ADVANCES IN ARTERIAL STIFFNESS ASSESSMENT
- DOI
- 10.1016/j.artres.2011.10.003How to use a DOI?
- Keywords
- Arterial stiffness; Pulse wave velocity; Wave analysis; Wave reflection; Ultrasound
- Abstract
Although the clinical relevance of arterial stiffness is increasingly recognized, the applicability of arterial stiffness for individual cardiovascular risk assessment is hampered due to technical and physiological difficulties. Arterial stiffness is not constant with blood pressure and not constant over the arterial tree. Currently, stiffness is commonly assessed in individuals over a long trajectory and neglects the pressure dependency. To circumvent these problems, we developed a technique to measure pulse wave velocity (PWV) locally using multiple M-line ultrasound. In the common carotid artery, PWV can only be measured using the dicrotic notch of the distension waveform as fiducial time-point, because the systolic foot is subjected to reflective interference. Dicrotic notch PWV provides a measure of stiffness at near systolic pressure level, which is intrinsically different from systolic foot PWV measured at diastolic pressure. To investigate the effect of pressure on local stiffness, we quantified carotid distensibility coefficients for the diastolic and systolic pressure ranges separately. We found that the diastolic-systolic difference in carotid distensibility varies significantly between individuals and is an independent determinant of left ventricular mass index. Moreover, this pressure dependency appears to increase with age (like arterial diameter), suggesting that this property could be used as a marker for structural remodeling of the artery wall. Biomechanically, the pressure dependency of stiffness directly affects pressure and flow waveform characteristics and their phase relation. Ignoring this may lead to overestimation of the impact of wave reflections on central blood pressure. Our work shows that pressure dependency of arterial stiffness can and should be accounted for to evaluate its implication for pressure augmentation and wave separation analysis.
- Copyright
- © 2011 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Background
Aging and cardiovascular diseases are associated with stiffening of the arteries1–3 which is associated with increased systolic blood pressure and pulse pressure and, consequently, an increased load on the heart.4,5 There is increasing evidence that in patients with borderline hypertension arterial stiffening may precede the development of hypertension.6 In a variety of studies it has been shown that arterial stiffening is associated with the risk for coronary artery disease and stroke.2,7–9 Moreover, local changes in arterial stiffness might be indicative of atherosclerosis.4,10 a degenerative process of the arterial wall involved in many cardiovascular diseases, like myocardial infarction and stroke. Therefore, arterial stiffness is a good candidate for the assessment of cardiovascular risk. Indeed, recently arterial stiffness has been included in the guidelines of the European Society of Hypertension and Cardiology for risk assessment.11
Characteristics of the arterial tree
The arterial tree is composed of elastic and muscular arteries, which differ in collagen, elastin and smooth muscle content. The central elastic arteries are generally less stiff than the muscular arteries and, therefore, deliver the largest contribution to the buffering capacity of the arterial system. Moreover, vascular remodeling might differ between muscular and elastic arteries, leading to spatial differences in age-related changes in arterial stiffness.4 In addition, atherosclerotic lesions might change the mechanical properties of a specific artery locally. Therefore, it is important to measure arterial stiffness locally. Arterial stiffness differs not only between arterial segments but also changes with blood pressure.12–15,16 This intrinsic property of the arterial system may have serious consequences for quantitative assessment of arterial stiffness and possibly antihypertensive treatment.17–20
Current stiffness assessment
