Impact of human immunodeficiency virus infection on arterial stiffness and wave reflections in the early disease stages
- DOI
- 10.1016/j.artres.2009.08.001How to use a DOI?
- Keywords
- Acquired immunodeficiency syndrome; Aortic stiffness; Cytokines; Vascular biology; Wave reflections
- Abstract
Background: Human immunodeficiency virus (HIV) infection is associated with subclinical inflammation and increased cardiovascular risk. Arterial stiffness and enhanced wave reflections are markers of cardiovascular disease and independent predictors of cardiovascular risk. The effect of HIV infection, per se, on aortic stiffness and wave reflections has not been clearly defined.
Methods: We studied 51 adults with a recent HIV infection, free of antiretroviral treatment and AIDS diagnosis, as well as 35 controls matched for age, sex and smoking status. Carotid-femoral pulse wave velocity (PWV) and timing of the reflected wave (Tr) were measured as indices of aortic stiffness, while aortic augmentation index (AIx) and augmented pressure (AP) were measured as indices of wave reflections.
Results: While PWV was similar in the two populations, Tr was significantly lower in HIV-infected subjects compared to controls (by 16.5ms, p=0.002). In addition, AIx and AP were decreased (by 6.4%, p=0.048 and by 3.3mmHg, p=0.010, respectively) in subjects with HIV infection. Moreover, HIV-infected patients compared with controls had increased values of hs-CRP [1.37 (0.85–2.53) vs. 0.75 (0.41–1.90) mg/l, p=0.007] and interleukin-6 [1.90 (0.91–3.9) vs. 1.28 (0.80–2.65) pg/ml, p=0.048]. Tr was negatively correlated with hs-CRP (r=−0.283, p=0.010) and interleukin-6 (r=−0.278, p=0.018).
Conclusions: Our study provides evidence of decreased wave reflections and similar aortic stiffness, as assessed by PWV, in the early stages of HIV infection in treatment-naive patients compared to controls. Subclinical inflammation and resultant peripheral vasodilatation constitute potential mediators of the whole pathophysiological process.
- Copyright
- © 2009 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
With successful antiretroviral therapy, patients infected with human immunodeficiency virus (HIV) are living longer. However, accumulating data suggest that HIV-infected patients experience unexpectedly high rates of cardiovascular (CV) disease.1–3
The pathogenesis of increased CV risk in this population and the role of HIV infection per se as a risk factor for accelerated arteriosclerosis remain unclear. Arterial stiffness and enhanced wave reflections are markers of CV disease and independent predictors of CV risk.4 Despite reports with increased aortic stiffness in HIV-infected patients receiving antiretroviral therapy, as well as in HIV treatment-naïve patients, results are, at least seemingly, discordant.5–8 Data on wave reflections have been limited to HIV patients on antiretroviral therapy showing either no difference in relation to the presence of lipodystrophy9 or increased values in infected patients compared to controls.5 Inflammation affects large-artery stiffness and wave reflections both through causal10 and associative11,12 relationships. The effect of HIV infection per se on aortic stiffness and wave reflections has not been clearly defined. For this purpose, in the present case-control study, we studied patients with a recent HIV diagnosis who had never received antiretroviral treatment and did not meet criteria of AIDS diagnosis (C stage of the Centers for Disease Control and Prevention classification).13
Methods
Study population
We studied 70 adult patients with documented HIV infection who had never received antiretroviral treatment or had a diagnosis of AIDS.13 Patients were recruited from the outpatient Infectious Diseases Unit, University of Athens, Hippokration Hospital and arterial function studies were performed at the Peripheral Vessels Unit of the 1st Department of Cardiology of Athens Medical School at Hippokration Hospital. The mode of infection of our study population was distributed as follows: homosexual contact, 66%; heterosexual contact, 21%; and unidentified cause, 13%. The median duration of HIV diagnosis was 0.67 years [range 0.23–2]. The mean CD4T-lymphocyte count was 486±121per mm3 and the mean log10 of HIV-1 RNA was 4.36±0.69. In order to avoid confounding factors that affect arterial function, potential participants with atherosclerotic cardiovascular and valvular disease, diabetes mellitus or impaired glucose tolerance, familial dyslipidemia, increased serum creatinine concentration or overt proteinuria and any other clinically significant concurrent systemic disease were excluded. Moreover, none of the participants had received any medical treatment nor had any history or clinical/laboratory evidence of any other underlying concurrent infection or inflammation 1 month prior to the study entry. HIV-negative control subjects matched with patients for age, sex and smoking status were recruited among the staff working at Hippokration Hospital. Overall, a total of 51 HIV-infected adults and 35 controls fulfilling the inclusion criteria were selected for participation.
