The effects of central arterial pressure and autonomic dysfunction on elevations in N-terminal pro-B-type natriuretic peptide (NT-proBNP) in men with diabetes
- DOI
- 10.1016/j.artres.2008.03.002How to use a DOI?
- Keywords
- Arterial stiffness; Augmentation index; Autonomic neuropathy; NT-proBNP
- Abstract
Background: In diabetes, left ventricular dysfunction independent of coronary disease is common and is associated with elevations in NT-proBNP. Our aims were to determine the relative importance of central aortic pressure, cardiovascular autonomic function and arterial stiffness as predictors of elevated NT-proBNP.
Methods: Fifty males with diabetes mellitus and 21 males with IGT were studied. Arterial stiffness and wave reflections were assessed by measuring aortic and brachial pulse wave velocity (PWV) and augmentation index (AIX). Cardiovascular autonomic function was assessed by measurements of heart rate variability following standard manoeuvres.
Results: Comparing diabetes versus IGT subjects (mean ± SD), both aortic PWV (9.7 ± 2.4 versus 8.2 ± 1.4 m/s, p < 0.01) and cardiovascular autonomic dysfunction (autonomic score 2.3 ± 1.3 versus 1.6 ± 1.0, p < 0.01) were greater in diabetes subjects. NT-proBNP levels correlated with central and brachial systolic pressure (r = 0.74, p < 0.0001 and r = 0.66, p < 0.0001, respectively), aortic PWV (r = 0.43, p < 0.01), AIX (r = 0.55, p < 0.0001), and autonomic function (r = 0.37, p < 0.01). Multiple regression analysis amongst diabetic subjects showed central systolic blood pressure to be the strongest predictor of NT-proBNP concentrations.
Conclusions: Elevated central arterial pressure is a strong predictor of NT-proBNP concentrations in diabetic men without clinically apparent left ventricular dysfunction. This is indicative of the key influence of unfavourable large artery haemodynamics on the development of left ventricular dysfunction in diabetes.
- Copyright
- © 2008 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Diabetes mellitus increases the risk of heart failure independently of underlying coronary artery disease, and many believe that diabetes in its own right may lead to a specific form of ‘diabetic cardiomyopathy’.1 The underlying pathogenesis of diabetic cardiomyopathy is still incompletely understood but may involve abnormalities in cardiovascular autonomic function,1,2 together with pathological changes within the arterial tree leading to large artery stiffening and increased central aortic pressure, 3–5 both of which promote the development of mal-adaptive myocardial remodelling.6 A method of identifying patients with diabetes at highest risk of developing left ventricular dysfunction is desirable in order to allow effective risk stratification and prioritisation of treatment. There has therefore been considerable interest in the use of brain natriuretic peptide as a potential screening tool to identify patients with subclinical heart failure.
B-type natriuretic peptide (BNP) and the N-terminal fragment of the prohormone (NT-proBNP), an active metabolite of BNP with a longer half-life, are cardiac neurohormones produced by the ventricles in response to volume expansion and pressure overload.7,8 Elevated levels of both these peptides are commonly found in patients with systolic heart failure and absolute levels have been shown to be highly predictive of prognosis.9 Furthermore, recent evidence suggests that plasma BNP has prognostic value for future cardiovascular events and death, even in patients with preserved left ventricular function.10 In patients with diabetes and preserved left ventricular function, elevated levels of BNP and NT-proBNP commonly occur, particularly in association with left ventricular hypertrophy and hypertension.11 The underlying mechanisms responsible for elevated BNP and NT-proBNP levels in diabetes patients without clinical evidence of left ventricular dysfunction are unclear but may involve abnormalities in large artery function. Indeed, recent studies have demonstrated a link between BNP and aortic stiffness in healthy Japanese subjects,12 healthy participants in the Framingham Heart Study13 and in patients with coronary artery disease,14 but to date, there has been little study of this association in diabetes and impaired glucose tolerance (IGT) subjects. Given that premature large artery stiffening is increasingly recognised as an important contributor to cardiovascular complications in diabetes, 15 it is possible that altered arterial function plays a pivotal role in the pathogenesis of subclinical left ventricular dysfunction and elevated NT-proBNP secretion in diabetes.
