Non-invasive estimates of central systolic blood pressure: Comparison of the Centron cBP301 and SphygmoCor devices
- DOI
- 10.1016/j.artres.2012.07.001How to use a DOI?
- Keywords
- Blood pressure; Aortic pressure; Centron; SphygmoCor; Transfer function; Pulse wave analysis
- Abstract
Background: Central systolic blood pressure (cSBP) may be more predictive of cardiovascular events than brachial BP. Therefore, non-invasive methods of determining central BP, which are suitable for routine clinical use, are required. The aim of this study was to compare estimates of cSBP provided by the Centron cBP301 with those obtained with the widely used SphygmoCor system.
Methods: In 60 subjects (30 females), age range 22–90 years, brachial BP was measured using the Centron device and then cSBP estimated using the Centron, and then SphygmoCor. In a subset of 16 subjects (8 females), measurements were repeated at rest and following the administration of glyceryl trinitrate (GTN).
Results: There was a strong correlation (r = 0.98; P < 0.001) between the estimates of cSBP obtained with each device. There was also good agreement between devices, with a mean difference (±SD) of 0.2 ± 3.5 mmHg (P = 0.5). Similarly, the devices were highly correlated and in good agreement following the administration of GTN, with the mean difference in cSBP ranging from 0.5 ± 3.9 mmHg to 2.3 ± 3.7 mmHg, across the measurement period.
Conclusion: The Centron cBP301 and SphygmoCor devices produce similar estimates of cSBP, both at rest and in response to a pharmacological challenge. The Centron device is potentially suitable for routine clinical monitoring of central BP.
- Copyright
- © 2012 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The prognostic value of brachial BP is firmly established.1 However, systolic and pulse pressures vary throughout the arterial tree, and emerging evidence supports the hypothesis that central pressure may be more predictive of cardiovascular risk than brachial pressure.2–5 Whilst invasive measurements of central pressure remain the gold standard, a variety of non-invasive devices for determining central pressure are now available.
Perhaps the most widely used method for non-invasive determination of central pressure is provided by the SphygmoCor system, which records the radial pulse waveform using applanation tonometry and then applies a validated6 transfer function to derive the central pressure. Although this method provides comparable results to direct (invasive) central pressure measurements, it requires a certain degree of technical expertise which can be time-consuming and may not, therefore, be suited to routine clinical use. More recently, techniques based on oscillometry at the brachial artery have been developed and validated7–9 which require limited technical expertise and time.
The Centron cBP301 device has recently been developed as an alternative to applanation tonometry. This device derives the cSBP from brachial pressure waveforms recorded from a standard oscillometric cuff, using a prospectively validated brachial-aortic transfer function.10 The aim of the current study was to compare estimates of cSBP provided by the Centron device with those of the widely used SphygmoCor system.
Methods
Subjects
Subjects were recruited from the staff and volunteer database of the Vascular Research Clinic at Addenbrooke’s Hospital. For the main study, 60 subjects were recruited, to obtain 3 well-balanced age groups: 20 subjects aged <35 years; 19 subjects between 35 and 65 years; and 21 subjects aged >65 years. Medical treatment was not withheld for the measurements. Sixteen of the subjects then underwent further measurements before, and following the administration of GTN. Local Research Ethics Committee approval was obtained and informed consent given by each participant. Exclusion criteria were cardiac arrhythmias at presentation, arteriovenous fistula in the arm or unstable clinical presentation.
Devices
Centron cBP301
The Centron cBP301 (Centron Diagnostics, Kent, UK) device measures cuff pressure from a standard brachial cuff and applies a validated11 oscillometric algorithm (SunTech Advantage™ A+, SunTech Medical, North Carolina, USA) to determine the systolic, diastolic and mean brachial BP (bBP). The cuff is then re-inflated to a pressure between the mean arterial pressure and SBP, and oscillations in the cuff pressure recorded over a period of several seconds. The waveforms are calibrated to the previously determined bSBP and bDBP and a validated10 generalised transfer function applied to estimate the cSBP.