Local elastic properties of a blood vessel are usually quantified by the distensibility coefficient. The distensibility coefficient is derived from the locally measured diameter waveform and pulse pressure values obtained from another artery, e.g. the radial or brachial artery,6 assuming a linear relationship between cross-section and distending pressure. However, central pulse pressure may differ substantially from peripheral blood pressure in shape and in value. Toward the periphery pulse pressure is amplified, because of tapering,21 wave reflections and a progressive increasing arterial stiffness, due to an increase of smooth muscle content in the artery wall.22 Waddell et al. found a difference between systolic pressures as measured in the carotid and brachial arteries of 8 mmHg in healthy controls, for patients with severe coronary artery disease this difference decreased to 2 mmHg.23 This disease (and age) related difference between carotid and brachial systolic blood pressure implies that carotid systolic pressure can only be estimated from peripheral blood pressure, for example as proposed by van Bortel et al.24
Pulse wave velocity (PWV), the speed at which the arterial pulse wave propagates along the arterial system, is a measure of arterial stiffness. The method most commonly used is the PWV measured over the carotid-femoral arterial trajectory, and is calculated as the ratio between the distance between the measurement sites and the transit time delay between these two sites. The carotid-femoral PWV (cfPWV) represents PWV averaged over a long segment composed of arteries with different mechanical characteristics. Although population studies have shown that increased cfPWV, indicating enhanced arterial stiffness, is a predictor of coronary heart disease and stroke,2,7 a recent study revealed that cfPWV is of only minor additional value for cardiac risk stratification in the elderly free from coronary heart disease.25 The assessment of cfPWV is subject to error, because of lack of standardization of the method,26 e.g. the different timing algorithms used to assess the transfer time cannot be used interchangeably.27 Moreover, since the carotid and femoral pulse waves travel in opposite directions, the use of the distance between the carotid and femoral artery will cause overestimation of the PWV measured. An additional source of error is the distance measurement (normally made with a tape measure), assuming a straight arterial segment. Because of the above, a direct comparison between local stiffness calculated from the distensibility coefficient, and converted to PWV by means of Bramwell–Hill equation,28 and cfPWV is questionable.29
Local pulse wave velocity
An alternative for cfPWV is local PWV, which can be measured by means of an ultrasound scanner with high temporal and spatial resolution.30–32 In this technique multiple diameter waveforms are recorded simultaneously at fourteen locations equidistantly spaced over 16.4 mm. Linear regression is performed on the time-reference points and their corresponding positions of each diameter waveform, the reciprocal of the regression slope being a direct measure of the PWV (Fig. 1). In a phantom set-up, we showed that local PWV by means of ultrasound can be obtained with good precision (coefficient of variation 0.5%) and accuracy (relative error 3%).31 Using the same technique, we observed that the systolic foot, signaling the incident wave, is followed within about 40 ms by a reflected wave propagating in opposite direction.16 These early wave reflections lead to inaccurate local PWV estimates, because the systolic foot detection is subject to reflective interference (Fig. 1f).16 Indeed the expected age-related differences in carotid artery stiffness could not be detected by means of the local PWV based on the systolic foot transit time.30 However, when using the dicrotic notch as time-reference point, local PWV could detect age-related difference in arterial stiffness.30 Compared to cfPWV, local PWV requires an improved temporal as well as spatial resolution, because of the short transit time; a PWV of 5 m/s converts to a transit time of only 3.3 ms over a distance of 16.4 mm. Therefore, we have to consider some technical and physiological aspects that influence precision, i.e., a measure of reproducibility, as well as accuracy (or bias), i.e., the degree of closeness to the actual value.

