Adducin and its relation to cardiovascular system
- DOI
- 10.1016/j.artres.2010.10.034How to use a DOI?
- Keywords
- Adducin; Arterial stiffness; Heart; Stroke
- Abstract
Adducin is a cytoskleton protein, which functions within the cells as a heterodimer composed of a combination of three subunits, α, β and γ. Each of these subunits is coded by genes (ADD1, ADD2, ADD3) mapping to different chromosomes. Mutation in ADD1 Gly460Trp polymorphism is associated with increased Na+K+-ATPase activity and increased renal tubular sodium reabsorption, which leads to salt-sensitive hypertension. A number of studies focused on possible association between these genes alone, or together with the interaction with other modulatory genes or with the environment, in relation to cardiovascular phenotypes. Variations in adducin genes were shown to modulate intermediate phenotypes such as arterial distensibitity, carotid IMT and left ventricle diastolic function. Moreover, some studies demonstrated an association between adducin genes and coronary artery disease, stroke and mortality. We showed that genetic variations in ADD1, ADD3 and angiotensin II receptor type-1 receptor interactively influenced the elastic properties of brachial and femoral arteries. Concerning the antihypertensive treatment, it was speculated that carriers of mutated ADD1 460Trp allele might benefit from diuretic use, however the present findings on this subject remain inconsistent. In conclusion, adducin might play an important role in the cardiovascular system.
- Copyright
- © 2010 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Adducin is a heterodymeric cytoskeleton protein, the three subunits are encoded by genes (ADD1, ADD2, ADD3) mapping to three different chromosomes. Mutation of the α-adducin gene (ADD1) entails increased Na+K+-ATPase activity,1,2 and increased renal tubular reabsorption.3 Variation in the Na+K+-ATPase activity and in the intracellur Na+ concentration might influence sodium-dependent transmembranous Ca2+ transport in vascular smooth muscle cells and via this mechanism might affect arterial tone.4
Previous studies in a randomly selected Flemish population (FLEMENGHO) demonstrated that an interaction between the genes encoding ADD1 (Gly460Trp polymorphism), the angiotensin-converting enzyme (ACE) and the angiotensin II type-1 receptor influenced the distensibility, cross-sectional compliance, and intima-media thickness of the femoral artery.5,6 Cwynar et al. reported interaction between the ADD1 Gly460Trp polymorphism and the A386G polymorphism in the γ-adducin subunit (ADD3). Peripheral and central pulse pressures were higher in carriers of both the ADD1 Trp allele and the ADD3 G allele.7 Moreover, in the prospective study from the FLEMENGHO population, the combination of ACE DD homozygosity and mutated ADD1, worsened cardiovascular prognosis to a similar extent as classical risk factors.8
This review article summarizes our results related to association between adducin polymorhisms and arterial properties and puts these into perspective against other published studies.
Adducin and arteries
Previous findings of the FLEMENGHO population showed interactions between the ADD1, ACE, and aldosterone synthase genes in relation to femoral intima-media thickness5 and carotid and femoral distensibility.6 We hypothesized that arterial properties might be related not only to interaction of ADD1 with ACE, but also interaction of ADD1 with two other adducin subunits genes or the genes encoding various components of the renin–angiotensin system. We addressed this question in randomly selected participants of the FLEMENGHO study.