SphygmoCor
The SphygmoCor (AtCor Medical, Sydney, Australia) device uses applanation tonometry at the radial artery to non-invasively record radial pressure waveforms. A validated6 generalised transfer function is then applied to estimate the cSBP. Radial artery waveforms are typically calibrated to the bSBP and bDBP obtained with an external sphygmomanometer. Therefore, in the current study, the radial pressure waveforms were calibrated using the bSBP and bDBP values obtained with the Centron device. This approach allowed a direct comparison of the derived central pressure values between devices, without the confounding influence of different calibration pressures.
Protocol
All assessments were made during a single visit to the clinic. Baseline characteristics were recorded and then subjects were seated in a quiet, temperature-controlled environment for at least 10 min prior to measurement. Brachial and central BP were measured using the Centron cBP301 and then central pressure measured using the SphygmoCor. All readings were then repeated at least twice more in the same arm, to achieve three high quality pairs of readings which were used in subsequent analyses. All readings were made by the same trained operator (AMM). A subset of individuals were then rested supine for a further 10 min. As in the main study, brachial and central BP were measured using the Centron cBP301 and then central pressure assessed using the SphygmoCor. A 500 μg tablet of GTN (Bristol Laboratories, Hertfordshire, UK) was placed under the tongue for 3 min and then removed. The BP measurements were then repeated at 3, 5, 10 and 15 min following GTN administration. All readings were made by the same trained operator (LMD).
Statistics
Data were analysed using the method of Bland and Altman, to demonstrate the difference in values between the two devices. Pearson’s correlation coefficient (r) was used to determine correlation. The mean difference and standard deviation were calculated and paired Student’s t-tests used to assess significance of the difference between absolute values. Data are presented as means ± SD, unless otherwise indicated, and P <0.05 was considered significant.
Results
Measurements were obtained in 60 subjects (30 women), between the ages of 22 and 90 years. The characteristics of the study sample are shown in Table 1. Three estimates of cSBP were obtained using each device in each subject, yielding a total of 180 paired readings. There was close correlation between the estimates of cSBP from the Centron cBP301 and SphygmoCor devices (r = 0.98; P < 0.001; Table 2; Fig. 1). The difference in absolute values between devices was not statistically significant and the standard deviation of the differences was low, indicating good agreement (mean difference 0.2 ± 3.5 mmHg; P = 0.5; Table 2; Fig. 2). Comparing the values of cSBP obtained with each device, 89% were within 5 mmHg, 99% were within 10 mmHg and 100% were within 15 mmHg. The maximum absolute difference between paired values was 13 mmHg. The Bland–Altman plot did not indicate any systematic bias in the estimation of cSBP (Fig. 2). Mean arterial pressure (MAP) values obtained with the Centron device were significantly lower, on average, than those obtained with the SphygmoCor (mean difference −5.15 ± 3.22 mmHg, P < 0.001), although the correlation between devices was high (Table 2). There was also a small, but significant difference in heart rate between devices (mean difference 1.8 ± 4.6 bpm, P < 0.001; Table 2).