(a) Example of 14 arterial distension waveforms of a subject, displayed with an off-set (bottom trace is proximal registration). (b) Corresponding acceleration waveforms, the circles indicate the characteristic time-points of the systolic foot (SF) and the dicrotic notch (DN). (c) The same 14 acceleration waveforms but over a short time interval around the systolic foot. (d) The same 14 acceleration waveforms but over a short time interval around the dicrotic notch. (e) Example of linear regression between time-point of the dicrotic notch (dependent variable) and the M-line position (independent variable). The pulse wave velocity (PWVdn) is the reciprocal of the slope of the regression line. (f) An example of backward propagating reflective waves, interfering with the systolic foot detection in the most distal traces of the acceleration waveform
Technical aspects of local pulse wave velocity assessment
Temporal resolution
In a phantom set-up we showed that the method is sensitive to the frequency characteristics of the filter applied to the distension waveforms.31 If small wave perturbations in the distension waveform are not filtered out adequately due to a too high cut-off frequency of the low pass filter, the precision of the PWV estimate degrades strongly (coefficient of variation went up from 0.5% to 10.5%).31 These small wave perturbations can be induced by arterial wave reflections. In contrast, lowering the cut-off frequency of the low pass filter too much will increase the effect of reflected waves on the incident waves and the accuracy of the PWV estimate will deteriorate.16
Spatial resolution
To reduce the effect of changes in speckle pattern in the ultrasound signal, a large spatial (transmural) window should be used to track arterial wall displacement. Assuming constant wall volume, wall thickness will decrease inversely with an increase in diameter. Consequently, a large spatial window will cover wall parts exhibiting gradually decreasing displacements, resulting in a lower distension estimate. Since in multiple M-line systems this phenomenon will occur for all lines similarly, a large spatial window will probably hardly affect the accuracy of PWV estimates, but will improve precision.
Distance dictated by the ultrasound probe
A major advantage of the proposed local PWV measurement is that it does not require distance measurements as the propagation distance is determined by the spacing between the piezo-electrical elements of the ultrasound probe. In a phantom set-up we showed that the residual of the PWV estimate shows a peculiarly regular pattern, likely caused by misalignment of piezo-electrical elements,31 causing a slight variation in ultrasound beam orientation and, hence, a bias in the PWV estimates. Although this bias increases with increasing PWV, the error (bias divided by true PWV) is independent of the PWV, but will be larger for arteries located at a larger distance from the ultrasound probe.
Active array of ultrasound probe
In our local PWV method less than half of the ultrasound probe is used: only 16.4 mm out of a total of 40 mm. A larger active array range will increase the wave transit time and, hence, the PWV estimate will become less sensitive to random noise. On the other hand distension waveforms measured closer to the reflections site, as might occur when the active array of the probe is larger, may encounter greater interference of reflections, biasing the PWV estimate.
Number of M-lines
By reducing the interspacing between the M-lines, a larger number of M-lines is obtained without increasing the active array size. The originally selected distance between the M-lines in multiple M-mode matches the focal width of the ultrasound beam. Hence, a further decrease of the interspacing makes the M-lines statistically dependent on each other. Consequently, a decreased interspacing will only hardly improve the precision of the PWV estimate.
Online feedback
The quality of the pulse wave velocity estimate, i.e. its standard error, is directly related to the regression coefficient. Although non-random noise, like reflective interference, might decrease the quality of the PWV estimate, it does not necessarily deteriorate the regression coefficient. The ultrasound system used in our studies16,30,31 did neither provide real-time feedback about the distension waveforms or its derivatives nor standard error readings. Online presentation of the distension waveforms, as in more recent systems, may optimize the quality of the recordings and in combination with the online display of the regression coefficient the accuracy of the pulse wave velocity estimate.33
Physiological aspects of local pulse wave velocity
Pulse wave velocity in stiff arteries
The quality of the PWV estimate may be sensitive to the degree of arterial stiffness itself. Increased arterial stiffness is associated with decreased distension and, therefore, noise on the distension waveform will be more pronounced, affecting the accuracy with which time-reference points in the distension waveforms can be identified. Moreover, higher pulse wave velocities are associated with lower transit times, and the influence of random noise on transit time measurements will be more dominant. In addition, with increased PWV reflections will arrive earlier in the cardiac cycle and the PWV estimated may be subject to interference of reflected waves.
