Pre-existing Cerebral Small Vessel Disease in Young Patients with Acute Ischemic Stroke caused by Large Artery Atherosclerosis or Small Artery Occlusion
Hui Li and Dan Du are both the co-first author of this study.
- DOI
- 10.2991/artres.k.210511.001How to use a DOI?
- Keywords
- Young; pre-existing cerebral small vessel disease; acute ischemic stroke; large artery atherosclerosis; small artery occlusion
- Abstract
Objective: To explore the characteristics of pre-existing Cerebral Small Vessel Disease (CSVD) in young patients with Acute Ischemic Stroke (AIS) caused by large artery atherosclerosis or small artery occlusion.
Methods: A total of 400 patients with suspected stroke or transient ischemic attack who aged 18–45 years old were included in this retrospective study. Demography data, vascular risk factors and primary CSVD were compared between patients with AIS and non-stroke or between patients with different subtypes of AIS.
Results: The levels of cerebrovascular risk factors were significantly higher in patients with AIS than those with non-stroke (p < 0.05). The majority of patients with large artery atherosclerotic stroke or small artery occlusive stroke were male. The age was slightly younger and the NIH stroke scale (NIHSS) score at admission were higher in large artery atherosclerotic stroke group than small artery occlusive stroke group. White matter hyperintensities, moderate and extensive enlarged perivascular space, 1–3 points of total score were more commonly observed in patients with AIS than those with non-stroke (p < 0.05). Male and diabetes were the risk factors of lacune infarctions (p < 0.05). Male, hypertension and primary CSVD were the risk factors of both of strokes (p < 0.05). The enlarged perivascular space notably increased the risk of large artery atherosclerotic stroke (p = 0.033).
Conclusion: Male, age, hypertension, diabetes and pre-existing CSVD were the risk factors of young patients with AIS. For young adults, once asymptomatic CSVD abnormalities were detected, cerebrovascular risk factors should be screened and pre-existing prevention measures for stroke should be taken.
- Copyright
- © 2021 The Authors. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The researches of stroke have long focused on the elderly, and the young stroke (18–45 years old) is a relatively neglected field. According to available data, the young stroke only account for less than 5% of all in western countries [1]. However, in developing countries, its proportion reaches 19–30% [2,3]. The incidence of young stroke is gradually increasing since the 1980s [4–6]. Researches on young stroke make more economic sense, because adults with young stroke are likely to be disabled during their most productive working years and have higher long-term mortality than their non-stroke peers [3,7,8].
Lots of western studies have suggested that cardioembolism, carotid artery dissection and unexplained stroke may be the main etiological types of stroke in young people [9,10]. However, in some Chinese studies, large artery atherosclerosis and small artery occlusion are still considered as the most common causes of young stroke [11,12]. Stroke caused by small artery occlusion, also called lacunar infarction, is one of the pathological changes of Cerebral Small Vessel Disease (CSVD). CSVD often coexists with large artery atherosclerosis, and the latter has been proven to increase the risk of stroke in the elderly [13–15]. Whether CSVD increases the risk of stroke is still controversial. A previous multicenter study in Korea has shown that the presence and severity of CSVD increase the risk of stroke [16]. Nevertheless, another prospective, multicenter clinical trial, Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS), has indicated that CSVD do not increase the risk of stroke [17].
Cerebral small vessel disease is a pathology that affects microcirculation of the brain, and it is a major cause of cognitive impairment and dementia worldwide [18]. In recent years, CSVD is often found in youth without typical vascular risk factors during the assessment of benign neurological disorders, including headaches, dizziness and vertigo, nonspecific cognitive or mood disorders. Consequently, understanding the effect of asymptomatic CSVD on stroke is conducive in taking positive pre-existing prevention measures to reduce the risk of stroke. Until now, there are few studies on the relationship between CSVD and Acute Ischemic Stroke (AIS) in young people.
Therefore, the purpose of this study was to explore the characteristics of pre-existing CSVD in young patients with AIS caused by large artery atherosclerosis or small artery occlusion.
2. SUBJECTS AND METHODS
2.1. Patients
This study was approved by the local Medical Ethics Committee of Wuhan Central Hospital (NO. [2020]201). A total of 400 patients aged 18–45 years old who were treated in our hospital with suspected stroke or Transient Ischemic Attack (TIA) from January 2018 to January 2020 were enrolled in this retrospective study. Neuroimaging data of all patients were obtained from Picture Archiving and Communication Systems. Inclusion criteria: (1) Patients aged 18–45 years old; (2) Patients underwent brain Magnetic Resonance Imaging (MRI, including DWI, T1 and T2 weighted sequence), head and neck vascular imaging after admission; (3) Patients with complete cardiovascular data and a clear etiological diagnosis of stroke prior to discharge. Exclusion criteria: (1) Stroke caused by cardioembolism, undetermined cause, or other infrequent stroke etiologies (non-atherosclerotic arteriopathies, genetic disorders, coagulation disorders, systemic diseases, neoplasms, and infections; (2) Patients with hemorrhagic stroke; (3) Patients with brain tumor, infection, degeneration, metabolism, poisoning or mental disease; (4) Patients with pre-existing atherosclerotic cerebral infarction or cerebral hemorrhage; (5) Patients whose images with artifacts. Of the 1956 patients with suspected stroke or TIA, 1556 were excluded, and 400 patients were included in this study. The process of case screening was shown in Figure 1.