Study design
The patients were studied supine after a 15-min rest, had fasted for at least 6h and had abstained from smoking, alcohol, and caffeinated beverages in the 12h before the study. All vascular studies were performed in a quiet, temperature-controlled room at 23°C. The study protocol included anthropometric and metabolic determinations, evaluation of aortic elastic properties and wave reflections, as well as measurement of high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6). Blood was drawn at the completion of vascular studies. The study protocol complies with the Declaration of Helsinki, was approved by our institutional ethics committee and all participants gave written informed consent.
Evaluation of aortic elastic properties and wave reflections
Pulse wave velocity
The pulse travels at a higher velocity in a stiff aorta and vice versa. Carotid-femoral pulse-wave velocity (PWV), an established index of aortic stiffness 4,14,15 was calculated from measurements of pulse transit time and the distance travelled between two recording sites (PWV=distance[m]/transit time[s]) using a validated non-invasive device (Complior; Artech Medical, Pantin, France) that allows online pulse-wave recording and automatic calculation of PWV.16 Two different pulse waves were obtained simultaneously at two sites (at the base of the neck for the common carotid and over the right femoral artery) with two transducers. The distance was defined as (distance from the suprasternic notch to femoral artery) – (distance from carotid artery to the suprasternic notch).
Central pressures and indices
The augmentation index (AIx) of the central (aortic) pressure waveform was measured as an index of wave reflections. AIx is a composite measure of the magnitude of wave reflections and arterial stiffness, which affects timing of wave reflections. The pressure waveform recorded at any site of the arterial tree is the sum of the forward-travelling waveform generated by pump ejection and the backward-travelling “echo” of the incident wave reflected at peripheral sites. The merging point of the incident and reflected waves can be identified on the pressure waveform as an inflection point.14 Augmented pressure (AP), the pressure added to the incident wave by the returning reflected wave, represents the pressure boost with which the left ventricle must cope that is caused by wave reflection. The AIx is defined as the AP divided by pulse pressure and is expressed as a percentage. Larger values of AIx indicate increased wave reflections from the periphery and/or earlier return of the reflected wave as a result of increased PWV (due to increased arterial stiffness) and vice versa. Because AIx is influenced by changes in heart rate, it was also corrected for a steady heart rate of 75 beats/min (AIx75). Timing of the reflected wave (Tr), i.e., the time the pulse wave needs to travel to the periphery and return to meet the incident wave is an index of PWV and was also calculated. All indices were measured by using a validated, commercially available system (SphygmoCor; AtCor Medical, Sydney, Australia) that employs the principle of applanation tonometry and appropriate acquisition and analysis software for non-invasive recording and analysis of the arterial pulse. The technique has been described in detail previously.14,17 In brief, from radial artery recordings, the central (aortic) arterial pressure was derived with the use of a generalized transfer function that has been shown to give an accurate estimate of the central arterial pressure waveform and its characteristics.14,17 Waveforms of radial pressure were calibrated according to sphygmomanometric systolic and diastolic pressure measured in the brachial artery because there is practically negligible pressure pulse amplification between the brachial and the radial artery.14
Measurement of inflammatory markers
Immediately after acquisition of venous blood, plasma or serum was separated by centrifugation (3000g at 4°C for 15min), placed in aliquots, and stored at −70°C for the measurement of inflammatory markers. Hs-CRP was measured by immunonephelometry (Dade Behring). High-sensitivity IL-6 was measured with specific ELISA (R&D Systems). Measurement of IL-6 was performed in 25 out of 35 control subjects.
Statistical analysis
SPSS statistical package, release 12.0 (SPSS Inc., Chicago, Illinois, USA) was used for all statistical analyses. Normality was tested using the Kolmogorov–Smirnov criterion. Logarithmic transformation was performed for skewed distributions before any parametric analysis. Significant differences between the study sub-groups were determined using the Student independent-samples t test or the chi-square test where appropriate. Analysis of covariance (ANCOVA) was performed in order to detect any influence of mean arterial pressure on PWV differences between HIV-infected subjects and controls. Correlation analyses were performed using the Pearson’s correlation coefficient. Descriptive statistics were arithmetic means±SD or medians (interquartile range) for skewed data. Statistical significance was set at p<0.05.
