agonistic antibody and recombinant GITRL lead to increased CD4+CD25− T-cells proliferation, GITR is introduced as a co-stimulatory receptor.13 GITRL expressed on ECs, B cells and monocytes, induces NF-KB activation, increases CD4+CD25− conventional T-cells expansion, survival, and enhances cytokine secretion.14 GITR expression on recently activated CD4+ and CD8+ T-cells leads to their increased expansion and cytokine secretion.15 Moreover, GITR expression is necessary for virus-specific clonal expansion and persistence of memory CD8+ T-cells in vivo.16
GITR interaction with its ligand, affects both conventional CD4+CD25-T-cells and Treg cells.17 Previous studies have shown that CD4+CD25+ Treg cells express high levels of GITR on their surface.9,12 Co-culture of conventional T-cells with CD4+CD25+ Treg cells in the presence of anti-GITR antibody, abrogates suppressor activity of Treg cells.13 Also injection of the same antibody to adult mice leads to autoimmune gastritis due to Treg cells depletion.14,18 In spite of originally suggested GITR immunomodulatory role,19 later studies suggest that GITR ligation with GITRL causes decreased Treg cell suppressor function and their expansion.20 However, there is still much controversy regarding GITR function on Treg cells. As a more accepted view, GITR engagement activates effector as well as Treg cells, however, does not participate in the mechanisms of Treg suppression.6 In addition, GITR-KO Treg cells are shown to maintain their suppressive function.21
In a previous study, we showed that CD4+CD45RO+ memory T-cells of patients with atherosclerosis produce IL-17 both ex-vivo and in-vitro.22 Here we report a high level of GITR expression on the CD4+CD45RO+ memory and CD4+CD45RO− effector T-cells of the patients with atherosclerosis as compared to the controls. Moreover, a notable percentage of the memory CD4+GITR+ T-cells produced IL-17 in the patients group.
Methods
Subjects
This study was approved by the ethics committee of Shiraz University of Medical Science (SUMS). The participants were informed about the aim of this study as well as safety and security measures and then a written informed consent was obtained. After informed consent, 20 ml heparinized blood was obtained from each of the 9 non-diabetic, non-smoker patients (5 men and 4 women aged 50–60 yrs, mean = 57.13 ± 3.7 yrs) who were diagnosed with coronary artery disease confirmed by carotid angiography and had hypertension [systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg] and dyslipidemia according to the established guidelines.23
Control group consisted of 8 non-diabetic, non-smoker, normotensive, normolipemic individuals with normal/insignificant coronary artery disease confirmed by carotid angiography (4 men, 4 women aged 50–60 yrs, mean = 44.5 ± 5.16 yrs) from each of whom 20 ml blood was also obtained. None of the patients and controls was on the Statin therapy.
Peripheral blood mononuclear cells (PBMCs) isolation
PBMCs were isolated by density gradient centrifugation (Ficoll–Paque PLUS, GE Healthcare Europe, GmbH, Germany) and cryopreserved in 10% dimethylsulfoxide (DMSO; Sigma–Aldrich) in fetal bovine serum (FBS Biosera, UK).
Flowcytometric analysis of memory T-cell subsets
For enumeration of Th17 and Th1 cells as well as the expression of co-inhibitory molecules, PBMCs (5 × 105 cells) were washed and stimulated with plate coated purified anti-CD3 (BD Pharmingen, 62.5 μg/ml) and soluble purified anti-CD28 (BD Pharmingen, 0.5 mg/ml) or phorbol myristate acetate (PMA, 50 ng/ml, Sigma) plus ionomycin (ION, 250 ng/ml, Sigma) at 37 °C and 5% CO2. Golgi-stop was added after 1 h and the cells were incubated for another 15 h at 37 °C and 5% CO2. Then the cells were washed and stained using conjugated antibodies: anti-CD45RO-FITC (BD Pharmingen), anti-CD4-PerCP (BD Pharmingen), and either anti-IFN-γ-APC (BD Pharmingen) plus anti-PD-1-PE (BD Pharmingen) or anti-GITR-APC (BD Pharmingen) plus anti-IL-17-PE (BD Pharmingen), and were incubated in 4 °C for 30 min. The cells were subsequently washed and resuspended in PBS containing 10% FBS. For each sample, 1 × 105 cells were required by FACScalibur flowcytometer. Live lymphocytes were gated on forward and side scatter and further analyzed for surface marker and cytokine expression. Flowcytometry analysis was carried out by flowjo software (version 7.6.2).