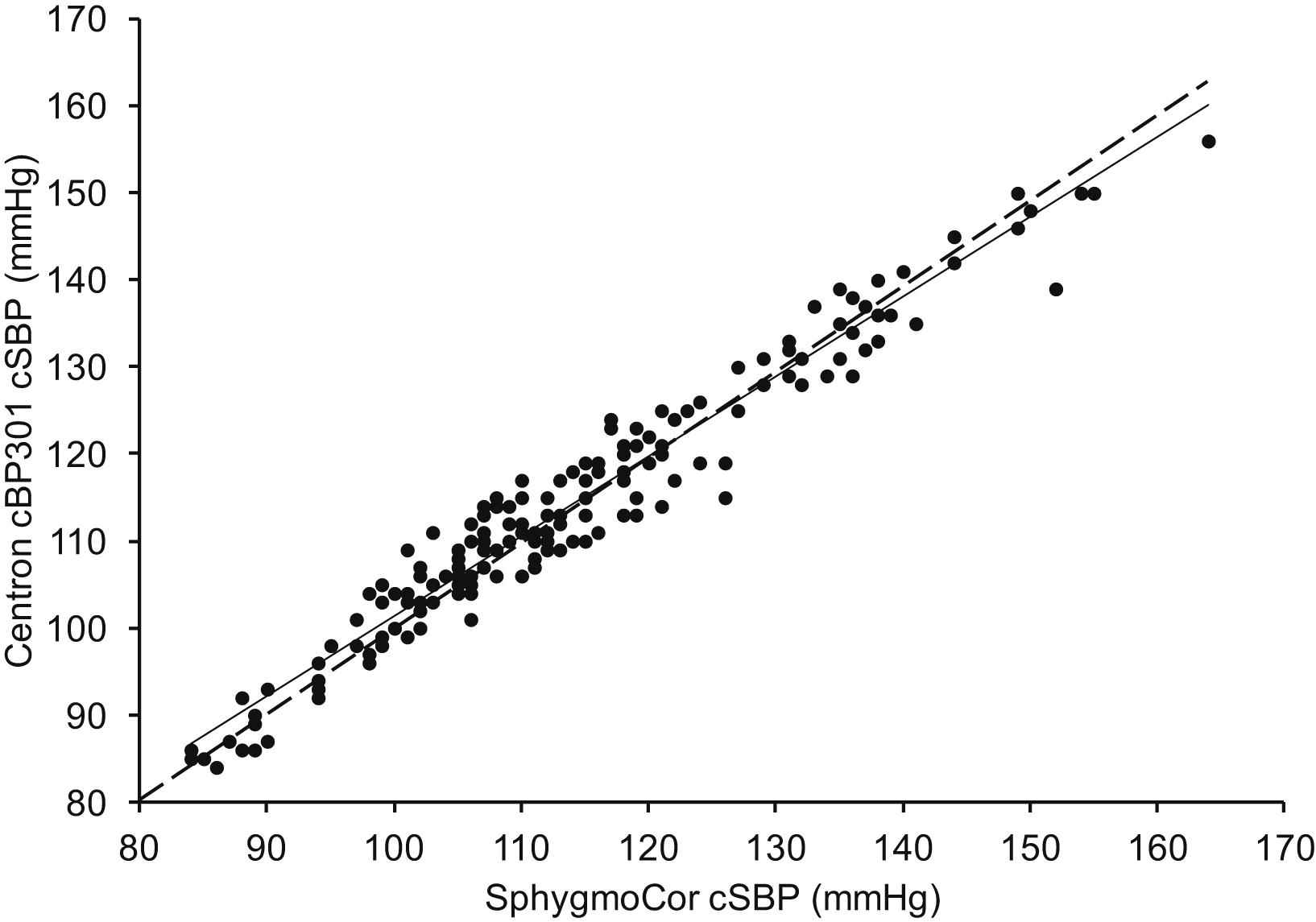
Correlation between cSBP values provided by the SphygmoCor and Centron cBP301. Regression equation: y = 0.9176x + 9.5684. Line of identity is indicated by dashed line.