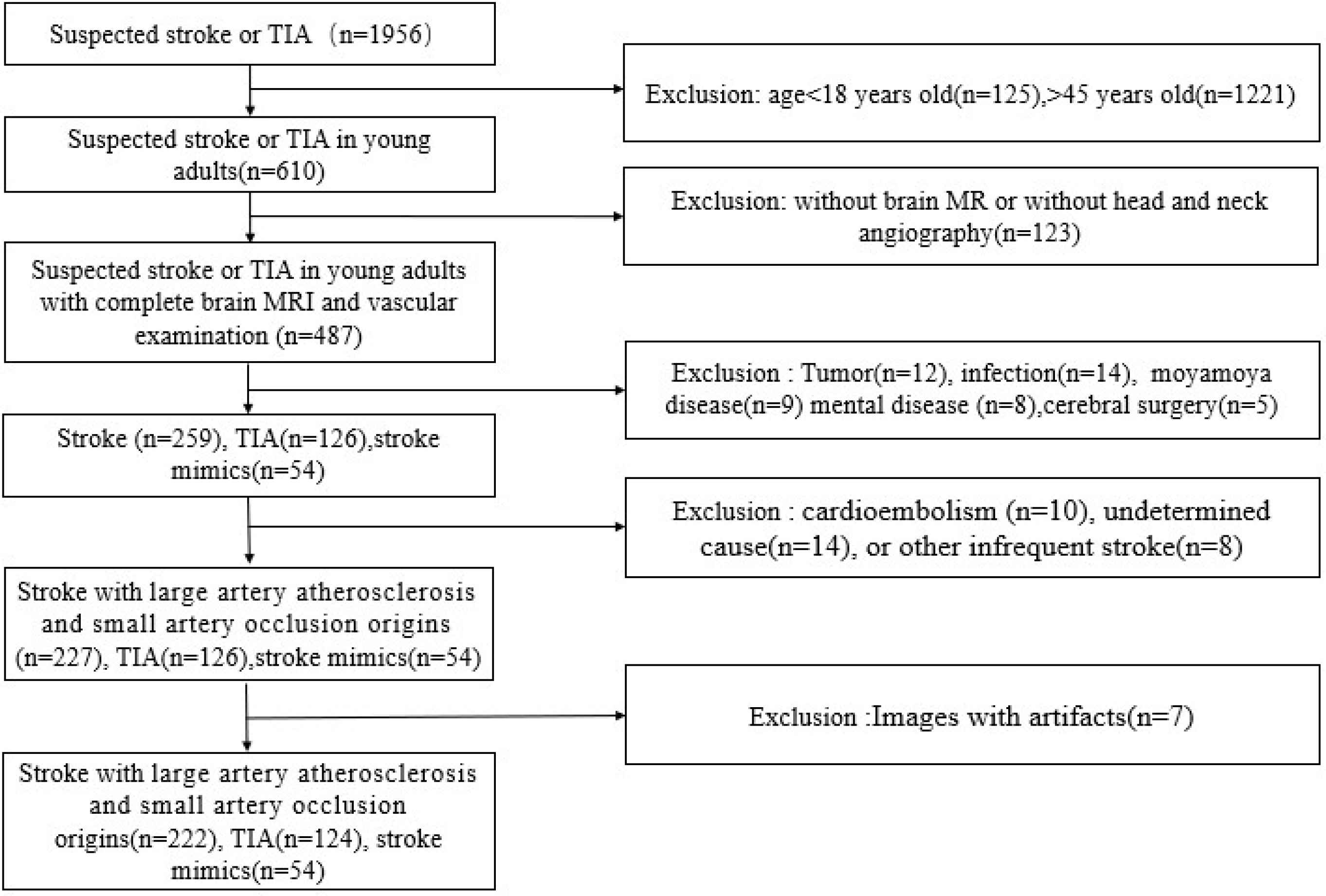
The flow chart of research objects selection.
2.2. Etiological Classification of Acute Ischemic Stroke and Definition of Pre-existing CSVD
The diagnosis of AIS was determined by specific neuropathological symptoms and definitive MRI evidence. AISs were divided into five subtypes according to Trail of Org 10172 in Acute Stroke Treatment (TOAST) etiological classification, including large artery atherosclerotic stroke, stroke caused by cardioembolism, small artery occlusive stroke, other strokes with clear cause and strokes with undetermined cause. According to the main etiological characteristics of stroke in Chinese youth, we only selected patients with large artery atherosclerotic stroke and small artery occlusive stroke in the present study. Pre-existing CSVD was defined as one or more signs of chronic Lacune Infarctions (LI), White Matter Hyperintensities (WMH) and Enlarged Perivascular Space (EPVS). Due to the limited application of susceptibility weighted imaging in the assessment of AIS in most hospitals in China, we only selected CSVD signs (LI, WMH and EPVS) available from routine MRI sequences to ensure feasibility and transferability of the results on clinical practice.