The aims of the present study were to: (1) determine the relative importance of wave reflection, central aortic pressure, and conduit artery stiffness as predictors of NT-proBNP levels; and (2) determine the extent to which cardiovascular autonomic function influences circulating levels of NT-proBNP.
Patients and methods
Subjects and protocol
In this observational study, 50 male patients with diabetes mellitus (35 type 2, 15 type 1) (age range 39–75 years) and 21 male patients with IGT (age range 42–69 years) were recruited consecutively from the hospital-based diabetes clinic at Torbay Hospital, UK. Patients with clinical evidence of atherosclerotic cardiovascular disease (coronary disease, cerebrovascular disease and peripheral vascular disease), clinical or echocardiographic evidence of left ventricular impairment or significant renal impairment (estimated GFR < 30 ml/min) were excluded from the study. Eligible patients were assessed on the basis of clinical history and examination, electrocardiogram and laboratory results. Thirty-seven patients within the diabetes group were receiving insulin and 28 patients were receiving antihypertensive medication for hypertension. Of those receiving anti-hypertensive medications, 26 were receiving angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers; 11 were receiving calcium channel blockers; 14 were receiving diuretics and 6 were receiving beta-blockers. Fourteen patients in the IGT group were receiving antihypertensive medication for hypertension (9 were receiving angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers; 3 were receiving calcium channel blockers; 5 were receiving diuretics and 2 were receiving beta-blockers). Twelve patients in the diabetes group and 4 patients in the IGT group were active cigarette smokers. Amongst patients with diabetes, 19 patients had evidence of diabetic retinopathy, 14 had documented peripheral neuropathy and 6 had micro- or macro-albuminuria. Forty out of 50 diabetic subjects and 16 out of 21 IGT subjects were receiving statin treatment. All patients were studied on a single occasion after a 12 h fast and medications were omitted on the morning of study.
Aortic and brachial pulse wave velocity (PWV) and arterial pulse wave analysis were performed in all subjects. Height and weight were recorded using standard methods and body composition was determined by bioelectrical impedance analysis. After 10 min supine rest, blood pressure was measured in the dominant arm and radial waveforms were recorded for pulse wave analysis. Following a further 10 min of supine rest, carotid, femoral and radial artery waveforms were recorded for the measurement of carotid to femoral and carotid to brachial PWV. Cardiovascular autonomic function tests were then performed. Finally, fasting blood samples (10 ml venous blood for each subject) were drawn on the morning of study. The study had approval from the local research ethics committee and all subjects gave informed consent to participate in the study.
Vascular measurements
Blood pressure measurement
Blood pressure (BP) was recorded after 10 min rest at the brachial artery of the dominant arm using a validated semi-automated osscilometric device in the supine position. All haemodynamic measurements were recorded in a quiet, temperature-controlled room.
Pulse wave analysis
Pulse wave analysis was performed using the Sphygmocor system (SphygmoCor, Atcor Medical, Sydney, Australia) as previously described.16 Briefly, this method allows non-invasive generation of a central aortic waveform from that recorded at the radial artery, using a validated generalised transfer function.17 The augmentation index (defined as the difference between the second and first peaks of the central arterial waveform, expressed as a percentage of the pulse pressure) was determined as a measure of systemic stiffness and taken from the ascending aortic waveform.
Pulse wave velocity (PWV)
Pulse wave velocity is the gold standard procedure and validated method for assessing arterial stiffness.18 In brief, aortic and brachial pulse wave velocity was measured as the time taken for the pulse to travel from a 3 lead electrocardiogram-gated signal to the first upstroke of the pulse wave at the carotid, radial and femoral site. Between-observer variation and within-observer variation was consistent with previously published data.18
Cardiovascular autonomic function
Cardiovascular autonomic function tests were performed by a single operator through the use of continuous ECG monitoring following standard manoeuvres.19,20 Determination of heart rate variability (HRV) using R–R intervals (measured by a single operator from a limb lead ECG trace) was used as a measure of cardiovascular autonomic function. The following manoeuvres were performed:
- (1)
Heart rate response to a single deep breath with the result taken as the difference between the maximum and minimum heart rate during the procedure.