Wave reflections in the arterial system
We showed that an inflection point in the upstroke of the carotid artery distension waveform is propagating backwards and consequently signals a reflected wave. By using the locally measured PWV and the round trip time, the distance from the measurement site to the reflection site, i.e., the time difference between the incident wave, signaled by the systolic foot, and the reflected wave, signaled by the inflection point, can be determined. In this way the distance from the measurement site in the common carotid artery to the site of reflection was estimated to be at about 13 cm.16 However, the reverse process, i.e. the use of the round trip time to calculate PWV, as proposed by Qasem and Avolio,34 however, is inappropriate.35 There are many reflection sites in the arterial tree, not one36 while wave reflections are continuously generated due to arterial tapering.37 Even in case of a single reflection site, inter-individual differences in time appearance of the inflection point in the distension waveform have to be appreciated due to variations in anatomy and/or artery wall properties. The latter was confirmed experimentally by Westerhof et al. demonstrating that a fixed reflection site in the arterial tree is elusive.38
Frequency dependency of wave velocity
The acceleration waveform, from which the PWV is obtained using either the systolic foot or the dicrotic notch as time-reference point,30 has a dominant frequency of about 30 Hz.16 The phase velocity is a frequency dependent variable39 and, hence, the observed PWV corresponds to the phase velocity at about 30 Hz. The phase velocity at higher frequencies (>5 Hz) is very close to the wave speed derived with the Moens-Korteweg equation for an artery with a purely elastic wall carrying an in-viscid fluid. Therefore, our local PWV method provides a direct measure for the stiffness of an artery and is directly related to the distensbility coefficient, using the Bramwell–Hill equation.28,30
Pressure dependency of arterial stiffness
Apart from the described methodological differences between dicrotic notch local PWV and cfPWV, the innate pressure dependency of arterial stiffness has a differential effect on both assessments. Dicrotic notch local PWV is measured at near systolic arterial pressure, whereas cfPWV is measured near diastolic pressure. The pressure dependency of arterial stiffness has to be considered when determining arterial elastic properties and the load on the left ventricle. By measuring intra-arterial pressure and common carotid artery diameter waveforms simultaneously in patients undergoing coronary angiography, we observed that the degree of pressure dependency may differ substantially among individuals. Moreover, arterial stiffness at systolic pressure exhibited a much stronger positive correlation with age (0.28 m/s per year) than arterial stiffness at diastolic pressure (0.08 m/s per year).40 To further analyze the degree of pressure dependency of arterial stiffness we quantified carotid distensibility coefficients for the diastolic