The key finding of our study was that brachial diameter decreased (P = 0.0001), while brachial distensibility increased with the ADD3 G allele, and that these associations were confined to ADD1 GlyGly homozygotes (Fig. 1).9 In the family-based analyses, we did not find any evidence for population stratification (0.07 ≤ P ≤ 0.96). Transmission of the ADD3 G allele was associated with a smaller brachial diameter in all 342 informative offspring (P = 0.0085) as well as in offspring homozygous for ADD1 Gly allele (n = 209; P = 0.018). ADD1 and ADD2, alone or in combination with each other, were not associated with the arterial properties in the three arterial beds. This was also true for ADD1 and ADD3 in relation to the carotid and femoral phenotypes.9
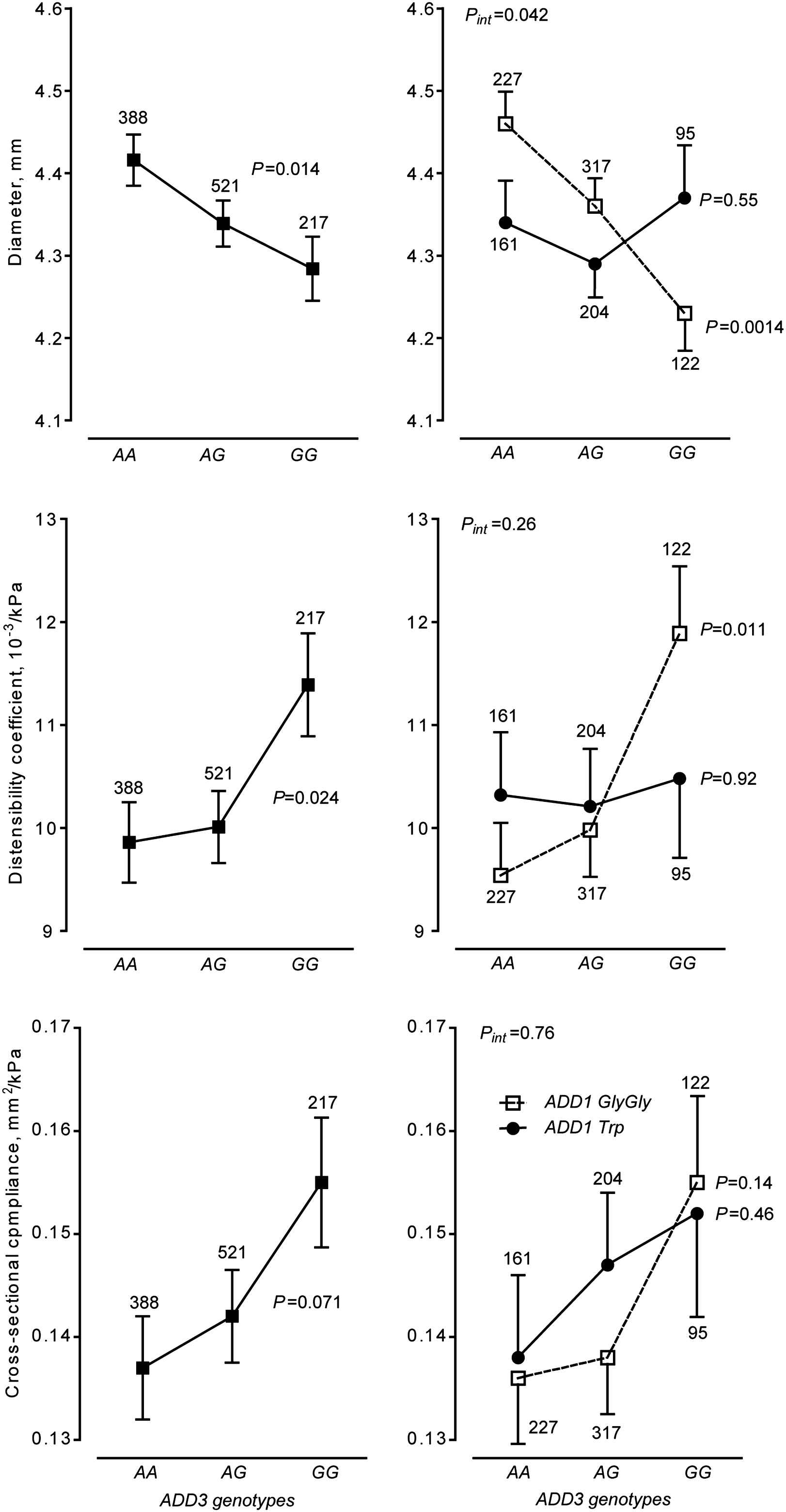
Diameter, distensibility and cross-sectional compliance of brachial artery in relation to ADD1 Gly460Trp and ADD3 A386G polymorphisms The analyses were adjusted for the observer, sex, age, body mass index, mean arterial pressure, heart rate, smoking, alcohol intake and the use of antihypertensive drugs and account for family clusters. Values are least square means ± SEs. The number of subjects contributing to each plotted point are given. P-values are linear trend across the ADD3 genotypes. Pint indicates the significance of the 2-way interaction between ADD1 and ADD3.