Results
Demographic and anthropometric characteristics
Due to matching, age, sex and smoking status distribution were similar in the two study sub-groups. HIV-infected subjects compared with controls had significantly lower body mass index (by 3kg/m2, p=0.003), while there was no difference regarding waist circumference (Table 1).
| HIV (+) patients (n=51) | Control subjects (n=35) | p | |
|---|---|---|---|
| Age, years | 33.1±8 | 33.2±8 | 0.93 |
| Males (%) | 92 | 88.5 | 0.71 |
| Height (m) | 1.76±0.07 | 1.78±0.07 | 0.69 |
| Body mass index (kg/m2) | 23.7±3 | 26.7±5.2 | 0.003 |
| Waist circumference (cm) | 83.5±9.3 | 86.7±11.4 | 0.25 |
| Brachial SBP (mmHg) | 125.7±12 | 126.9±11.6 | 0.66 |
| Brachial DBP (mmHg) | 72.5±9.2 | 77.3±10.7 | 0.033 |
| Brachial Pulse Pressure (mmHg) | 53.2±8.5 | 49.5±7.5 | 0.045 |
| Mean Arterial Pressure (mmHg) | 90.8±9.5 | 93.8±10.5 | 0.11 |
| Glucose (mg/dl) | 90.8±12.8 | 90.5±14.3 | 0.94 |
| Total cholesterol (mmol/L) | 4.48±0.8 | 5.00±0.81 | 0.014 |
| LDL-cholesterol (mmol/L) | 2.83±0.74 | 3.27±0.81 | 0.024 |
| HDL-cholesterol (mmol/L) | 1.01±0.24 | 1.24±0.23 | <0.001 |
| Triglycerides (mmol/L) | 1.25±1.16 | 1.06±0.67 | 0.45 |
| hs-CRP (mg/l) | 1.37 (0.85–2.53) | 0.75 (0.41–1.90) | 0.007 |
| IL-6 (pg/ml) | 1.90 (0.91–3.9) | 1.28 (0.80–2.65)a | 0.05 |
Data are expressed as means±SD or medians (interquartile range) for skewed distributions or as a percentage, SBP & DBP=systolic & diastolic blood pressure; LDL=low-density lipoprotein; HDL=high-density lipoprotein; hs-CRP=high-sensitivity C-reactive protein; IL-6=Interleukin 6. Bold letters are used in order to emphasize the existence of a significant p value.
For IL-6 n=25.
Demographic, clinical and laboratory parameters in HIV-infected patients and in control subjects.
Biochemichal parameters and inflammatory markers/mediators
HIV-infected subjects compared to controls exhibited significantly lower levels of total cholesterol (by 20.2mg/dl, p=0.014), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) (by 17mg/dl, p=0.024 and by 8.7mg/dl, p<0.001 respectively) while the higher values of serum triglycerides in the former population were not statistically significant. Moreover, HIV-infected patients compared with controls had significantly increased values of hs-CRP [1.37 (0.85−2.53) vs. 0.75 (0.41–1.90) mg/l, p=0.007] and IL-6 [1.90 (0.91–3.9) vs. 1.28 (0.80–2.65) pg/ml, p=0.048] (Table 1).
Hemodynamic and arterial function variables
Heart rate was higher in HIV-infected patients (by 6.4bpm, p=0.012) compared to controls. HIV-infected subjects had lower diastolic brachial blood pressure (BP) (by 4.8mmHg, p=0.003) and higher brachial pulse pressure (by 3.7mmHg, p=0.045), whereas there was no difference regarding systolic BP and mean arterial pressure (Table 1). Aortic diastolic BP was significantly lower in HIV-infected subjects compared to controls (by 5.7mmHg, p=0.017), aortic systolic BP was marginally decreased (by 4.6mmHg, p=0.059) while there was no difference in aortic pulse pressure. PWV was similar in the two populations even after adjustment for mean arterial pressure (adjusted p=0.20). However, Tr was significantly lower in HIV-infected subjects compared to controls (by 16.5ms, p=0.002). In addition, AIx and AP were lower (by 6.4%, p=0.048 and by 3.3mmHg, p=0.010, respectively) in subjects with HIV infection (Table 2). AIx75 was lower in HIV patients compared to controls (by 3.8%) to a suggestive level of statistical significance (p=0.21).