2.3. Clinical Data
Clinical data, including demographic data, cerebrovascular risk factors, NIHSS scores and therapeutic approach after admission, were also collected. Demographic data included age and gender. Cerebrovascular risk factors covered blood pressure, blood glucose, Triglycerides (TG), Total Cholesterol (TC), High-density Lipoprotein Cholesterol (HDL-C), Low-density Lipoprotein Cholesterol (LDL-C) and homocysteine. Therapeutic approaches referred to oral antiplatelet drugs, intravenous thrombolysis, endovascular treatment and other treatments.
2.4. MRI Acquisition
1.5T Philips Ingenia scanner (Philips, The Netherlands) and 3.0T Siemens Skyra scanner (Siemens, Erlangen, Germany) were used for brain scan. The images contained T1-weighted, T2-weighted, T2 Fluid Attenuated Inversion Recovery (T2-FLAIR), and DWI sequences. The sequence parameters of 1.5T MRI were as follows: T1-weighted images [Repeat Time (TR) = 2500 ms, Echo Time (TE) = 25 ms, Field of View (FOV) = 220 × 198 mm2, matrix = 320 × 256, slice thickness = 5 mm, layer spacing = 1 mm). T2-weighted images (TR = 5000 ms, TE = 118 ms, FOV = 220 × 199 mm2, matrix = 320 × 256, slice thickness = 5 mm, layer spacing = 1 mm). T2 FLAIR sequence (TR = 9000 ms, TE = 172 ms, FOV = 220 × 220 mm2, matrix = 192 × 256, slice thickness = 5 mm, layer spacing = 1 mm). DWI sequences (TR = 5000 ms, TE = 83 ms, FOV = 240 × 240 mm2, matrix = 128 × 128, slice thickness = 5 mm, layer spacing = 1 mm, b-value = 0, 1000). The sequence parameters of 3.0T MRI were as follows: three-dimensional T1WI (TR = 2530 ms, TE = 3.43 ms, TI = 1100 ms, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 1.3 mm3, flip angle = 8°, 144 sagittal sections) were obtained on the sagittal plane by using the magnetized rapid gradient echo technology. T2WI (TR = 6000 ms, TE =125 ms, FOV = 230 × 230 mm2, flip angle = 90°, slice thickness = 5 mm, layer spacing = 1 mm, 80 axial sections). T2-FLAIR (TR = 8500 ms, TE = 81 ms, FOV = 230 × 230 mm2, flip angle = 150°, slice thickness = 5 mm, layer spacing = 1 mm, 80 axial sections). DWI sequences (TR = 4040 ms, TE = 64 ms, FOV = 220 × 220 mm2, matrix = 128 × 128, slice thickness = 5 mm, layer spacing = 1 mm, b-value = 0, 1000).
2.5. Pre-existing CSVD MRI Abnormalities Analysis
Pre-existing CSVD MRI abnormalities were evaluated by an attending radiologist (Hui Li, MM) and a radiology professor (Yuanliang Xie, PhD). About 50 cases were randomly selected for consistency test. LI was defined as round or ovoid lesions with the diameter from 3 to 20 mm in the basal ganglia, internal capsule, centrum semiovale or brainstem, with CSF signal density on T2WI and T2-FLAIR, without increased signal on DWI imaging [19]. The presence of LI was scored 1, otherwise scored 0. WMH was diagnosed and scored by the revised version of the visual scale of Fazekas [20]. The Fazekas scale included Periventricular White Matter Hypersignal (PVWMH) score and Deep White Matter Hypersignal (DWMH) score. PVWMH score was defined as follows: none (0, no lesions), mild (1, caps or a pencil-thin lining), moderate (2, smooth halo) and severe (3, irregular lesions extending to deep white matter). DWMH was scored as follows: none (0, no lesions), mild (1, punctuate foci), moderate (2, beginning confluent foci) and severe (3, large confluent lesions). EPVS was defined as small (<3 mm) punctate (if perpendicular to the plane of scan) or linear (if longitudinal to the plane of scan) lesions with signal intensity similar to that of cerebrospinal fluid on all sequence spaces and without a T2-hyperintense rim on FLAIR imaging. A three-category ordinal scale (mild, 0–10; moderate, 10–25; extensive, >25) was used to evaluate the severity of EPVS at basal ganglia level [21].
Total CSVD score was calculated based on LI, WMH, and EPVS referred to the ordinal scale developed by Klarenbeek et al. [22]. One point was awarded in the following cases: (1) one or more lacune infarctions were present; (2) DWMH Fazekas score 2 or 3, or PVWMH Fazekas score 3; (3) EPVS in the basal ganglia or centrum semiovale was scored 2 or 3. Then the three subscores were summed up to generate a total CSVD score that ranged from 0 to 3.
2.6. Statistical Analysis
SPSS version 22.0 (NY, USA) was used to perform the statistical analysis. The categorical/dichotomous variables were analyzed using χ 2 test, and the linear-by-linear association was used for orderly classification variables. Continuous variables were analyzed by Mann–Whitney U-test. The impact of age, gender, risk factors on the risk of development of CSVD MRI abnormalities and the subtypes of AIS were assessed using logistic regression analysis. Odds Ratio (OR) and 95% CI were reported. p < 0.05 was considered to statistically significant. Kappa value was used to evaluate the consistency between the surveyors. Kappa value <0.40 indicated that the reliability of the data was poor, while kappa value >0.75 suggested high credibility.