Flowcytometric analysis of GITR expression
For the analysis of GITR expression on memory T-cell subsets, PBMCs (5 × 105 cells) were washed and stimulated with plate-coated purified anti-CD3 (BD Pharming, 62.5 μg/ml) antibody and soluble purified anti CD28 (BD Pharmingen, 0.5 μg/ml) at 37 °C and 5% CO2. After 1 h Golgi-stop was added and cells were incubated for another 15 h at 37 °C and 5% CO2. Then the cells were washed and stained for surface markers using conjugated antibodies: anti-CD45RO-FITC (BD Pharmingen), anti-CD4− PerCP (BD Pharmingen), anti-GITR-APC (BD Pharmingen) in 4 °C for 30 min. The cells were subsequently washed and resuspended in PBS containing 10% FBS. Live lymphocytes were gated by forward and side scatter and analyzed for surface marker expression.
Statistical analysis
The Statistical analyses were performed using SPSS software (version 15, Chicago, IL) and GraphPad prism (version 5, La Jolla, CA). Mann–Whitney U test was used for non-parametric comparison of the medians; therefore, results were demonstrated as median and range. P-values less than 0.05 were considered significant. The subpopulations with less than 100 events were not included in the analysis.
Results
GITR expression on the CD4+ T-cell subpopulations
The frequencies of GITR expressing CD4+ T-cell subsets were also analyzed in the non-stimulated condition and after stimulation with anti-CD3 and anti-CD28 antibodies for patient and control groups. The representative dot plots of stimulated T-cells and the gating strategy used to detect the frequency of GITR+ T-cells are shown in Fig. 1A.
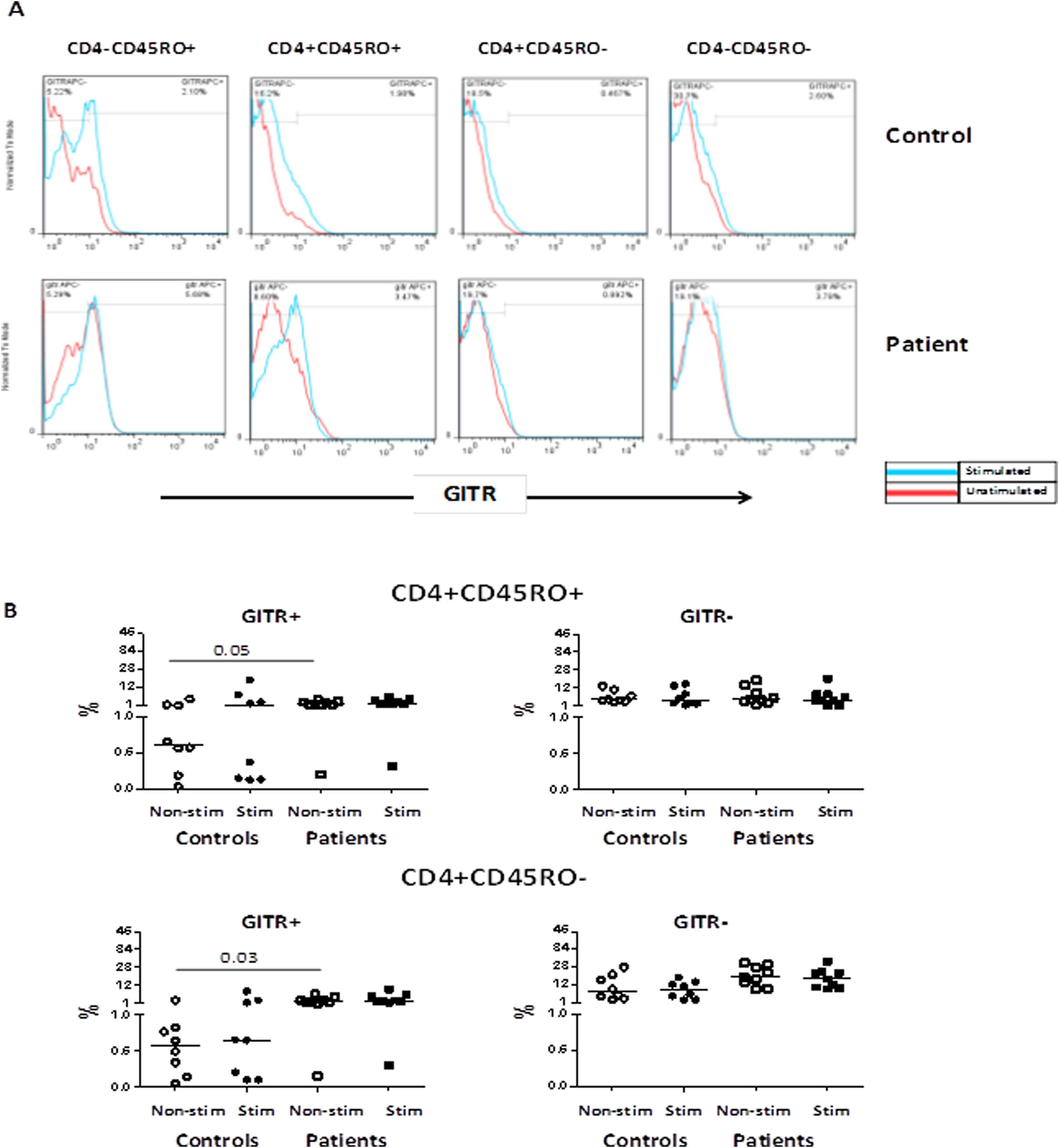