and systolic pressure ranges separately, utilizing existing non-invasive measurement, i.e. ultrasound based diameter waveforms and tonometric pressure waveforms. In a healthy population (Asklepios cohort, aged 35–55 yrs) we found that the diastolic-systolic difference in distensibility appears to be significantly associated with left ventricular mass index, independently of age, sex, blood pressure, cfPWV and metabolic risk factors.41 Moreover, this diastolic-systolic difference in distensibility appears to increase with age (like arterial diameter), suggesting that this property could be used as a marker for structural remodeling of the artery wall.42
Pressure dependent arterial stiffness and central hemodynamics
We showed that the pressure dependency of arterial stiffness can differ substantially between individuals. Biomechanically, the pressure dependency of arterial stiffness directly affects the arterial pressure and flow waveform characteristics and their phase relationship. In a lumped parameter model of heart and circulation without wave reflections, we analyzed the effect of pressure dependent arterial stiffness on the pressure and flow waveforms (Fig. 2, unpublished results). Note that in a circulation with significant pressure dependent arterial stiffness the arterial and left ventricular pressure waveforms are of the “A-type”, i.e. pressures are higher in late than in early systole, whereas with constant arterial stiffness a “C-type” pressure waveform is observed.43 In wave analysis the direct effects of a pressure dependent arterial stiffness on pressure and flow are commonly neglected. For example, attributing the pressure above an inflection point in the pressure waveform completely to reflections, as is done by the augmentation index, will overestimate the impact of reflections on pressure. Similarly, wave separation analysis by means of Fourier analysis44 will lead to overestimation of the amplitude of the reflected waves, because this analysis is based on linear systems theory and, hence, assumes that arterial stiffness is constant with pressure. In the cfPWV measurement, transit time delay is determined using the systolic foot as fiducial time-point and, therefore, provides a measure for diastolic stiffness, underestimating average stiffness. In subjects with pronounced pressure dependent arterial stiffness cfPWV may, thus, not fully reflect the left ventricular afterload, the subject is exposed to. This might explain why cfPWV is of only minor additional value for cardiovascular risk prediction.25
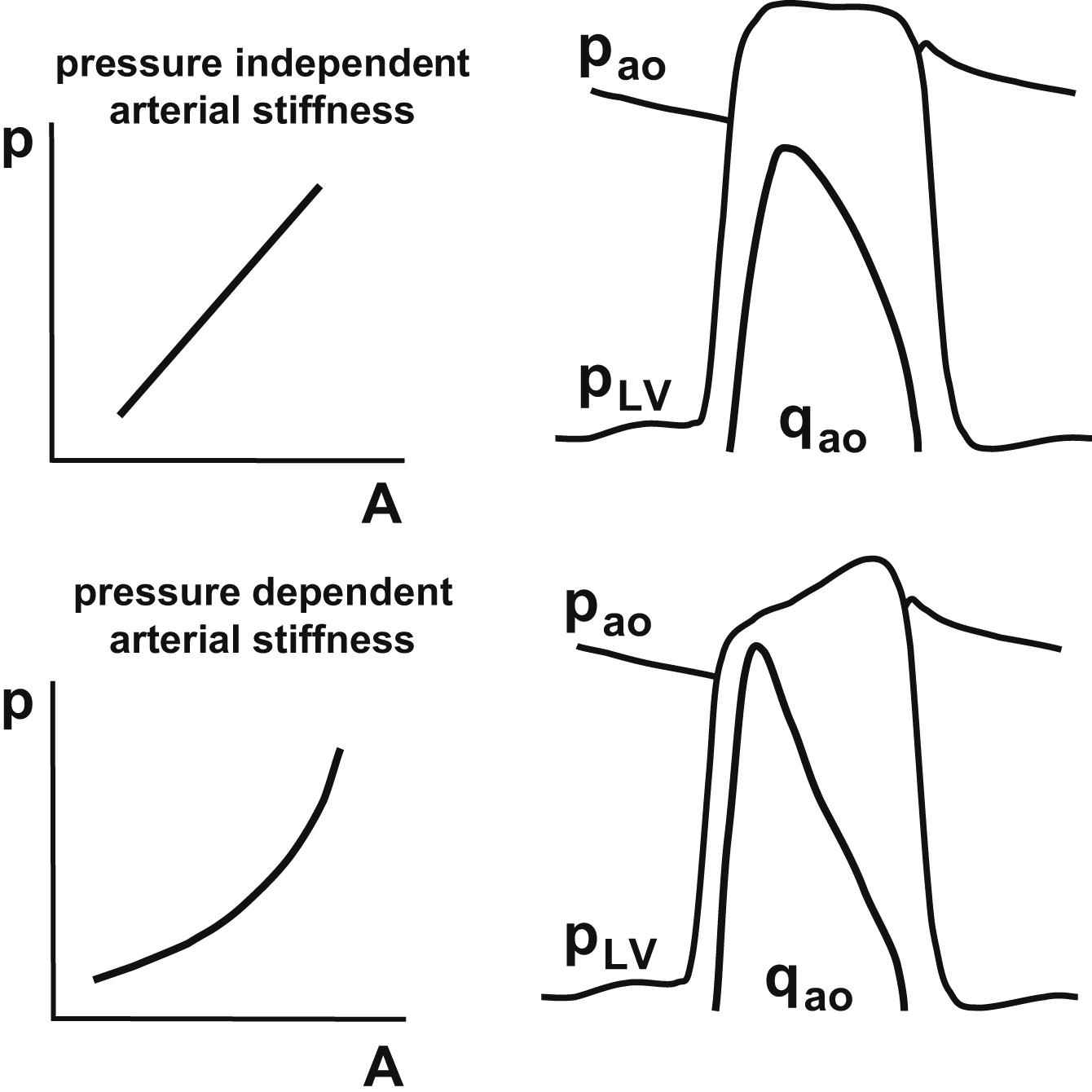
Consequences of a higher degree of pressure dependence of arterial stiffness for hemodynamics. Note that stroke area, pulse pressure and average ΔA/Δp over the entire pressure (p) and area (A) range are the same for both cases. The tracings illustrate the respective differences in left ventricular pressure (pLV), aortic pressure (pao) and flow (qao) waveforms. Note that in case substantial pressure dependent arterial stiffness, arterial pressure is higher in late than in early systole.