3. RESULTS
3.1. Consistency between the Surveyors
The pre-existing CSVD MR abnormalities obtained twice by physician 1 (Hui Li, MD) were compared, and it showed a good reliability with kappa value of 0.81 for the presence of LI, 0.89 for PVWMH, 0.85 for DWMH, and 0.76 for EPVS. The consistency test between the surveyors also indicated satisfactory consistency with kappa values of 0.79 for the presence of LI, 0.87 for PVWMH, 0.89 for DWMH, and 0.85 for EPVS.
3.2. Clinical Characteristics of Young Adults with AIS
A total of 400 patients with suspicious stroke or TIA underwent brain MRI, head and neck angiography examination. About 222 patients (55.5%) were diagnosed with AIS, including 130 (58.6%) with large artery atherosclerotic stroke and 92 (41.4%) with small artery occlusive stroke. There were 178 patients (44.5%) with non-stroke, including 124 (69.7%) with TIA and 54 (30.3%) with stroke mimics.
Compared with non-stroke group, patients with AIS had higher blood pressure (p = 0.000), blood glucose (p = 0.000), TG (p = 0.000), TC (p = 0.000), HDL-C (p = 0.000), homocysteine (p = 0.000), NIHSS score (p = 0.012) at admission and LDL-C (p = 0.002). The majority of patients with AIS received antiplatelet therapy (84.6% vs. 93.5%).
The majority of patients with large artery atherosclerotic stroke and small artery occlusive stroke were male (89.2% vs. 87.0%). Compared with patients with small artery occlusive stroke, the age of those with large artery atherosclerotic stroke was slightly younger (p = 0.027), the blood glucose (p = 0.023) and NIHSS score (p = 0.003) at admission were significantly higher. There were no significant differences in gender, systolic blood pressure, blood lipid and homocysteine at admission between the two subtypes of stroke (p > 0.05) (Table 1).
| Clinical variables | Total (n = 400) | Acute ischemic stroke (n = 222) | Non-stroke (n = 178) | p-value | |||
|---|---|---|---|---|---|---|---|
| Large artery atherosclerotic stroke (n = 130) | Small artery occlusive stroke (n = 92) | p-value | Total (n = 222) | ||||
| Age | 40 (35–43) | 40.5 (37–43) | 43 (41–43) | 0.027* | 42 (39–43) | 39 (30–43) | 0.105 |
| Male (%) | 294 (73.5) | 116 (89.2) | 80 (87.0) | 0.604 | 196 (88.3) | 98 (55.1) | 0.000* |
| Blood pressure | 146 (129–173) | 151 (136–167) | 160 (145–175) | 0.211 | 156 (142–170) | 125 (120–140) | 0.000* |
| Blood glucose | 5.42 (4.86–6.89) | 5.74 (5.01–9.66) | 5.23 (4.64–6.40) | 0.023* | 5.63 (5.01–7.71) | 4.95 (4.78–5.52) | 0.000* |
| Triglyceride | 1.62 (1.04–2.44) | 1.86 (1.25–2.64) | 2.11 (1.2–2.22) | 0.537 | 1.87 (1.20–2.32) | 1.52 (0.73–0.97) | 0.000* |
| Total cholesterol | 4.68 (4.00–5.24) | 4.89 (4.1–5.2) | 4.64 (4.2–5.26) | 0.108 | 4.83 (4.17–5.21) | 4.5 (3.92–5.18) | 0.000* |
| HDL-C | 1.10 (0.95–1.25) | 1.1 (0.97–1.21) | 1.13 (0.83–1.45) | 0.107 | 1.11 (0.96–1.25) | 1.22 (1.08–1.43) | 0.002* |
| LDL-C | 2.89 (2.33–3.78) | 3.06 (2.64–3.60) | 2.91 (2.51–3.28) | 0.419 | 2.97 (2.64–3.52) | 2.56 (2.23–3.29) | 0.000* |
| Homocysteine | 13.55 (10.45–20) | 13.65 (10.75–26.95) | 16.10 (10.90–20.10) | 0.643 | 13.9 (10.9–23.1) | 11.6 (9.73–15.08) | 0.000* |
| NIHSS score | 3 (1–5) | 5 (2–7) | 2 (0–2) | 0.003* | 4 (1–5) | 1 (0–1) | 0.012* |
| Treatments (%) | |||||||
| Intravenous thrombolysis | 4 (1.0) | 2 (1.5) | 2 (2.1) | – | 4 (1.8) | – | – |
| Intraarterial thrombectomy | 6 (1.5) | 6 (4.6) | 0 | – | 6 (2.7) | – | – |
| Intraarterial thrombolysis | 4 (1.0) | 4 (3.1) | 0 | – | 4 (1.8) | – | – |
| Antiplatelet | 278 (69.5) | 110 (84.6) | 86 (93.5) | – | 196 (88.3) | – | – |
| Others | 18 (4.5) | 8 (6.2) | 4 (4.3) | – | 12 (5.4) | – | – |
p < 0.05.