| HIV+ patients (n=51) | Control subjects (n=35) | p | |
|---|---|---|---|
| Central SBP (mmHg) | 106.7±10 | 111.3±11.8 | 0.059 |
| Central DBP (mmHg) | 72.9±9 | 78.6±10.9 | 0.017 |
| Central pulse pressure (mmHg) | 33.7±5 | 32.7±5.8 | 0.38 |
| Heart rate (bpm) | 75.4±11 | 69±9 | 0.012 |
| Pulse wave velocity (m/s) | 6.4±0.8 | 6.3±1 | 0.74 |
| AIx (%) | 2.5±14 | 8.9±14 | 0.048 |
| AIx75 (%) | 2.8±13.7 | 6.6±13.2 | 0.21 |
| Augmented pressure (mmHg) | 1±5.1 | 4.3±4.6 | 0.010 |
| Tr (ms) | 152±17.5 | 168.5±24.5 | 0.002 |
Data are expressed as means±SD SBP & DBP=systolic & diastolic blood pressure; AIx=augmentation index, AIx75 =AIx corrected for heart rate; Tr=timing of the reflected wave. Bold letters are used in order to emphasize the existence of a significant p value.
Aortic stiffness and wave reflections parameters in HIV-infected patients and in control subjects.
In the entire study population, PWV correlated positively with age (r=0.348, p=0.001), body mass index (r=0.295, p=0.008), aortic systolic (r=0.472, p<0.001) and diastolic BP (r=0.528, p<0.001), brachial systolic (r=0.377, p=0.001) and diastolic BP (r=0.516, p<0.001), as well as with hs-CRP (r=0.243, p=0.0130) and LDL-cholesterol (r=0.374, p=0.001) and correlated negatively with Tr (−0.352, p=0.001). AIx correlated positively with age (r=0.480, p<0.001), body mass index (r=0.249, p=0.024), waist circumference (r=0.249, p=0.049), aortic systolic BP (r=0.246, p=0.026) and pulse pressure (r=0.292, p=0.008) and correlated negatively with brachial pulse pressure (−0.289, p=0.008) and heart rate (r=−0.311, p=0.005). Moreover, Tr correlated positively with HDL (r=−0.297, p=0.011) and correlated negatively with age (r=−0.404, p<0.001), AIx (r=−0.387, p<0.001), PWV (r=−0.352, p=0.001), aortic systolic (r=−0.270, p=0.014) and diastolic BP (r=−0.229, p=0.039), brachial diastolic BP (r=−0.273, p=0.013), as well as with log hs-CRP (r=−0.283, p=0.010) and log IL-6 (r=−0.278, p=0.018). Heart rate also correlated with IL-6 (r=0.266, p=0.026).
Discussion
This is the first study, to the best of our knowledge, to show that patients in the early stages of HIV infection who had never received antiretroviral treatment or had a diagnosis of AIDS exhibit decreased wave reflections and similar levels of aortic stiffness, as assessed by PWV, compared to controls. These findings elucidate pathophysiological links and may have implications for the overall CV risk profile in these patients.
A growing body of evidence indicates in recent years, that people with HIV infection, especially those on antiretroviral therapy, are at a significantly greater risk for coronary heart disease and myocardial infarction than uninfected people of the same age.1–3 Moreover, a high proportion of asymptomatic myocardial ischemia was found in HIV infected adults, irrespectively of their antiretroviral treatment status.18 Interestingly, interruption of antiretroviral treatment was associated with an increased short-term risk of CV disease in the Strategies for Management of Antiretroviral Therapy (SMART) Study, highlighting the complicated nature of the mechanisms underlying CV risk in such patients.19 Dyslipidemia and endothelial dysfunction are among the main underlying factors directly associated with high CV risk.20–22
Arterial stiffness is an independent marker of CV disease and a predictor of the corresponding risk.4 Assessment of arterial wall properties is increasingly used as a surrogate marker of future events in various clinical settings including HIV-infected patients. Results in this latter setting have been diverse owing to several factors. Carotid wall stiffness has been found to be increased in HIV-infected children.20 As regards aortic stiffness, in HIV-infected patients receiving antiretroviral therapy PWV has been shown to be both increased5,7,8 and unaffected.7 In HIV treatment-naïve individuals with or without AIDS, Schillaci et al. recently reported that these patients have greater values of PWV than healthy controls.6 Our finding of unaltered PWV, in HIV treatment-naïve individuals is not necessarily in contrast with the aforementioned report, since the infection in our population had been more recently acquired according to the shorter duration of diagnosis.