- (2)
Heart rate response to 6 consecutive breaths over 1 min with the result taken as the mean difference in the maximum and minimum heart rate during the last 3 breathing cycles.
- (3)
Heart rate response to standing with the result taken as the difference between the maximum and minimum heart rate occurring after the change in posture.
- (4)
Heart rate response to Valsalva’s manoeuvre, which was performed by the patient blowing into a mouthpiece connected to an aneroid manometer at a pressure of 40 mmHg for 15s. The result was taken as the difference between the maximum heart rate during strain and the minimum heart rate following release.
- (5)
Blood pressure response to standing was determined by recording the blood pressure in the supine position and then in the upright standing position with the result taken as the difference in systolic pressure in mmHg.
Generation of an overall autonomic score20 was used to grade cardiovascular parasympathetic and sympathetic function. The scoring system was as follows: HRV < 8 beats/min during single deep breath scores 1; HRV < 7 beats/min during 6 consecutive deep breath scores 2; HRV < 17 beats/min during lying to standing scores 1; HRV < 13 beats/min during Valsalva’s manoeuvre scores 1; blood pressure change with posture (systolic fall > 25 mmHg or diastolic fall > 10 mmHg) scores 1. The sum of these scores constituted the overall autonomic score for an individual subject.
Biochemical measurements
Serum lipids, plasma glucose, HbA1c and NT-proBNP (Roche Diagnostics GmbH, Mannheim; inter-assay CV 2.7% and intra-assay CV 3.2%) were measured in the fasting state using conventional commercial assays. Plasma was stored at −80 °C.
Statistical analysis
All statistical analyses were performed using SPSS (version 14) for Windows. Data are expressed as mean values ± SD. The unpaired t-test and ANOVA were used to evaluate differences between group means for normally distributed data. Correlation between variables was evaluated using Spearman’s and Pearson’s correlation coefficients and by stepwise multiple regression analysis. A p value of less than 0.05 was considered significant.
Results
Comparisons between diabetes and IGT patients
The physical and biochemical characteristics comparing diabetes subjects (type 1 and 2 diabetes combined) with IGT subjects are summarized in Table 1. Haemodynamic data and results of cardiovascular autonomic function comparing the 2 patient groups are shown in Table 2. The 2 groups were reasonably matched in terms of age, weight, brachial artery blood pressure and lipid parameters. Mean duration of diabetes was 11 years for patients with diabetes. NT-proBNP (mean log NT-proBNP) concentrations were similar in both patient groups. Three of the diabetes patients and 3 IGT patients had NT-proBNP concentrations that were frankly elevated according to assay-specific, age- and sex-adjusted normal laboratory ranges.
| Variable | Diabetes subjects (n = 50) | IGT subjects (n = 21) |
|---|---|---|
| Age (years) | 56 ± 10 | 58 ± 7 |
| Weight (kg) | 95 ± 23 | 93 ± 18 |
| Height (cm) | 176 ± 6 | 176 ± 9 |
| Waist circumference (cm) | 107 ± 16 | 105 ± 11 |
| Fat mass (%) | 31 ± 21 | 29 ± 6 |
| NT-proBNP (pg/ml) | 109 ± 230 | 98 ± 128 |
| HbA1c (%) | 8.6 ± 1.6** | 6.0 ± 0.5 |
| Total cholesterol (mmol/l) | 4.4 ± 1.1 | 4.4 ± 0.8 |
| LDL cholesterol (mmol/l) | 2.1 ± 0.8 | 2.0 ± 0.6 |
| HDL cholesterol (mmol/l) | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Triglyceride (mmol/l) | 2.2 ± 2.3 | 2.4 ± 1.9 |
Mean ± SD.
p < 0.01.