Does carotid-femoral pulse wave velocity also show pressure dependent arterial stiffness?
Despite previously mentioned problems with the acquisition of the cfPWV, it is worth investigating whether pressure related differences in arterial stiffness are revealed with cfPWV. Compared to local PWV cfPWV has the benefit of a long trajectory and, hence, less sensitive to the precision of the transit time measurements. In a preliminary analysis performed on 28 presumed healthy volunteers, aged 24–76 yrs, the cfPWV measured at dicrotic notch level (cfPWVDN) had a stronger association with age than PWV measured at about diastolic pressure (cfPWVSF, Fig. 3). The observed increase in cfPWVSF of 0.06 m/s per year is close to the values of cfPWVSF as previously reported.29,40,45 On the other hand, cfPWVDN increased with 0.22 m/s per year, which closely matches the 0.28 m/s per year increase of systolic carotid stiffness as reported above.40 cfPWVDN, as measure of arterial stiffness, however, is sensitive to changes in hemodynamic conditions during the measurement, because the position of the dicrotic notch, signaling the closure of the aortic valve,46 is modulated by changes in ejection period and heart rate. The precision and accuracy of the method will improve if both carotid and femoral waveforms are measured simultaneously rather than sequentially. However, waveform distortion due to wave reflections and tapering may affect dicrotic notch detection at the femoral site and will deteriorate the precision and accuracy of cfPWVDN and perhaps invalidate the estimate. If these technical issues could be addressed adequately, the diastolic-systolic difference in carotid-femoral PWV could be used in addition to the carotid diastolic-systolic difference in stiffness, to comprehensively evaluate arterial elastic properties.