Regarding wave reflections, HIV-treated patients exhibit increased values compared to uninfected subjects,9 while no difference was observed between HIV-treated patients with lipodystrophy and those without, although duration of antiretroviral therapy was a predictor of AIx.5 Our study is the first to show decreased wave reflections indices in HIV-treatment naïve patients without AIDS diagnosis compared with controls, possibly owing to the impact of HIV infection per se. The fact that wave reflections are decreased despite a lower transit time could be attributed to marked peripheral vasodilatation and this corroborated by previous studies from our laboratory.10 Notably, the treatment-naïve patients in our study had a heart rate similarly increased to those in the study conducted by Schillaci et al.,6 despite matching for other parameters, and this is most likely due to HIV infection itself. This increase in heart rate may account, but not entirely, for the decrease in AIx and central BP values. A hypothesis unifying previously published and present findings and incorporates factors such as duration of infection and antiretroviral treatment is presented in Figure 1. Accordingly, while increased CV risk cannot be inferred from the present findings in the particular setting studied, the results of the present and previous studies taken together indicate that risk is multifactorial involving infection per se, duration of infection, viral load and treatment.
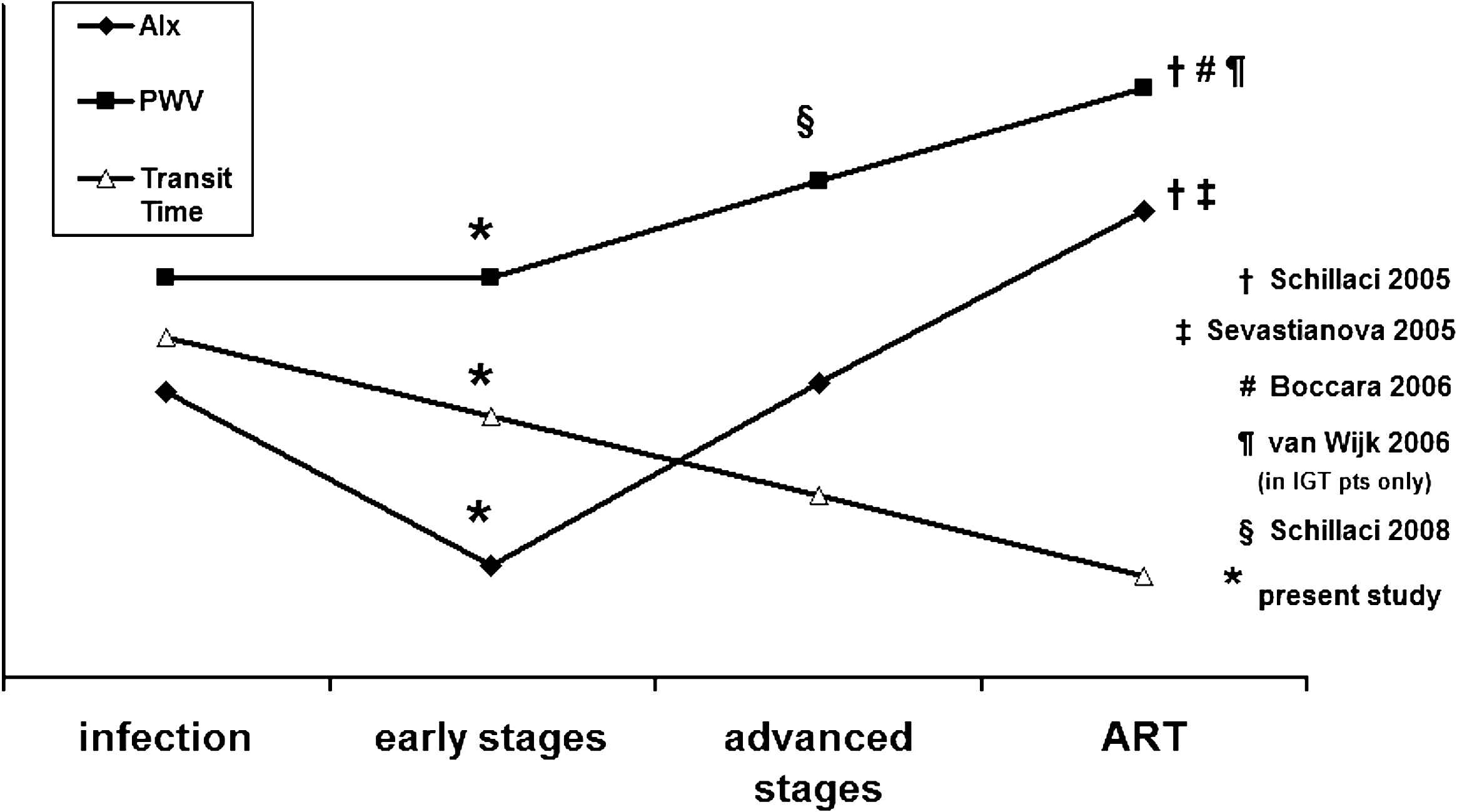
Diagram unifying results of previously published reports and of present study. Impairment of arterial function indices is related to the stage of the disease and the administration of antiretroviral therapy. AIx=augmentation index, PWV=pulse wave velocity, ART=antiretroviral therapy.