Physical and biochemical characteristics of diabetes and IGT subjects
| Variable | Diabetes subjects (n = 50) | IGT subjects (n = 21) |
|---|---|---|
| Brachial systolic blood pressure (mmHg) | 144 ± 22 | 136 ± 22 |
| Brachial diastolic blood pressure (mmHg) | 81 ± 15 | 81 ± 10 |
| HR (beats/min) | 73 ± 14* | 66 ± 10 |
| Aortic systolic pressure (mmHg) | 127 ± 20 | 125 ± 21 |
| Aortic diastolic pressure (mmHg) | 81 ± 9 | 82 ± 10 |
| Augmentation index (%) | 21 ± 12 | 26 ± 10 |
| Augmentation index (HR corrected) (%) | 20 ± 8 | 21 ± 10 |
| Aortic pulse wave velocity (m/s) | 9.7 ± 2.4** | 8.2 ± 1.4 |
| Brachial pulse wave velocity (m/s) | 8.1 ± 1.2 | 7.7 ± 1.2 |
| Autonomic score | 2.3 ± 1.3** | 1.6 ± 1.0 |
| HR variability with single deep breath (beats/min) | 13 ± 8 | 17 ± 19 |
| HR variability with 6 consecutive deep breaths (beats/min) | 11 ± 7 | 13 ± 11 |
| HR variability with postural change | 7 ± 8 | 6 ± 12 |
| Systolic blood pressure difference with postural change (mmHg) | 7 ± 9 | 5 ± 7 |
| HR variability with Valsalva (beats/min) | 21 ± 20 | 22 ± 20 |
Mean ± SD.
p < 0.05;
p < 0.01.
HR, heart rate.
Haemodynamic parameters and autonomic function tests in diabetes and IGT subjects
Aortic stiffness as defined by carotid–femoral PWV was greater in diabetes patients versus IGT patients (9.7 ± 2.4 m/s versus 8.2 ± 1.4 m/s, p < 0.01) although there were no statistically significant differences between brachial artery stiffness as defined by carotid–radial PWV. Central aortic pressures did not differ significantly between the 2 groups and augmentation index (AIX), a measure of systemic wave reflection, was similar in both groups. There were statistically significant differences in autonomic function between the 2 patient groups, with diabetes patients having a greater degree of autonomic dysfunction than IGT patients, as evidenced by a higher mean autonomic score (2.3 ± 1.3 versus 1.6 ± 1.0, p < 0.01). The prevalence of cardiovascular autonomic neuropathy, as defined by an autonomic score ≥3, amongst diabetes patients was 21 out of 50 (42%) and 4 out of 21 (19%) amongst IGT patients.
Univariate relationships amongst diabetes and IGT subjects
In these analyses, inter-relationships were tested for measures of vascular function, autonomic function and concentrations of NT-proBNP. Amongst the diabetes subjects, there were statistically significant correlations between NT-proBNP and central systolic blood pressure (r = 0.74, p < 0.0001), central pulse pressure (r = 0.79, p < 0.0001), brachial systolic blood pressure (r = 0.66, p < 0.0001), aortic stiffness (r = 0.43, p < 0.01) (Fig. 1), augmentation index (r = 0.55, p < 0.0001) and autonomic function (autonomic score, r = 0.37, p < 0.01 and heart rate variability during Valsalva, r = −0.41, p < 0.01) (Fig. 2). For aortic stiffness, there were statistically significant correlations with autonomic function (autonomic score, r = 0.40, p < 0.01 and heart rate variability during Valsalva, r = −0.54, p < 0.0001) (Fig. 2) and duration of diabetes (r = 0.35, p < 0.05). Augmentation index also correlated with autonomic function (autonomic score, r = 0.31, p < 0.05 and heart rate variability during Valsalva, r = −0.41, p < 0.01).