Comparison of age, gender, vascular risk factors and treatments between ischemic stroke and non-stroke, as well as the subtypes of stroke
3.3. Pre-existing CSVD MRI Abnormalities in Young Ischemic Stroke
Pre-existing CSVD MRI abnormalities were evaluated for the presence of LI, WMH and EPVS, CSVDm (number of the types of CSVD imaging markers), LI score, WMH score, EPVS grading and CSVD total score. Pre-existing CSVD MRI abnormalities were present in 95.4% of patients with large artery atherosclerotic stroke and 93.5% with small artery occlusive stroke. Three and two kinds of pre-existing CSVD MRI abnormalities were more common in patients with large artery atherosclerotic stroke and small artery occlusive stroke, while one abnormality was rare. LI (64.6% vs. 71.7%), WMH (89.2% vs. 87.0%) and EPVS (83.1% vs. 82.6%) were commonly observed in both patients with large artery atherosclerotic stroke and small artery occlusive stroke, and most of them had mild to moderate WMH and EPVS.
The presence of pre-existing CSVD in patients with AIS was more common than non-stroke group (94.6% vs. 76.4%, p = 0.000). Overall, WMH were found in 83% patients and there was significantly more common in patients with AIS compared to non-stroke group (88.3% vs. 76.4%, p = 0.011). Significantly fewer mild (47.7% vs. 49.4%) but more moderate (27.9% vs. 14.6%) and extensive (18.9% vs. 12.4%) EPVS were observed in patients with AIS (p = 0.035). Patients with AIS got fewer 0 point (12.6% vs. 20.2%) and more 1 point (45.9% vs. 31.5%), 2 points (30.6% vs. 22.5%) and 3 points (5.4% vs. 2.2%) of total score than non-stroke group (p = 0.014) (Table 2).
| CSVD abnormalities | Total (n = 400) | Acute ischemic stroke (n = 222) | Non-stroke (n = 178) | p-value | |||
|---|---|---|---|---|---|---|---|
| Large artery atherosclerotic stroke (n = 130) | Small artery occlusive stroke (n = 92) | p-value | Total (n = 222) | ||||
| Pre-existing CSVD (%) | 346 (86.5) | 124 (95.4) | 86 (93.5) | 0.750 | 210 (94.6) | 136 (76.4) | 0.000* |
| CSVDm (%) | 0.192 | 0.664 | |||||
| 1 | 20 (5.8) | 12 (9.2) | 2 (2.2) | – | 14 (6.3) | 6 (3.4) | – |
| 2 | 132 (38.2) | 42 (32.3) | 32 (34.8) | – | 74 (33.3) | 58 (32.6) | – |
| 3 | 194 (56.0) | 70 (53.8) | 52 (56.5) | – | 122 (55.0) | 72 (40.4) | – |
| LI (%) | 236 (68.2) | 84 (64.6) | 66 (71.7) | 0.156 | 150 (67.6) | 86 (48.3) | 0.110 |
| WMH (%) | 332 (96) | 116 (89.2) | 80 (87.0) | 0.745 | 196 (88.3) | 136 (76.4) | 0.011* |
| EPVS (%) | 298 (86.1) | 108 (83.1) | 76 (82.6) | 0.783 | 184 (82.9) | 114 (64.0) | 0.318 |
| CSVD score | |||||||
| LI score | 0.157 | 86 (48.3) | 0.110 | ||||
| 0 | 110 (31.8) | 40 (30.8) | 20 (21.7) | – | 60 (27.0) | 50 (28.1) | – |
| 1 | 236 (68.2) | 84 (64.6) | 66 (71.7) | – | 150 (67.6) | 86 (48.3) | – |
| WMH score | 0.478 | 0.241 | |||||
| 0 | 14 (4.0) | 8 (6.2) | 6 (6.5) | – | 14 (6.3) | 0 (0) | – |
| 1 and 2 | 288 (83.2) | 104 (80.0) | 66 (71.7) | – | 170 (76.6) | 118 (66.3) | – |
| 3 and 4 | 36 (10.4) | 8 (6.2) | 12 (13.0) | – | 20 (9.0) | 16 (9.0) | – |
| 5 and 6 | 8 (2.3) | 4 (3.1) | 2 (2.2) | – | 6 (2.7) | 2 (1.1) | – |
| EPVS grading | 0.914 | 0.035* | |||||
| 1 | 194 (56.1) | 62 (47.7) | 44 (47.8) | – | 106 (47.7) | 88 (49.4) | – |
| 2 | 88 (25.4) | 36 (27.7) | 26 (28.3) | – | 62 (27.9) | 26 (14.6) | – |
| 3 | 64 (18.5) | 26 (20.0) | 16 (17.4) | – | 42 (18.9) | 22 (12.4) | – |
| Total score | 0.292 | 0.014* | |||||
| 0 | 64 (18.5) | 22 (16.9) | 6 (6.5) | – | 28 (12.6) | 36 (20.2) | – |
| 1 | 158 (45.7) | 54 (41.5) | 48 (52.2) | – | 102 (45.9) | 56 (31.5) | – |
| 2 | 108 (31.2) | 42 (32.3) | 26 (28.3) | – | 68 (30.6) | 40 (22.5) | – |
| 3 | 16 (4.6) | 6 (4.6) | 6 (6.5) | – | 12 (5.4) | 4 (2.2) | – |
p < 0.05.