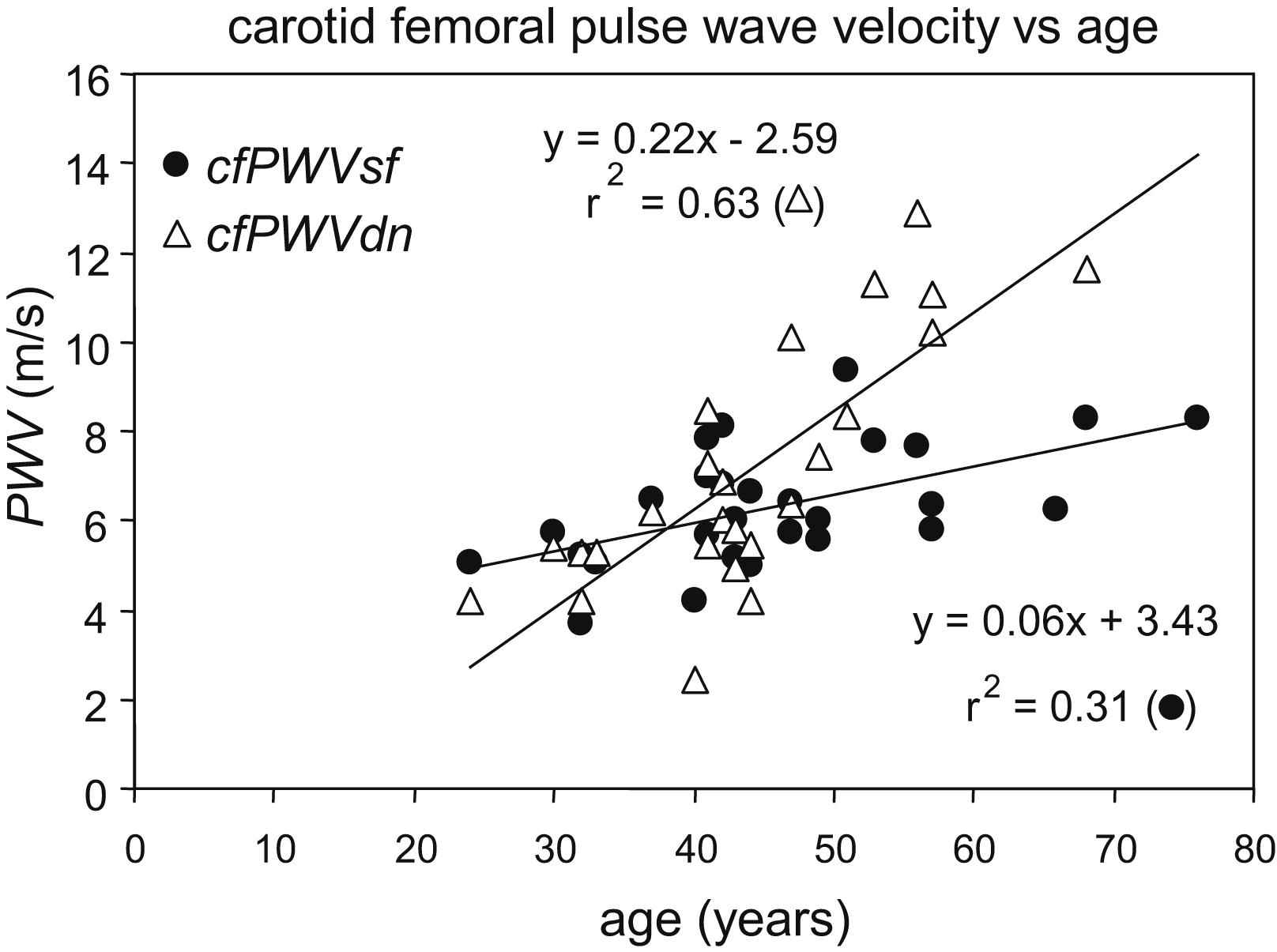
Relation between carotid-femoral pulse wave velocity obtained using systolic foot (cfPWVSF, dots) or dicrotic notch (cfPWVDN, triangles) and age.
Conclusion
Pulse wave velocity can be measured locally at the common carotid artery by means of multiple M-line ultrasound. Our method utilizes the dicrotic notch as time-reference point and, therefore, measures arterial stiffness at near systolic pressure level, which is intrinsically different from systolic foot PWV measured at diastolic pressure. The pressure dependency of arterial stiffness appears to increase with age and is associated with arterial dilatation and structural remodeling the left ventricle. Ignoring that the innate pressure dependency of stiffness directly affects arterial pressure and flow waveform characteristics and their phase relation may lead to overestimation of the impact of wave reflections on central blood pressure. Our work shows that pressure dependency of arterial stiffness can and should be quantified to evaluate has implications for pressure augmentation and wave separation analysis.
Conflict of interest
None.
References
Cite this article
TY - JOUR AU - Evelien Hermeling AU - Robert S. Reneman AU - Arnold P.G. Hoeks AU - Koen D. Reesink PY - 2011 DA - 2011/11/06 TI - ADVANCES IN ARTERIAL STIFFNESS ASSESSMENT JO - Artery Research SP - 130 EP - 136 VL - 5 IS - 4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.10.003 DO - 10.1016/j.artres.2011.10.003 ID - Hermeling2011 ER -