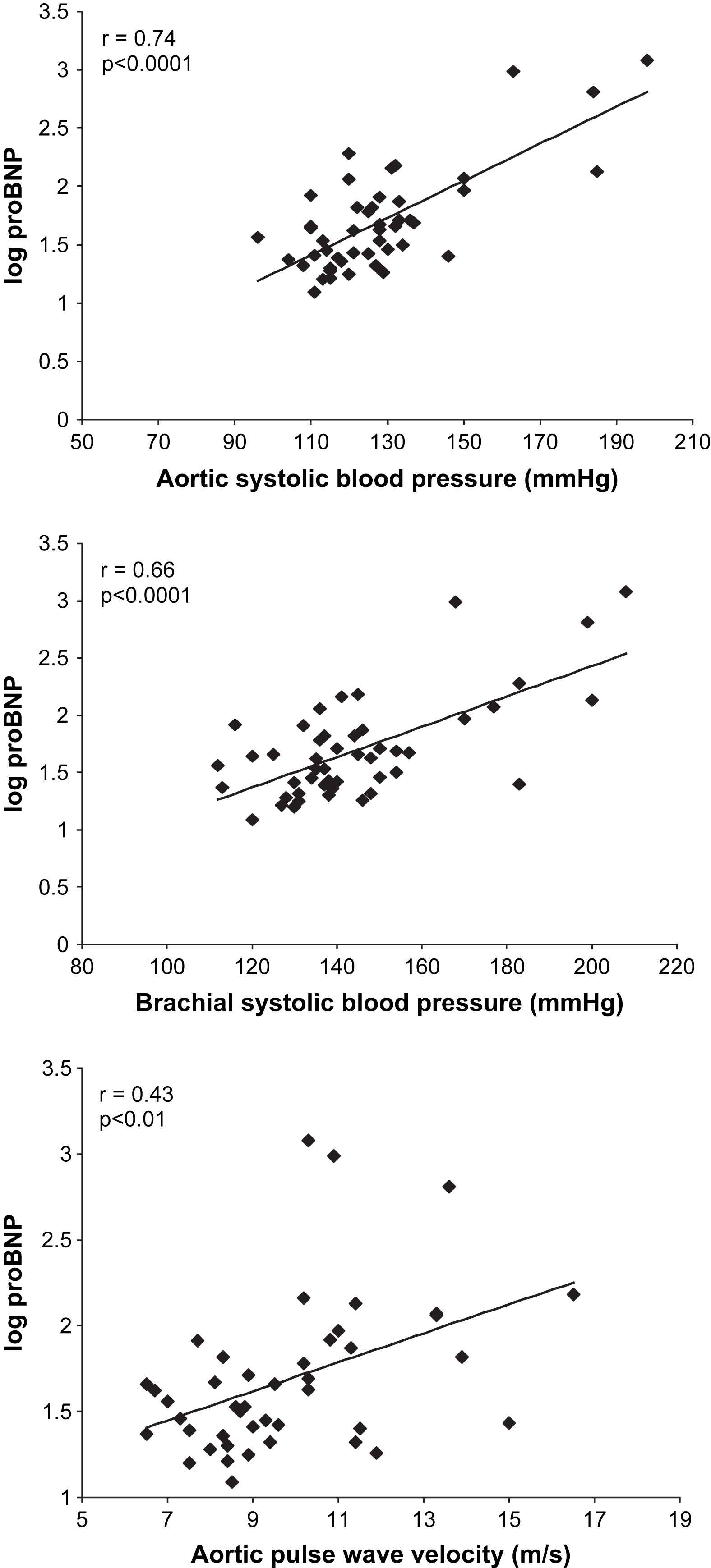
Relationship between NT-proBNP and arterial parameters amongst diabetes subjects alone.

Relationships between heart rate variability during Valsalva, NT-proBNP and aortic pulse wave velocity (PWV) amongst diabetes subjects alone.
Amongst IGT subjects, NT-proBNP correlated with central systolic blood pressure (r = 0.52, p < 0.05), central pulse pressure (r = 0.53, p < 0.05) and brachial systolic blood pressure (r = 0.50, p < 0.05), but statistically significant correlations were not noted with augmentation index (r = 0.33, p = 0.14) or aortic stiffness (r = 0.31, p = 0.19).