Comparison of pre-existing CSVD MRI abnormalities between ischemic stroke and non-stroke, as well as subtypes of stroke
3.4. Relationship between Pre-existing CSVD MRI Abnormalities and Age, Gender, Risk Factors, Subtypes of Ischemic Stroke
Pre-existing CSVD MRI abnormalities were observed in almost 86.5% of all subjects (LI 68.2%, WMH 96.0%, EPVS 86.1%), and 56.1% of them had three types and 38.2% had two types. CSVD MRI abnormalities were increased with age. LI were observed in 70% patients over 35 years old, the OR for developing LI was 10.61 times (p = 0.001) compared to the patients under 35 years old. The presence of WMH and EPVS accounted for 92.7% and 84.0% of patients over 35 years old, and the OR for developing WMH and EPVS were 4.598 times (p = 0.026) and 5.205 times (p = 0.048) respectively compared with those under 35 years old. LI were present in 64.6% of male patients. Male was more likely to develop lacunar infarction than female up to 4.12 times (p = 0.017). 77% of diabetic patients developed LI up to 5.58 times (p = 0.005) compared with non-diabetic patients (Table 3 and Figure 2).
| Variables | LI | WMH | EPVS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Adjusted OR (95% CI) | p-value | No. (%) | Adjusted OR (95% CI) | p-value | No. (%) | Adjusted OR (95% CI) | p-value | |
| >35 (Yes vs. No) | 210 (70.0) | 10.61 (2.54–44.29) | 0.001* | 278 (92.7) | 4.598 (1.365–12.258) | 0.026* | 252 (84.0) | 5.205 (1.013–26.751) | 0.048* |
| Male (Yes vs. No) | 190 (64.6) | 4.12 (1.29–13.18) | 0.017* | 254 (86.4) | 0.904 (0.174–4.688) | 0.904 | 230 (78.2) | 0.929 (0.133–6.471) | 0.941 |
| Diabetes (Yes vs. No) | 94 (77.0) | 5.58 (1.67–18.62) | 0.005* | 110 (90.2) | 0.317 (0.091–1.099) | 0.070 | 106 (86.9) | 1.907 (0.289–12.583) | 0.503 |
| Hypertension (Yes vs. No) | 170 (69.1) | 0.644 (0.207–2.003) | 0.447 | 228 (92.7) | 0.541 (0.110–2.658) | 0.449 | 208 (84.6) | 0.290 (0.047–1.786) | 0.182 |
| High triglycerides (Yes vs. No) | 118 (67.8) | 1.479 (0.507–4.311) | 0.474 | 152 (87.4) | 1.255 (0.377–4.175) | 0.711 | 142 (81.6) | 2.850 (0.525–15.483) | 0.225 |
| High total cholesterol (Yes vs. No) | 32 (72.7) | 1.938 (0.293–12.826) | 0.493 | 40 (90.9) | 0.993 (0.200–4.944) | 0.993 | 38 (86.4) | 1.835 (0.327–11.684) | 0.482 |
| Hyperhomocysteinemia (Yes vs. No) | 44 (78.6) | 2.390 (0.775–7.368) | 0.129 | 52 (92.9) | 1.349 (0.893–2.037) | 0.155 | 50 (89.3) | 1.231 (0.231–6.562) | 0.808 |
p < 0.05.
Binary logistic regression analysis for the risk factors of LI, WMH and EPVS
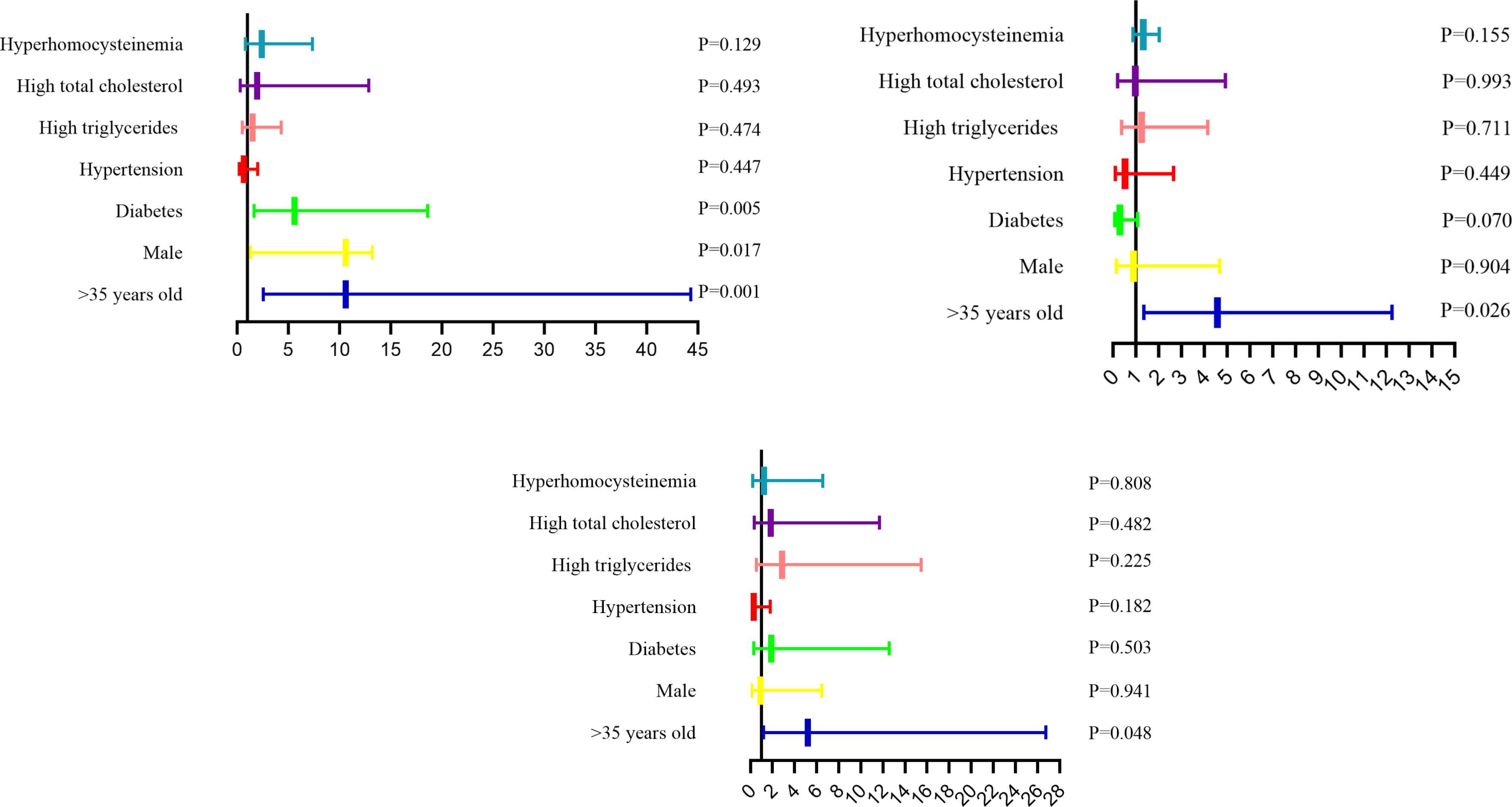
The analysis of risk factors for development of LI, WMH and EPVS, respectively. p < 0.05 presented independent risk factors.