Multiple plausible pathophysiological mechanisms may account for our findings. Acute inflammation leads to an increase in arterial stiffness and a decrease in wave reflections,10 while in chronic systemic inflammatory diseases aortic stiffness is increased and data on AIx are not consistent, possibly due to different disease stages.23,24 As regards HIV infection, although it causes immunosuppression with attenuated inflammatory response to certain opportunistic infections, HIV infection in the chronic phase is characterized by subclinical, low-grade inflammation.25,26 HIV and its proteins, such as gp120, Tat, and Nef induce expression of inflammatory cytokines and increase endothelial permeability through enhanced expression of IL-6.25–28 Furthermore, although the role of CRP in induction of vascular functional changes is not straightforward,12,29 it has been shown that levels of this acute phase protein are associated with HIV disease progression independent of CD4 lymphocyte counts and HIV RNA levels.30 Based on the association of IL-6 and hs-CRP with Tr, along with the positive correlation of IL-6 with heart rate, one could suggest that HIV infection per se affects arterial function through activation of pro-inflammatory pathways.
Arterial stiffness is dynamically dependent on vascular tone 31 and recently it was shown that HIV infects smooth muscle cells in the atherosclerotic plaque both in vivo and in vitro,32 thus highlighting the complicated nature of the mechanisms underlying vascular changes in HIV infection.
Abnormal lipid metabolism may account for the arterial changes observed in our study.33 Indeed, HIV infection itself is associated with dyslipidemia and changes include an early decrease in HDL cholesterol and elevations in triglycerides, while LDL cholesterol decreases later in the course of the disease.25,34 In our study population, HDL was significantly lower in HIV-infected patients and was positively correlated to Tr.
With respect to the limitations of our study, its cross-sectional design does not allow conclusions on causality regarding the observed relationships. Additionally, HIV-infected patients were significantly leaner than controls despite matching for age and sex. Although this may account partly for the changes observed, it could also be viewed as an inherent feature of the disease since it appears to be consistent among studies.6
In conclusion, our study provides evidence of decreased wave reflections and similar PWV in the early stages of HIV infection in treatment-naive patients free from cardiovascular disease and AIDS diagnosis compared to controls. Subclinical inflammation and resultant peripheral vasodilatation are plausible mediators. These findings constitute potential mechanisms in a multifactorial paradigm explaining early atherosclerosis and vasculopathy reported in individuals infected with HIV. Thorough assessment of arterial elastic properties could be considered for HIV patients, even in the early stages of the disease, with the aim of implementing intensive lifestyle and risk factor interventions in order to prevent future events.
Conflict of interest
There is no conflict of interest.
Funding
None.
References
Cite this article
TY - JOUR AU - Charalambos Vlachopoulos AU - Helen Sambatakou AU - Dimitris Tsiachris AU - Ilias Mariolis AU - Konstantinos Aznaouridis AU - Nikolaos Ioakeimidis AU - Athanasios J. Archimandritis AU - Christodoulos Stefanadis PY - 2009 DA - 2009/10/09 TI - Impact of human immunodeficiency virus infection on arterial stiffness and wave reflections in the early disease stages JO - Artery Research SP - 104 EP - 110 VL - 3 IS - 3 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2009.08.001 DO - 10.1016/j.artres.2009.08.001 ID - Vlachopoulos2009 ER -