Multiple regression analysis amongst diabetic subjects
In order to determine independent predictors of NT-proBNP concentrations in diabetes patients, stepwise multiple regression analysis was performed with log NT-proBNP as the dependent variable and several potential predicting independent variables (age, central systolic pressure, brachial systolic pressure, aortic PWV, duration of diabetes, HbA1c, weight, estimated glomerular filtration rate and autonomic score) included. We generated 2 models: the first (Table 3a) included central systolic pressure as a predictor, and the second (Table 3b) included brachial systolic pressure as a predictor. These analyses revealed central systolic pressure (beta = 0.67, p < 0.0001) to be the strongest predictor of NT-proBNP concentration of all the variables studied. Multiple regression analysis for the IGT subject group was not undertaken because of the relatively small numbers within this group.
| Independent variable | Beta | t | p value |
|---|---|---|---|
| Central systolic pressure (mmHg) | 0.67 | 6.2 | < 0.0001 |
| Estimated glomerular filtration rate (ml/min) | −0.24 | −2.3 | 0.029 |
| Age (years) | 0.16 | 1.4 | 0.18 |
| Aortic PWV (m/s) | 0.053 | 0.50 | 0.62 |
| Autonomic score | −0.02 | −0.20 | 0.84 |
| Weight (kg) | −0.11 | −1.13 | 0.27 |
| Duration of diabetes (years) | −0.003 | −0.031 | 0.98 |
| HbA1c (%) | −0.07 | −0.67 | 0.51 |
Predictors of NT-proBNP concentrations: stepwise multiple regression analysis with log NT-proBNP as dependent variable and including central systolic pressure as an independent variable (R2 = 0.64)
| Independent variable | Beta | t | p value |
|---|---|---|---|
| Brachial systolic pressure (mmHg) | 0.52 | 4.4 | < 0.0001 |
| Age (years) | 0.34 | 2.8 | 0.007 |
| Estimated glomerular filtration rate (ml/min) | −0.22 | −1.66 | 0.10 |
| Aortic PWV (m/s) | −0.03 | −0.20 | 0.84 |
| Autonomic score | 0.03 | 0.23 | 0.82 |
| Weight (kg) | −0.07 | −0.55 | 0.59 |
| Duration of diabetes (years) | −0.02 | −0.14 | 0.89 |
| HbA1c (%) | −0.09 | −0.75 | 0.46 |
Predictors of NT-proBNP concentrations: stepwise multiple regression analysis with log NT-proBNP as dependent variable and including brachial systolic pressure as an independent variable (R2 = 0.52)
Discussion
Large artery stiffening is an important contributor to cardiovascular morbidity and mortality in patients with diabetes and IGT15 and is increasingly recognised to play an important pathophysiological role in the development of diabetic cardiomyopathy.1,3 Elevated circulating concentrations of the cardiac hormone B-type natriuretic peptide (BNP) and the N-terminal fragment of the prohormone (NT-proBNP), in the absence of clinically apparent left ventricular dysfunction, have also been shown to predict the development of clinical heart failure and cardiovascular mortality.9,10
In the present study, we have investigated determinants of large artery stiffening in diabetes and IGT patients and specifically examined the effects of conduit artery stiffness (aortic and brachial arteries), central arterial pressure and cardiovascular autonomic dysfunction on NT-proBNP concentrations in these patients. The main finding of the study is that central arterial pressure strongly and independently predicts elevations in circulating NT-proBNP in diabetic men without clinically apparent left ventricular dysfunction. Recent studies have demonstrated an association between aortic pulse wave velocity and NT-proBNP in healthy individuals in the Framingham cohort13 and in patients with coronary disease.14 However, to date, there has been little study of this relationship in diabetes and IGT subjects although Dawson et al.21 have recently reported an association between augmentation index and BNP in type 2 diabetes patients with preserved left ventricular function.