Male was 4.424 and 17.835 times more likely to develop large artery atherosclerotic stroke (4.424 times, p = 0.017) and small artery occlusive stroke (17.835 times, p = 0.001) compared to female. Hypertension significantly increased the risk of large artery atherosclerotic stroke (7.198 times, p = 0.000) and small artery occlusive stroke (23.119 times, p = 0.000). For large artery atherosclerotic stroke and small artery occlusive stroke, HDL-C and hyperhomocysteinemia were protective factors. The pre-existing CSVD could both increase the risk of large artery atherosclerotic stroke (5.249 times, p = 0.031) and small vessel occlusive stroke (3.182 times, p = 0.042). The pre-existing EPVS obviously increased the risk of large artery atherosclerotic stroke (9.989 times, p = 0.033) (Table 4).
| Variables | Large artery atherosclerotic stroke (n = 130) | Small artery occlusive stroke (n = 92) | Non-stroke (n = 178) | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| >35 years old (Yes vs. No) | 0.519 (0.136–1.976) | 0.336 | 1.844 (0.359–9.461) | 0.463 | 1 |
| Male (Yes vs. No) | 4.424 (1.309–14.955) | 0.017* | 17.835 (3.136–101.424) | 0.001* | 1 |
| Diabetes (Yes vs. No) | 1.801 (0.592–5.484) | 0.300 | 1.026 (0.361–2.920) | 0.961 | 1 |
| Hypertension (Yes vs. No) | 7.198 (2.509–20.653) | 0.000* | 23.119 (6.877–77.721) | 0.000* | 1 |
| High triglycerides (Yes vs. No) | 2.204 (0.867–5.603) | 0.097 | 1.277 (0.504–3.232) | 0.606 | 1 |
| High total cholesterol (Yes vs. No) | 0.178 (0.043–0.737) | 0.017* | 0.228 (0.072–0.725) | 0.012* | 1 |
| Hyperhomocysteinemia (Yes vs. No) | 0.298 (0.107–0.829) | 0.020* | 0.319 (0.120–0.848) | 0.022* | 1 |
| Pre-existing CSVD (Yes vs. No) | 5.249 (1.684–10.631) | 0.031* | 3.182 (1.202–7.248) | 0.042* | 1 |
| LI (Yes vs. No) | 0.561 (0.183–1.718) | 0.311 | 2.213 (0.703–6.963) | 0.175 | 1 |
| WMH (Yes vs. No) | 4.021 (0.153–6.294) | 0.320 | 3.984 (0.389–5.241) | 0.424 | 1 |
| EPVS (Yes vs. No) | 9.989 (1.208–82.576) | 0.033* | 3.084 (0.501–18.999) | 0.225 | 1 |
p < 0.05.
Multiple logistic regression analysis for the risk factors of subtypes of ischemic stroke
4. DISCUSSION
In the past, researches on young stroke mainly focused on exploring its risk factors. Young CSVD is mostly presented in the form of asymptomatic, which is a neglected field. There are few studies to explore the relationship between pre-existing CSVD and stroke. A post hoc analysis in older people, SAMMPRIS trial, showed a lack of association between CSVD and stroke recurrence [17]. However, our study showed that more than 90% of young patients with AIS (95.4% of large artery atherosclerotic stroke and 93.5% of small artery occlusive stroke) were co-existed with CSVD. An explanation for high frequency of young CSVD with AIS may be that patients are more susceptible to vascular risk factors than other people, and there might be a genetic predisposition to cardiovascular disease. Another possibility is that patients have been exposed to vascular risk factors for longer time than healthy controls [23]. Blood pressure, as the most common risk factor of cerebrovascular disease, has showed its association with subtle vascular brain injury so as to already reduce cerebral integrity in very early life [24].