(A) Representative histograms indicating GITR expression in CD4+ T-cells and CD4− T-cells subpopulations in one patient and one control individual in non-stimulated condition and after stimulation with anti-CD3 and anti-CD28 antibodies; (B) Comparison of the frequencies of memory and effector CD4+ T-cell based on the GITR expression between patients and controls.
The highest percentage of GITR expression was observed on the CD4+CD45RO+ memory T-cells and CD4+CD45RO− effector T-cells in non-stimulated condition in patients as compared with controls (Fig. 1B). The stimulation affected the GITR expression levels on controls in memory CD4+ T-cells (Fig. 1B). However, there was no difference in the GITR MFI between groups before and after stimulation.
Frequencies of IL-17 and GITR expressing CD4+CD45RO+ memory T-cells
The main difference between patients and controls regarding this population was that the IL-17 producing memory T-cells were enriched in GITR molecule in the patient group. As shown in Fig. 2A and B, in patients group (but not controls) the CD4+CD45RO+GITR+IL-17+ memory T-cells, produced IL-17 even before stimulation (0.22, 1.32 vs. 0.05, 1.88, P = 0.03). The frequency of CD4+CD45RO+GITR+IL-17− T-cell subset in non-stimulated condition in patients was non-significantly higher than controls group (1.37, 4.009 vs. 0.56, 3.143, P = 0.09) and had no significant change after stimulation (2.21, 5.47 vs. 1.32, 13.57, P = 0.08). After stimulation with anti-CD3 and anti-CD28 antibodies, the frequency of CD4+CD45RO+GITR+IL-17− T-cell subset increased in the control group (1.32, 13.57 vs. 2.21, 5.47, P = 0.6).