Elevations in levels of BNP and NT-proBNP are associated with a number of cardiac abnormalities including increased wall stretch due to volume expansion, pressure overload, left ventricular dysfunction and left ventricular hypertrophy.22 Since left ventricular mechanical function is heavily influenced by aortic pressure changes occurring during the cardiac cycle, it is likely that increased NT-proBNP production occurs, in part, as a consequence of increased systolic afterload derived from large artery stiffening. Indeed, haemodynamic changes associated with large artery stiffening, particularly elevated central aortic pressure are known to be the major determinants of left ventricular afterload,6 lead directly to the mal-adaptive changes of myocardial hypertrophy and contribute ultimately to the development of left ventricular failure.23,24
In the present study, although aortic stiffness (aortic PWV) was closely correlated with elevations in NT-proBNP, this association was not independent of other important confounding factors particularly systolic blood pressure, age and duration of diabetes. The reason why central arterial pressure and augmentation index appeared to be more efficient predictors of NT-proBNP levels than aortic stiffness is not entirely clear. Aortic PWV is considered to be the gold-standard measurement of large artery stiffness and is established as a predictor of cardiovascular mortality in essential hypertension and chronic renal failure.25,26 However, its measurement relates to stiffness in only a single artery, the aorta. In contrast, arterial pulse wave analysis provides a non-invasive measurement of central pressure and the contribution that wave reflection makes to peak aortic systolic pressure.16,17 Because the amplitude and timing of wave reflection is not only influenced by conduit artery stiffness but also by arterial/arteriolar tone in the smaller resistance vessels, wave reflection and central pressure probably provide a more global assessment of afterload. In addition, the fact that myocardial load and wall stretch (both important determinants of NT-proBNP production) are known to be highly dependent on central pressure6 provides further explanation for our observation that central systolic pressure and central pulse pressure were the strongest predictors of NT-proBNP levels of all the parameters examined in the present study.
Cardiovascular autonomic dysfunction is common in diabetes and, although the mechanisms remain only partly understood, has been implicated in the aetiology of several clinically important cardiac abnormalities including silent myocardial ischaemia, arrhythmias and left ventricular dysfunction.27 Indeed, several studies have shown cardiovascular autonomic neuropathy to be a powerful predictor of premature cardiovascular mortality in diabetes.27,28 In addition to its detrimental effect on cardiac function, cardiovascular autonomic neuropathy may also play a pathophysiological role in the development of premature large artery stiffening in diabetes. In addition to the findings of the present study, other investigators have also demonstrated a link between autonomic neuropathy and large artery function in non-insulin treated type 2 diabetes subjects5 and in women with type 1 diabetes.29 To date, there has been little study of the effects of cardiac autonomic neuropathy on BNP or NT-proBNP levels in diabetes and IGT. However, exogenous administration of BNP does influence cardiac autonomic function in an animal model30 and Yufu et al.31 have recently demonstrated that increased plasma BNP levels correlate with cardiac reflex parasympathetic dysfunction in type 2 diabetes subjects, suggesting a link between the BNP neurohormonal system and autonomic function. However, in the present study, although we have demonstrated that cardiovascular autonomic neuropathy is associated with elevations in NT-proBNP in diabetes subjects, we were unable to demonstrate an independent relationship between these parameters.
In conclusion, in men with type 1 and 2 diabetes, increased central arterial pressure is a strong independent predictor of elevations in NT-proBNP concentrations. Aortic stiffness and cardiovascular autonomic neuropathy may also play a role to a lesser extent. This association provides further evidence of the key influence of unfavourable large artery haemodynamics on the development of left ventricular dysfunction in diabetes.
Competing interests
None declared.
Acknowledgements
We thank the Biochemistry Department of Torbay Hospital for analysis of blood samples. We also thank Torbay Medical Research Fund for kindly supporting this study.
References
Cite this article
TY - JOUR AU - S. Bunce AU - A. Stride AU - C. Matthews AU - S. Shaw AU - J.C. Smith PY - 2008 DA - 2008/04/23 TI - The effects of central arterial pressure and autonomic dysfunction on elevations in N-terminal pro-B-type natriuretic peptide (NT-proBNP) in men with diabetes JO - Artery Research SP - 60 EP - 66 VL - 2 IS - 2 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2008.03.002 DO - 10.1016/j.artres.2008.03.002 ID - Bunce2008 ER -