Furthermore, this study indicated that, in young adults, the presence of pre-existing CSVD significantly increased the risk of large artery atherosclerotic stroke and small artery occlusion stroke. Similar results have been reported in a multicenter study from Korea [16]. However, the latter study focused on older people. The mechanism of pre-existing CSVD on stroke may be as follows. First, small vessel diseases increase media, lumen ratio and rarefaction in the microcirculation, leading to increased peripheral resistance [25]. The increase of peripheral resistance is the main factor leading to the increase of mean blood pressure, which will increase the stiffness and atherosclerosis of the great arteries [26]. Second, chronic CSVD damages collaterals and impairs the compensatory mechanism of artery occlusion that leads to higher risk of AIS [27].
Majority of young people with large artery atherosclerotic stroke and small artery occlusive stroke had two or three kinds of CSVD abnormalities at least. In this study, compared with non-stroke patients, patients with AIS had a higher proportion of moderate to severe EPVS, and the presence of EPVS significantly increased the risk of large artery atherosclerotic stroke. This is contrary to the conclusion of Zhang et al. [28]. The inconsistent results might be related to the selection of case population. Our study population was young people aged 18–45 years old, and there might be fewer mixed influence factors on EPVS, including age, systemic diseases and other related factors. On the contrary, Wang’s [29] latest study suggested that the severity of EPVS was related to intracranial and extracranial atherosclerosis, and Staals et al. [30] believed that the presence of EPVS was related to different stroke subtypes. The results of these studies were consistent with ours on some degree.
For young adults over 35 years old, the risk of LI, WMH and EPVS increased four to 10 times, which demonstrated that CSVD abnormalities were increased with age. It turned out that age was also an important risk factor for CSVD in young people, just like elderly CSVD [31,32]. In addition, gender was a risk factor for LI in young adults. Among males, the proportion of smokers and drinkers was significantly higher [33–35], indicating that males were more frequently exposed to the risk factors of CSVD and had a greater risk of lacunar infarctions. Type 2 Diabetes Mellitus was also considered as an important risk factor for CSVD in this study, which was consistent with the epidemiological and pathogenesis studies of CSVD in recent years [36–38], suggesting that diabetes mellitus was also a risk factor for LI. The causal relationship between diabetes and lacunar is yet to be determined, and a genetic predisposition to diabetes, maybe is related to lacunar stroke [36].
In young adults, hypertension is also a common risk factor for large artery atherosclerotic stroke and small artery occlusive stroke. In this study, 79.3% (176/222) of patients with young stroke had hypertension. The causes of hypertension in young people may be related to genetic factors, unhealthy diet and rest, and high intensity mental pressure. Chronic hypertension can lead to vascular wall atherosclerosis, fibrinoid necrosis, hyaline degeneration, microaneurysms and other pathological changes, leading to stroke [39,40].
The advantage of our study is that it has showed that pre-existing CSVD is very common in young adults with large atherosclerotic and small artery occlusive stroke, and pre-existing CSVD significantly increases the risk of ischemic stroke. Previous studies do not do specifical study among young population in the relationship of CSVD and stroke, and people may ignore the influence of asymptomatic CSVD on young stroke. Once asymptomatic CSVD are detected in young people, further screening for cerebrovascular disease risk factors and pre-existing stroke prevention measures is required. The shortcomings of this study are that it is a single-center study and the sample size is small, which could only explain the relationship of CSVD, stroke types and risk factors in a small scope of youths, a larger multi-center prospective study is needed to provide more insight in this proposition, and further clarify CSVD and the pathogenesis of stroke and causality.
5. CONCLUSION
In conclusion, this study showed that young adults with large artery atherosclerotic stroke or small artery occlusive stroke often coexisted with CSVD abnormalities. The presence of pre-existing CSVD in young patients increased the risk of large artery atherosclerotic stroke or small artery occlusive stroke, which indicated that CSVD may be involved in the pathogenesis of atherosclerosis. For young adults, once asymptomatic CSVD abnormalities are detected, cerebrovascular risk factors should be screened and pre-existing prevention measures for stroke should be taken.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
HL and DD carried out the study, participated in collecting data, statistical analysis, and drafted the manuscript. JC participated in collecting data and provided guidance on case classification. XW and YX participated in its design and helped to draft the manuscript. All authors read and approved the final manuscript.
FUNDING
No financial support was provided.
ETHICAL STANDARDS
This study was approved by the local Medical Ethics Committee of Wuhan Central Hospital (NO. [2020]201).
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Hui Li AU - Dan Du AU - Juan Chen AU - Xiang Wang AU - Yuanliang Xie PY - 2021 DA - 2021/05/16 TI - Pre-existing Cerebral Small Vessel Disease in Young Patients with Acute Ischemic Stroke caused by Large Artery Atherosclerosis or Small Artery Occlusion JO - Artery Research SP - 121 EP - 128 VL - 27 IS - 3 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.210511.001 DO - 10.2991/artres.k.210511.001 ID - Li2021 ER -