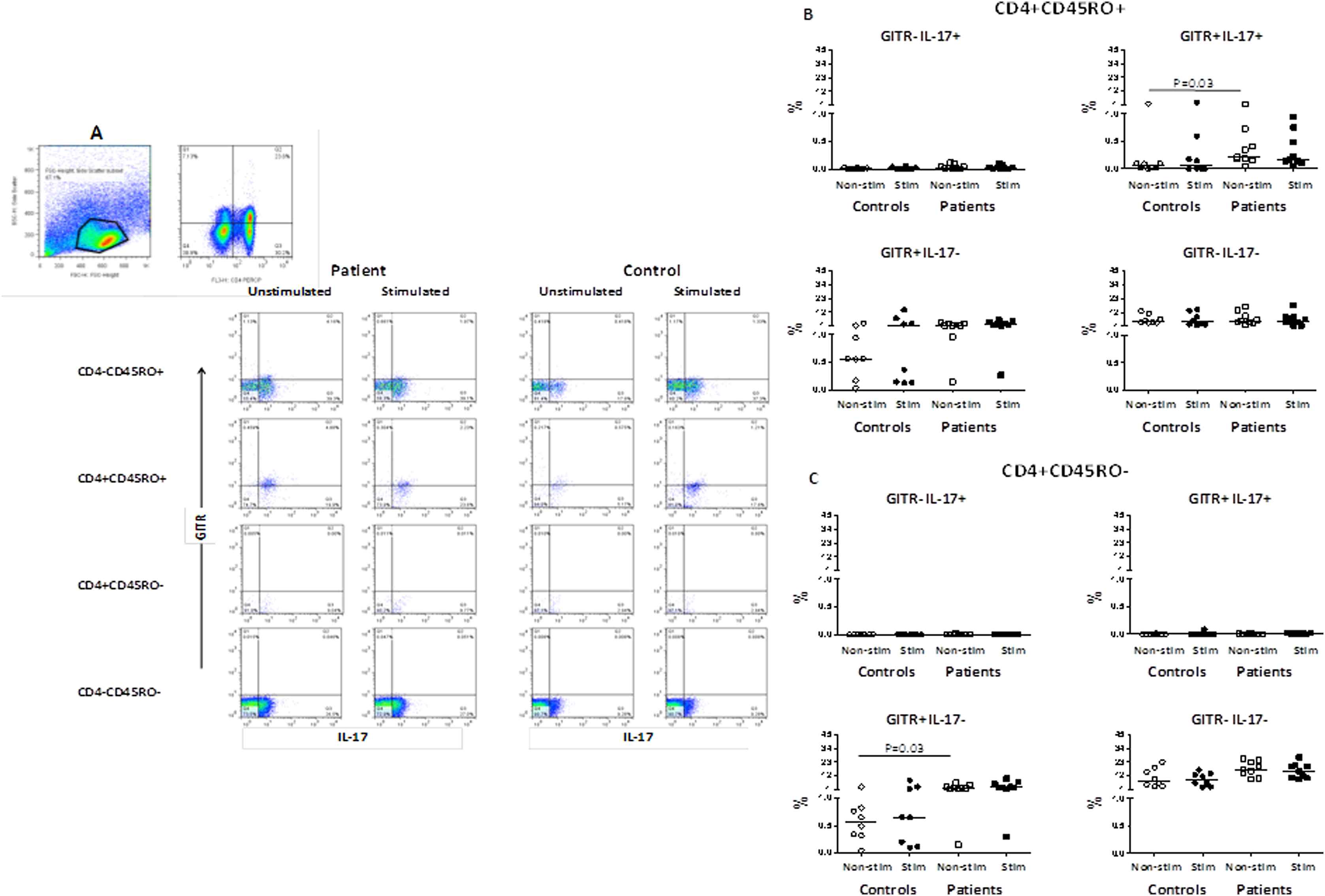
(A) Representative dot plots indicating GITR and IL-17 expression in CD4+CD45RO+ and CD4+CD45RO− T-cells in one patient and one control individual in non-stimulated condition and after stimulation with anti-CD3 and anti-CD28 antibodies, The gating was performed on lymphocytes followed by CD4 and CD45RO before gating for IL-17 and GITR molecules; (B) The frequencies of IL-17 and GITR expressing CD4+CD45RO+ T-cells (% total) in patients with atherosclerosis and in controls; (C) The frequencies of IL-17 and GITR expressing CD4+CD45RO− T-cells (% total) in patients with atherosclerosis and controls.
Frequencies of IL-17 and GITR expressing CD4+CD45RO− effector T-cells
As shown in Fig. 2A and C, the frequency of CD4+CD45RO-GITR+IL-17− T-cell subset in non-stimulated condition in patients was higher than in controls group (2.41, 6.44 vs. 0.57, 2.99, P = 0.03) and showed no significant change after stimulation (2.41, 6.44 vs. 2.56, 9.31, P = 0.07).