Moreover, we observed that femoral cross-sectional compliance (P = 0.020) and distensibility (P = 0.055) were higher in AT1R C allele carriers than in AT1R AA homozygotes, but that this association was only observed in the presence of the mutated ADD1 Trp allele, and not in ADD1 GlyGly homozygotes (P ≥ 0.16).10 In the family-based analyses, transmission of the AT1R C allele was associated with higher femoral distensibility in offspring carrying the mutated ADD1 Trp allele (n = 115, P = 0.022)10 (Fig. 2)
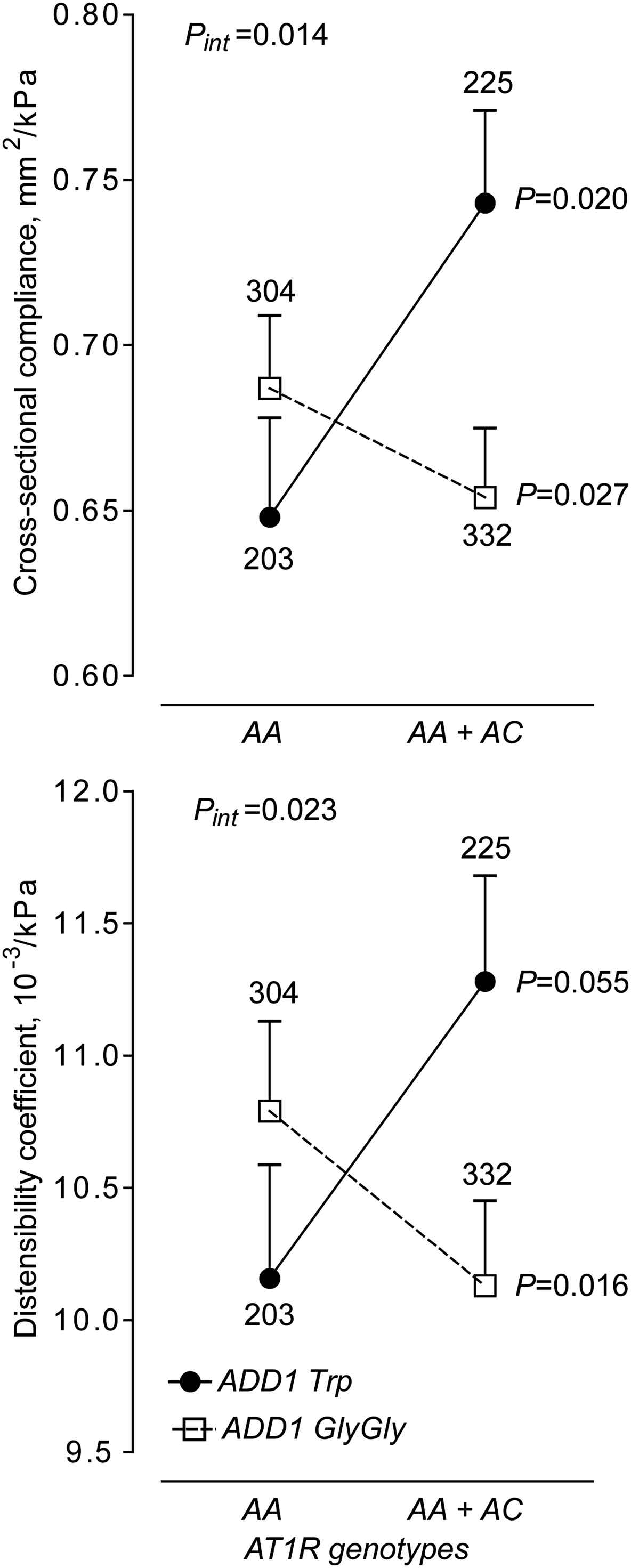
Femoral cross-sectional compliance and distensibility in relation to the ADD1 and AT1R genotypes The analyses were adjusted for the same covariates as in Fig. 1. Values are least square means ± SEs. The number of subjects contributing to each plotted point are given. P-values denote the siginificance of the difference between AT1R genotypes. Pint indicates the significance of the interaction between ADD1 and AT1R.
Our epidemiological study only allows speculation about the reasons why there might be an association between the properties of the brachial artery, a small muscular artery, and the ADD3 A386G polymorphism. One possible mechanism is that the polymorphism might affect the neurogenic tone of vascular smooth muscle cells. Indeed, in rat models, increased blood pressure was associated with a decrease in the hypothalamic levels of γ-adducin mRNA and protein.11,12 Furthermore, inhibition of γ-adducin by intracellular delivery of γ-adducin-specific antibodies increased the neuronal firing rate possibly via regulation of Na+,-K+-ATPase.11 On the other hand, given that the phenotypes representative of two other arterial segments did not shown any association with the adducin SNPs, suggests that local mechanisms in the brachial artery, rather than the central nervous system, might underlie the demonstrated findings.