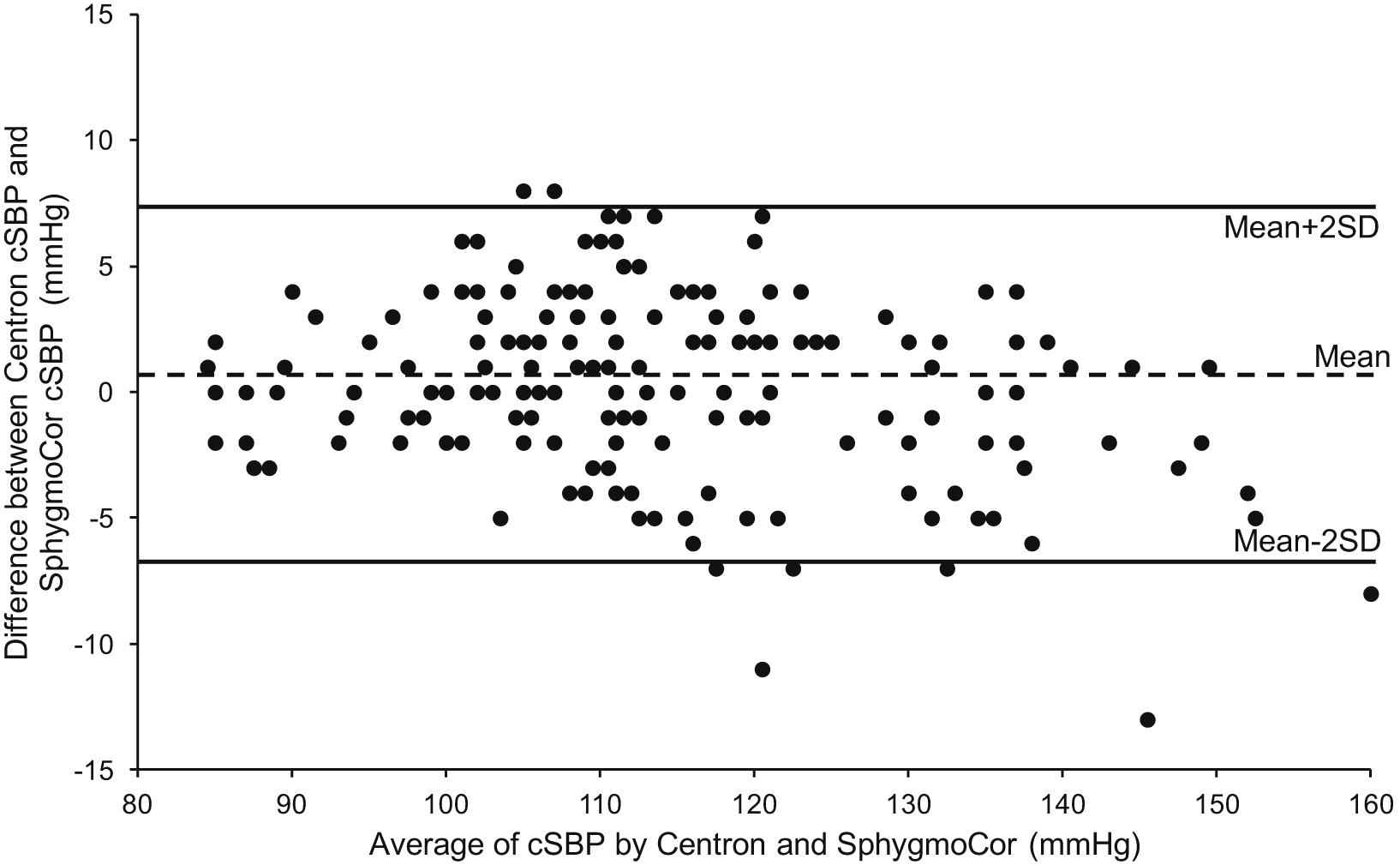
Bland–Altman plot showing agreement between estimates of cSBP provided by the SphygmoCor and Centron cBP301.
| Characteristics | |
|---|---|
| Age (years) | 50.6 ± 23.2 |
| Age range (years) | 22–90 |
| Gender (male:female) | 30:30 |
| Height (m) | 1.71 ± 0.10 |
| Weight (kg) | 74.2 ± 15.2 |
| BMI (kg/m2) | 25.5 ± 4.9 |
| Antihypertensives (yes:no) | 16:44 |
| Obese BMI (yes:no) | 11:49 |
| Systolic BP 0–99 (mmHg) | 2 |
| Systolic BP 100–129 (mmHg) | 35 |
| Systolic BP 130–159 (mmHg) | 20 |
| Systolic BP 160–179 (mmHg) | 3 |
Data are means ± SD or numbers.