PD-1 expression on the CD4+ and CD4− T-cell subpopulations
The frequencies of PD-1 expressing CD4+ T-cell subsets were also analyzed in the non-stimulated condition and after stimulation with anti-CD3 and anti-CD28 antibodies for patient and control groups. The representative dot plots of stimulated T-cells and the gating strategy used to enumerate PD-1 T-cells are shown in Supplementary Fig. 3A. The highest percentage of PD-1 expression in patients was observed on the CD4+CD45RO+ memory T-cells as compared with controls; however, the difference was not significant (Fig. 3B). There was no difference in the PD-1 MFI between groups before and after stimulation.
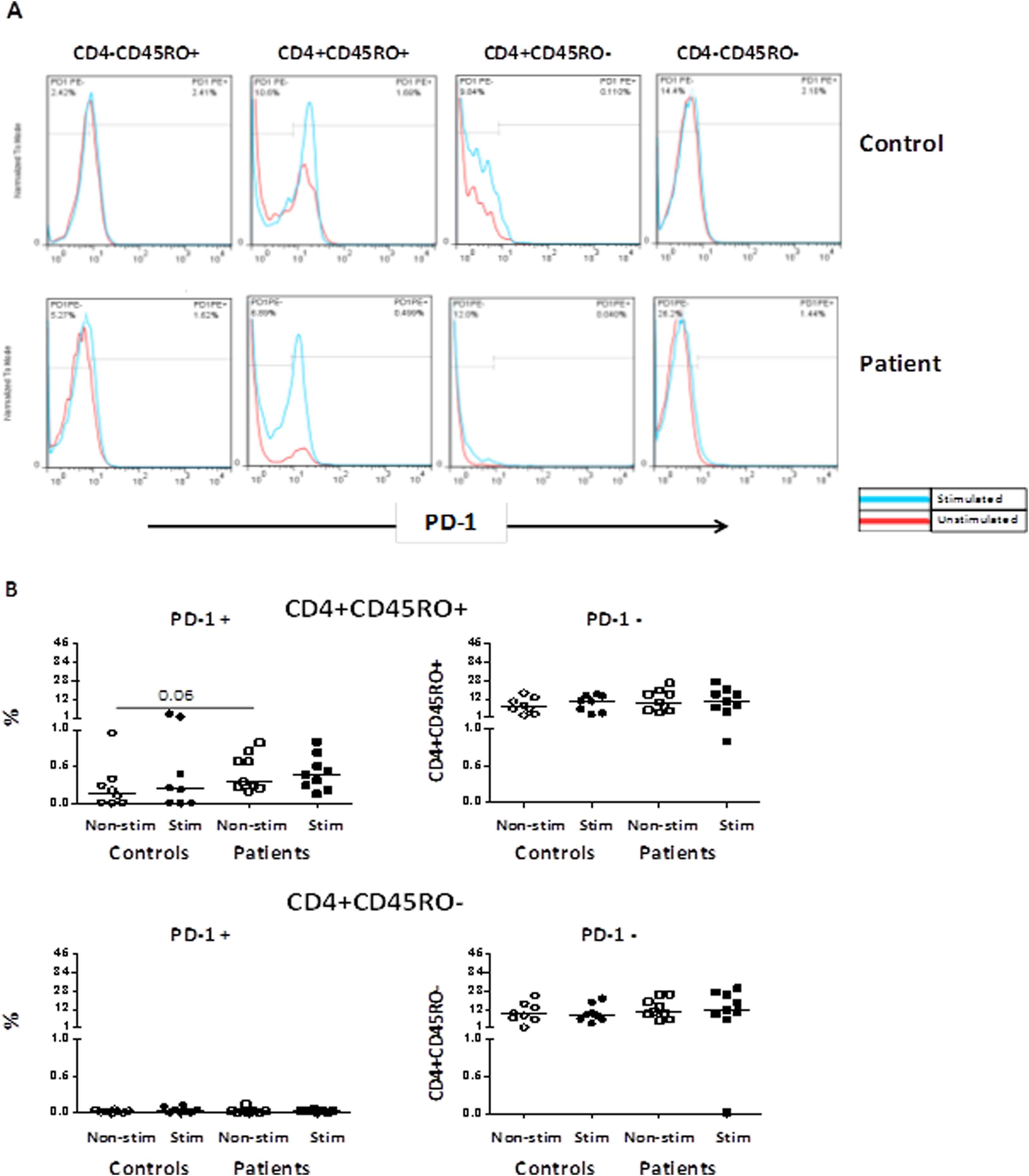
(A) Representative histograms indicating PD-1 expression in CD4+ T-cells and CD4− T-cells subpopulations in one patient and one control individual in non-stimulated condition and after stimulation with anti-CD3 and anti-CD28 antibodies; (B) Comparison of the frequencies of memory and effector CD4+ T-cell based on the PD-1 expression between patients and controls.