There is an apparent discrepancy between our analyses in which compliance of femoral artery was lower in the AT1R AA homozygotes carrying ADD1 Trp allele, whereas in other analyses of hypertensive subjects, arterial stiffness assessed as aPWV was increased with AT1R C allele.13,14 However, these studies cannot be compared. Compliance of artery is a parameter measured locally at one arterial site, while aPWW reflects characteristics of arterial segment between two sites of measurements, e.g. carotid and femoral artery. Elastic aorta and muscular femoral artery have different properties.15 Moreover, observations collected in a general population should not be extrapolated to hypertensive patients. Indeed, the aforementioned association between aPWV and AT1R C allele was found only in hypertensive, but not in normotensive, individuals.13 Finally, we demonstrated the effect of AT1R C allele only in carriers of mutated ADD1 Trp allele, which implicates gene–gene interaction.
Adducin and cardiovascular complications
In the FLEMENGHO participants, the left ventricular diastolic relaxation assessed by tissue Doppler imaging was modulated by genetic variations in ADD1 and ADD3.16 Below the age of 50 years, the lateral Ea/Aa ratio was higher in ADD1 Trp allele carriers than in GlyGly homozygotes, particularly in the presence of ADD3 GG homozygosity. In older subjects, these phenotype-genotype associations were not significant.16 In several studies, hypertensive ADD1 Trp allele carriers17–20 had increased risk of coronary artery disease. Moreover, in black patients with coronary artery disease, ADD1 variant was associated with an 8-fold excess risk of all-cause death.19
In 6471 participant of the prospective Rotterdam study,20 ADD1 variant carriers had increased risk of ischemic stroke (hazard ratio [HR], 1.29; 95% CI, 1.02 to 1.63), and this was true mainly in hypertensive subjects (HR, 2.32; 95% CI, 1.68 to 3.21). Moreover, in healthy Dutch women free from previous cardiovascular disease,21 the ADD1 Trp polymorphism was related to risk of stroke in later life. The risk of ischemic stroke was 10.9-fold higher among women with systolic hypertension compared to normotensive GlyGly homozygotes.21
An increased risk of stroke or coronary artery disease associated with variant ADD1 allele seems to be more pronounced in hypertensive subjects, in whom we can expect the role of gene–gene interaction or gene–environmental interaction. Indeed, in a FLEMENGHO population the combination of ACE DD homozygosity and mutated ADD1 worsened the cardiovascular prognosis to a similar extent as classical risk factors.8 In ADD1 Trp allele carriers, the multivariate-adjusted hazard ratios associated with ACE DD versus I were 1.72 (P = 0.007) for total mortality, 2.35 (P = 0.02) for cardiovascular mortality, 2.02 (P = 0.005) for all cardiovascular events, and 2.59 (P = 0.03) for heart failure. In contrast, these hazard ratios did not reach significance in ADD1 GlyGly homozygotes (0.08 ≤ P ≤ 0.90).8
Adducin and use of diuretics
ADD1 Gly460Trp polymorphism was associated with an increased renal sodium reabsorption3 and was linked to salt-sensitive hypertension.22 Thiazide diuretics promote renal sodium excretion. This action of diuretics counteracts the biological effect of the α-adducin polymorphism. Therefore, in ADD1 Trp carriers, it is supposed they can benefit from use of diuretics. This question was explored in several studies.19,23–26 However, results of these studies are inconsistent. In a population based case-control study, the Trp allele was associated with a lower risk of myocardial infarction in users of diuretics compared with users of other blood pressure lowering medication.23 In GENHAT, a genetic study24 conducted within a randomized clinical trial, women carrying Trp allele who used chlorthalidone had a greater risk of myocardial infarction, whereas men did not. The other three studies found no interaction between the α-adducin gene and the use of diuretics on the prevention of myocardial infarction or stroke.19,25,26 These conflicting results suggest that ADD1 genotype guided therapy with diuretics may not be a clinically or economically useful method to improve antihypertensive therapy.
Conclusion
From three genes encoding the adducin subunits, ADD1 Gly460Trp polymorphism is the most important for cardiovascular system. Mutation in ADD1 gene modulates arterial properties, diastolic function of left ventricle, and is associated with higher risk of stroke, coronary artery disease and increased mortality rate. Hypertensive background is important when considering effect of ADD1 Trp allele on stroke or coronary artery disease. Therefore, interaction of adducin with other genes or with environmental factors should be considered when study effect of this polymorphism on cardiovascular system.
References
Cite this article
TY - JOUR AU - Jitka Seidlerová PY - 2010 DA - 2010/12/12 TI - Adducin and its relation to cardiovascular system JO - Artery Research SP - 134 EP - 137 VL - 4 IS - 4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2010.10.034 DO - 10.1016/j.artres.2010.10.034 ID - Seidlerová2010 ER -