Subject characteristics of the cohort.
| Centron cBP301 | SphygmoCor | Difference | P | Pearson’s r | P | |
|---|---|---|---|---|---|---|
| Brachial SBP (mmHg) | 127.3 ± 16.1 | |||||
| Brachial DBP (mmHg) | 75.6 ± 9.9 | |||||
| Brachial PP (mmHg) | 51.7 ± 14.3 | |||||
| MAP (mmHg) | 86.8 ± 10.3 | 91.9 ± 10.7 | −5.2 ± 3.2 | <0.001 | 0.95 | <0.001 |
| cSBP (mmHg) | 114.0 ± 15.1 | 113.8 ± 16.0 | 0.2 ± 3.5 | 0.5 | 0.98 | <0.001 |
| Heart Rate (bpm) | 69.8 ± 11.5 | 68.0 ± 11.3 | 1.8 ± 4.6 | <0.001 | 0.96 | <0.001 |
Data are means ± SD. MAP = mean arterial pressure. cSBP = central systolic blood pressure.
Comparison of average blood pressure and heart rate between devices.
Sub-study
Measurements were obtained in 16 subjects (8 women), between the ages of 22 and 54 years. Administration of GTN caused a significant fall in cSBP after 3 min, as detected by the two devices (105.5 ± 7.7 mmHg–101.7 ± 7.0 mmHg, Centron; 106.8 ± 8.1 mmHg–101.2 ± 7.6 mmHg, SphygmoCor, Table 3). There was a close correlation between the estimates of cSBP from the Centron cBP301 and SphygmoCor devices and good agreement between the devices at each time point (Table 3).
| Centron cBP301 | SphygmoCor | Difference | P | Pearson’s r | P | |
|---|---|---|---|---|---|---|
| Baseline | 105.5 ± 7.7 | 106.8 ± 8.1 | 1.3 ± 4.6 | 0.3 | 0.83 | <0.001 |
| +3 min | 101.7 ± 7.0 | 101.2 ± 7.6 | 0.5 ± 3.9 | 0.6 | 0.86 | <0.001 |
| +5 min | 99.9 ± 7.0 | 98.6 ± 7.5 | 1.3 ± 4.4 | 0.3 | 0.82 | <0.001 |
| +10 min | 101.0 ± 8.0 | 98.6 ± 6.8 | 2.3 ± 3.7 | 0.2 | 0.89 | <0.001 |
| +15 min | 101.6 ± 8.4 | 100.0 ± 7.4 | 1.6 ± 3.9 | 0.1 | 0.89 | <0.001 |
Data are means ± SD.
Comparison of central systolic blood pressure between devices before and after administration of GTN.
Discussion
The aim of this study was to compare non-invasive estimates of cSBP, obtained using a newly described method based on cuff oscillometry at the brachial artery (Centron cBP301) with a widely used method based on hand-held tonometry at the radial artery (SphygmoCor). The results demonstrate a high degree of correlation and very good agreement between the devices, indicating that the Centron cBP301 provides comparable estimates of cSBP to the SphygmoCor. The portability of the Centron device, together with its limited requirement for technical expertise makes it potentially suitable for use in routine clinical practice.
Systolic pressure can differ by up to 40 mmHg between the brachial artery and the aorta.12 Moreover, there is considerable disparity in aortic pressure between individuals with similar levels of brachial pressure,13 indicating that aortic pressure cannot simply be estimated from brachial BP values alone. Recently, the utility of measuring central, rather than brachial BP, for the improved prediction of cardiovascular risk has been demonstrated.2–5 However, clinical decisions are unlikely to be based on central, rather than brachial pressure, without appropriate guidelines and a widespread availability of devices which allow central pressure to be assessed easily in routine clinical practice.
A variety of devices have been developed in order to determine the central BP non-invasively. One such device, which is widely used, is the SphygmoCor, which uses applanation tonometry to record waveforms at the radial artery and a generalised transfer function to derive the cSBP. The SphygmoCor has been compared against direct, invasive measurements of aortic BP and produces similar values under resting conditions and in response to GTN,6 which are well within the limits specified by the Association for the Advancement of Medical Instrumentation (AAMI; 5 ± 8 mmHg). Although the device is portable, the tonometry procedure utilised by the SphygmoCor does require a trained operator with some degree of technical expertise. However, the successful introduction of cSBP into everyday clinical practice, including the primary care setting, will rely on measurements which are rapid and simple to obtain, using an easily-transportable device.