Frequencies of IFN-γ and PD-1 expressing CD4+CD45RO+ memory T-cells
As shown in Fig. 4A and B, in the non-stimulated condition, the frequencies of CD4+CD45RO+IFN-γ–PD-1+ memory T-cells in patients were higher than the control group (0.26, 0.62 vs. 0.12, 0.38, P = 0.05). A percentage of patients CD4+CD45RO+ T-cells that did not express PD-1 were producing IFN-γ even before stimulation. The frequencies of CD4+CD45RO+PD-1–IFN-γ+ memory T-cells increased after stimulation with anti-CD3 and anti-CD28 antibodies in both controls (0.68, 1.37, P = 0.03), and patients (1.00, 6.11, P = 0.06).
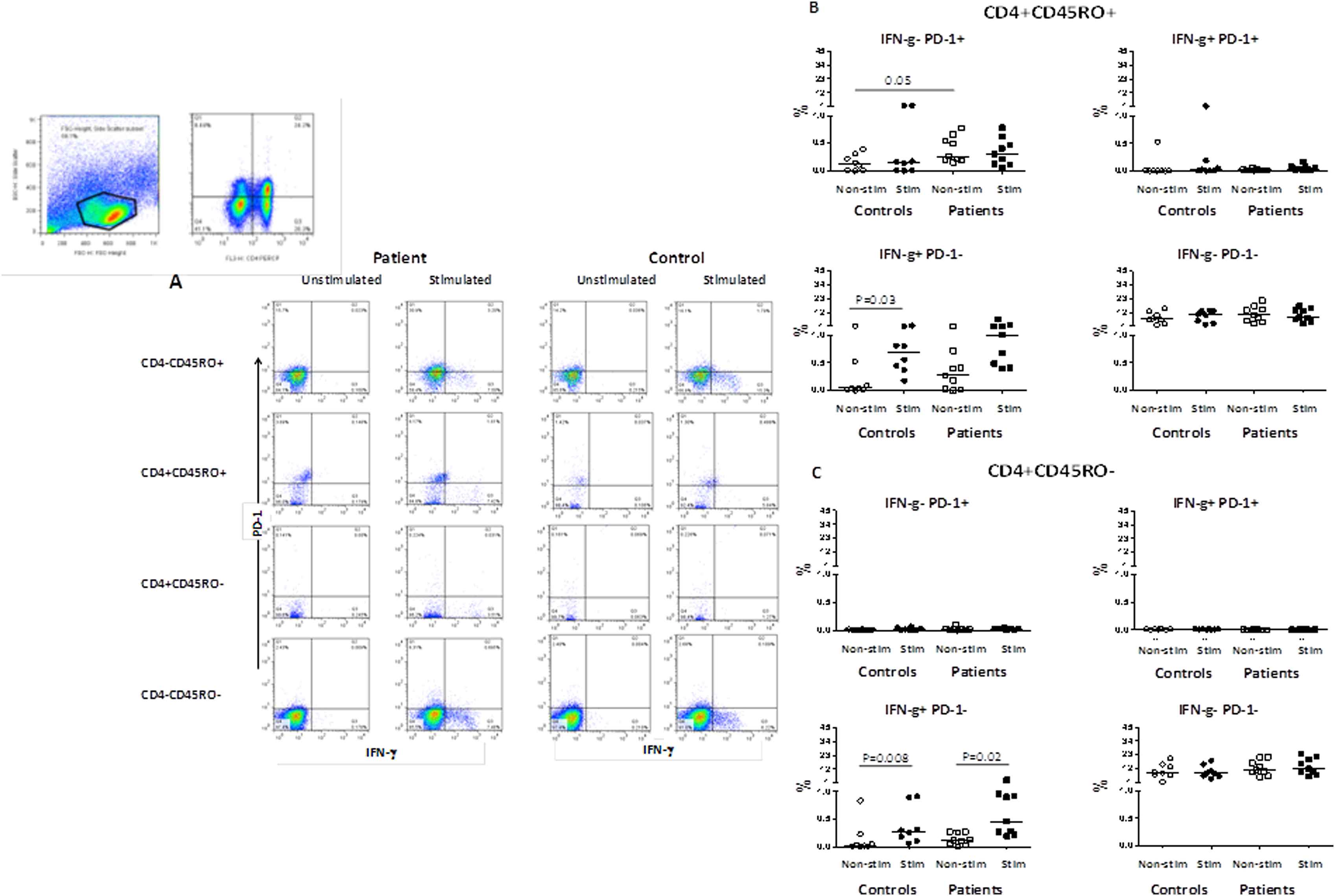
(A) Representative dot plots indicating IFN-γ and PD-1 expression in CD4+CD45RO+ and CD4+CD45RO− T-cells in one patient and one control individual in non-stimulated condition and after stimulation with anti-CD3 and anti-CD28 antibodies. The gating was performed on lymphocytes followed by CD4 and CD45RO before gating for IFN-γ and PD-1 molecules; (B) The frequencies of IFN-γ and PD-1 expressing CD4+CD45RO+ T-cells (% total) in patients with atherosclerosis and in controls; (C) The frequencies of IFN-γ and PD-1 expressing CD4+CD45RO− T-cells (% total) in patients with atherosclerosis and controls.
Frequencies of IFN-γ and PD-1 expressing CD4+CD45RO− effector T-cells