The Centron cBP301 is a new device, which uses standard cuff-based oscillometry to record pressure waves from the brachial artery. A generalised transfer function which has been optimised and prospectively validated against invasively determined aortic pressure10 is then applied to provide an estimate of the cSBP. Its design and instructions for use closely resemble those of a standard oscillometric device, meaning that the Centron cBP301 combines portability with ease of operation. In addition, the Centron cBP301 does not require a connection to an external computer, since the results are displayed on its own mini monitor.
Estimates of cSBP calculated by the Centron cBP301 and the SphygmoCor devices were highly comparable under resting conditions, and fell well within the AAMI criteria for equivalence. Peripheral waveforms were calibrated using the brachial SBP/DBP, although calibration using the brachial MAP/DBP may provide closer estimates of the true aortic pressure.8 Unfortunately, invasive measurements of cSBP were not available in this study; therefore, it is impossible to determine which method of calibration, or, indeed, which of the devices provides closer estimates of the true aortic pressure. Nevertheless, both devices have been prospectively validated against invasive measurements6,10 and the agreement between devices in the current study was high. Importantly, the current study included a wide age-range and hypertensive subjects were not excluded, meaning that the devices were compared over a broad range of blood pressures. Despite this, there was no discernible relationship between the level of blood pressure and the extent of agreement between the two devices, indicating a lack of any systematic bias in the data.
Wave reflections within the arterial system can exert a considerable impact on central pressure. The nitric oxide donor, GTN, substantially reduces wave reflections and central pressure,14 and in the current study, administration of GTN resulted in a significant reduction in cSBP measured with both devices. Importantly, the difference between devices in response to GTN was <2.5 mmHg across the entire measurement period, and equated to the values obtained at rest, indicating that the algorithms employed by each device for estimating cSBP coped well with acute changes in cSBP.
Although not the focus of the current study, the MAP values obtained with the two devices differed significantly. This is likely to be due to small differences in MAP between the brachial and radial arteries and the different methods of determining the MAP employed by each device. The oscillometric method used by the Centron device identifies the mean pressure directly from oscillations in the brachial cuff, whereas the SphygmoCor device calculates the mean pressure by integration of the arterial pressure waveform. In addition, the heart rate values obtained from the two devices also differed significantly, a finding which is likely to reflect the sequential, rather than simultaneous nature of the measurement protocol. Although heart rate exerts a potent influence on cSBP,15 the magnitude of the difference was very small (<2 beats/min) and is unlikely to have had a significant influence on the observed cSBP values.
In summary, the Centron cBP301 is a new, oscillometric BP device, incorporating non-invasive estimates of cSBP which are highly comparable to another, widely used tonometric method. The ease of use and portability of the Centron device make it widely applicable for routine clinical use, including the primary care setting.
Conflict of interest
The Clinical Pharmacology Unit has received unrestricted educational donations and equipment from AtCor Medical, Centron Diagnostics and IEM.
Acknowledgements
AMM was supported by the Wellington Medical Research Foundation. IBW holds a British Heart Foundation Senior Clinical Fellowship. This work was supported, in part, by the National Institute for Health Research Cambridge Biomedical Research Centre Award and Comprehensive Local Research Network.
References
Cite this article
TY - JOUR AU - Ann-Marie Mekhail AU - Lisa M. Day AU - Anna K. Goodhart AU - Ian B. Wilkinson AU - Carmel M. McEniery PY - 2012 DA - 2012/07/24 TI - Non-invasive estimates of central systolic blood pressure: Comparison of the Centron cBP301 and SphygmoCor devices JO - Artery Research SP - 109 EP - 113 VL - 6 IS - 3 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2012.07.001 DO - 10.1016/j.artres.2012.07.001 ID - Mekhail2012 ER -