As shown in Fig. 4A and C, a small percentage of CD4+CD45RO− effector T-cells were producing IFN-γ in patients even before stimulation. After stimulation, the frequency of CD4+CD45RO–IFN-γ+PD-1− effector T-cells increased in both patients (0.46, 3.17, P = 0.02) and controls (0.27, 0.85, P = 0.008) as compared with non-stimulated condition (0.11, 0.27 vs. 0.02, 0.83, P = 0.20).
Discussion
In this study, we found an increased expression of GITR molecule on memory (CD4+CD45RO+) and effector (CD4+CD45RO−) T-cells in the peripheral blood of patients with atherosclerosis compared with control subjects.
Most of previous studies have suggested that GITR is a co-stimulatory molecule which plays a major role in inflammatory conditions. Studies of lung inflammation in GITR−/− mice have shown lower inflammatory response, decreased NO production and iNOS activity as well as decreased expression of adhesion molecules and infiltration of inflammatory cells in the absence of GITR molecule. In addition, GITR absence decreases lung injury and joint injury in experimental mice models.24,25 GITR signaling is involved in the process of inflammation and inflammatory diseases where stimulation of GITR results in the activation of matrix metalloproteinase-9 (MMP-9) and expression of TNF-α in primary macrophages.26 GITR and GITRL expression are also shown to be distributed in the human atherosclerotic plaque specimens.26 Thus it is not surprising that GITR expression is increased on the peripheral blood CD4+ T-cells during atherosclerotic inflammation.
In our study, analyzing the CD4+ T-cells functional subsets showed that the GITR molecule is overexpressed on IL-17+ T-cells in the CD4+CD45RO+ memory subset and IL-17− T-cells in the CD4+CD45RO− effector subset in patients. Among CD4+ T-cell subsets, mostly Th17 cells secrete IL-17, by which they induce epithelial and myeloid cells to release chemokines and cytokines and recruit neutrophils and macrophages. In atherosclerotic inflammation, these cells can promote plaque instability and enhance pro-inflammatory and pro-atherogenic phenotype.26 In addition, Th1 cells, which produce IL-12 and IFN-γ, mediate the expression of type A and B scavenger receptors that can recognize modified and oxidized-LDL (ox-LDL).27 Th1 cells can induce ECs inflammation and foam cells formation in atherosclerosis.28 Our findings showed that IFN-γ was produced by the PD-1− subset of CD4+CD45RO+ memory and CD4+CD45RO− effector T-cells in patients (Fig. 3b). Stimulation by anti-CD3 and anti-CD28 increased IFN-γ production in both T-cell subsets in patients and controls. These findings are in line with previous reports showing that Th1 cells employ different means to aggravate the progression of atherosclerosis and lead to the plaque instability.29 A fraction of CD4+CD45RO+ memory T-cells in patients, however, expressed PD-1 and lacked the potential to produce IFN-γ after stimulation (Fig. 4b). The expression of PD-1 on memory T-cells is suggested to be associated with aging.30,31 It is; however, not clear if these PD1 expressing memory CD4+ T-cells have diminished TCR-mediated proliferation and/or are prone to senescence as reported previously.31 Having said so, the scarcity of PD-1+CD4+CD45RO− effector T-cells in both groups challenges the significance of PD-1 inhibition in this subset as opposed to other diseases.32,33
GITR expression plays diverse roles in different subtypes of T-cells. GITR triggering in the presence of anti-GITR antibody or soluble GITRL can inhibit the suppressor effect of T regulatory cells.34,35 It is already shown that Treg cell function in atherosclerosis is diminished and depletion of peripheral Tregs increases atherosclerotic lesion size and plaque vulnerability in early stages of atherosclerosis.36–38 Therefore, an increase in the expression of GITR molecule can be an accelerating mechanism in atherogenesis and can weaken the Treg function in combating unwanted inflammation.
In a previous study, we demonstrated that the percentage of CD4+ T-cells producing both IL-17 and IFN-γ was increased in patients with atherosclerosis. Inflammatory environment and IL-6 can reduce the expression of FOXP3 and induce IL-17 expression in Treg cells.33,39 Therefore, the lower IFN-γ MFI and higher IL-17 MFI observed in patients compared to controls can be suggestive of the Th1 to Th17 shift.22 In the current study, we observed that the CD4+CD45RO+IL-17+ memory T-cells are also GITR+. While CD4+CD25+ Treg cells express high levels of GITR on their surface, it is interesting to see if the GITR+ T-cells in atherosclerosis are IL-17 producing Tregs.9,12 In any case, the GITR expression and IL-17 production by CD4+CD45RO+ T-cells along with the generally reduced function of Tregs, provides a favorable milieu for progressive inflammation. It is worth mentioning that our study has several limitations; one of the limitations of our study is that it only uses one methodology to evaluate the expression of GITR and PD-1. A detailed evaluation of gene expression and confirmation of protein expression of these molecules would be more appropriate. Moreover, using stringent criteria and high cost of experiments limited the number of patients and controls which are included in this study, and has decreased the power of conclusions.
Conclusion
Our study provides data on the expression of GITR molecule on IL-17 producing memory T-cells in patients with positive coronary artery disease confirmed by carotid angiography as compared to control subjects. A population among memory T-cells, which expressed PD-1 and was not producing IFN-γ, was also increased in patients’ peripheral blood. These data support the involvement of IL-17 producing memory T-cells in the atherosclerosis and suggest the modified phenotype/function of T-cell subsets under chronic inflammation.
Declaration of interest
The authors declare no competing interest.
Acknowledgments
This project was financially supported by a grant (
Appendix A
Supplementary data
Supplementary data related to this article can be found at
References
Cite this article
TY - JOUR AU - Atefe Ghamar Talepoor AU - Negar Behnamfar AU - Mohammad Javad Zibaeenezhad AU - Mehrnoosh Doroudchi PY - 2017 DA - 2017/12/27 TI - IL-17 producing CD4+CD45RO+ T-cells in atherosclerosis express GITR molecule JO - Artery Research SP - 20 EP - 28 VL - 21 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.12.004 DO - 10.1016/j.artres.2017.12.004 ID - Talepoor2017 ER -
