Therapeutic Approaches for Blastic Plasmacytoid Dendritic Cell Neoplasm: Allogeneic Hematopoietic Cell Transplantation and Novel Therapies
Peer review under responsibility of IACH
- DOI
- 10.2991/chi.d.190218.001How to use a DOI?
- Keywords
- Blastic plasmacytoid dendritic cell neoplasm; Allogeneic hematopoietic cell transplant; Targeted therapies; Overall survival
- Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a clinically aggressive hematologic malignancy derived from precursors of plasmacytoid dendritic cells. There is no established standard therapy for BPDCN and the efficacy of conventional chemotherapy is limited, with an anticipated median overall survival ranging from 8 to 14 months. No randomized controlled trials have ever been performed to evaluate the benefit of frontline consolidation with an allogeneic hematopoietic cell transplant (allo-HCT) in BPDCN. Yet, offering an allograft has become the de facto option in BPDCN, and remains the only known long-term curative option for these patients, even in the modern era of targeted therapies. In our opinion, allo-HCT is recommended as part of frontline consolidation, especially in patients achieving first complete remission and who are deemed capable of tolerating the procedure, as published data show 3- to 4-year progression-free survival ranging from 69% to 74% in this population. Prompt referral to a transplant center, at the time of a diagnosis of BPDCN, is important to confirm allo-HCT candidacy and to initiate the process of identifying a suitable human leukocyte antigen (HLA)-compatible donor. Because disease relapse remains a major concern, additional strategies, such as post-allograft consolidation/maintenance therapy, are certainly needed to help further improve outcomes. Finally, patients deemed ineligible to receive an allo-HCT, due to lack of response and/or poor performance status, should be considered for enrollment in clinical trials.
- Copyright
- © 2019 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a clinically aggressive hematologic malignancy derived from precursors of plasmacytoid dendritic cells [1,2]. BPDCN is listed in the recently revised 2016 World Health Organization classification of myeloid neoplasms and acute leukemia [3]. The malignant cells generally express CD4+, CD56+, and CD123+ [4]. There is no established standard therapy for BPDCN, and treatment generally involves combination chemotherapy regimens commonly used to treat acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and high-grade non-Hodgkin lymphoma (NHL), in patients with systemic involvement [5,6]. Unfortunately, the efficacy of conventional chemotherapy is limited, with an anticipated median overall survival (OS) ranging from 8 to 14 months [6–8]. SL-401, a recombinant fusion protein composed of the catalytic and translocation domains of diptheria toxin fused to interleukin 3 (IL3), represents a targeted therapy directed against the IL3 receptor (CD123), which is commonly expressed in BPDCN [9]. In a pilot phase 1/2 study that included 11 patients with BPDCN, SL-401 yielded a major objective response in 7 (78%) of 9 evaluable cases; however, long-term durability was not observed in most patients, as responses were short-lived, with a median duration of only 5 months, with only one cycle of therapy for most patients treated on the study [9]. Results of the phase 2 study presented by Pemmaraju et al. demonstrated high overall response rates (ORRs) of 90%, and a median OS which had not been reached after prescribing SL-401 in the frontline setting [10]. Moreover, 45% of treated cases were able to proceed to a hematopoietic cell transplant (HCT), mainly allogeneic hematopoietic cell transplant (allo-HCT), even when the median age of patients was 70 years.
Allo-HCT is a potentially curative treatment modality for several malignant hematologic diseases and benign blood disorders [11–14]. There are no randomized controlled trials (RCTs) evaluating the benefit of frontline consolidation with allo-HCT in BPDCN [15]. Yet, offering an allo-HCT has become the de facto option for BPDCN patients who achieve an objective response to induction chemotherapy, chemoradiotherapy, and/or targeted therapies, given the historical poor survival outcomes and high rates of transformation to AML [15]. Allo-HCT is also offered beyond the frontline consolidation setting, but response rates are generally disappointing, similar to results seen for other aggressive hematologic malignancies [16].
Here, we provide an extensive review of the published literature on the evolving role of allo-HCT for the treatment of BPDCN, and summarize the outcomes of various promising therapies which are considered experimental at this time.
1.1. Epidemiology of BPDCN
BPDCN is an exceedingly rare disease with an estimated incidence of less than 1% [17]. It constitutes approximately 0.44% of all hematologic malignancies and 0.7% of lymphoma-like diseases involving the skin [6]. It is likely, however, that this represents an underestimate, due to the fact that BPDCN is often misdiagnosed and, likely, underreported, and also due to the changing nomenclature which has occurred over the years. For example, BPDCN was previously known as blastic natural killer (NK) cell lymphoma or CD4+/CD56+ hematodermic neoplasm [2].
There are no known environmental or genetic factors associated with BPDCN [8]. However, history of a preceding hematologic malignancy has been described in up to 20% of cases [5,6,18]. Moreover, no particular racial or ethnic group is considered at a higher risk of developing BPDCN [8].
BPDCN can occur at any age; however, most cases occur in patients older than 60 years [5,19,20]. Also, it occurs more commonly in men, with a reported male-to-female ratio of approximately 3:1 [8,21]. Pediatric/neonatal BPDCN appears to present with markedly distinct features which separate it from the more common adult BPDCN; hence, the focus of this review will be on BPDCN in the adult population [22].
1.2. Clinical Presentation
The most frequent clinical presentation of BPDCN is on the skin, with or without bone marrow (BM) involvement. The skin lesions are generally asymptomatic [5,8,21]. There is no specific diagnostic appearance of the skin lesions, which can be single or multiple, and can present as nodules and/or plaques, with or without associated erythema and/or ulcerations [8,21,23]. Thrombocytopenia and anemia are common manifestations of BPDCN involving the BM [6,8]. Additionally, in 20–25% of cases, BPDCN may manifest with a leukemic presentation without cutaneous involvement [6]. Other organs can also be affected by BPDCN including, but not limited, the central nervous system, lymph nodes, and the liver [6,8,24].
1.3. Treatment of BPDCN
No treatment is considered standard of care for induction therapy for BPDCN. We next summarize the commonly prescribed therapies for BPDCN including allo-HCT.
1.3.1. Conventional therapy
Localized skin involvement
The optimal treatment of primary cutaneous BPDCN without extracutaneous manifestations remains to be better defined [25,26]. There are anecdotal reports on cases treated with low-intensity systemic chemotherapy regimens in combination with localized radiation therapy (RT) [25], or with an intensive chemotherapy regimen which combines hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) alternating with methotrexate and cytarabine [26]. The use of RT alone has also been described in case reports, being prescribed to patients who refuse or are deemed too frail to tolerate chemotherapy [27]. Unfortunately, these patients experience early relapse, including systemic disease involvement, hence questioning the efficacy of RT as a single treatment modality in BPDCN [24,27,28]. Pemmaraju et al., among others, have found that skin-only BPDCN consistently has a poor outcome in their historical series, similar to BPDCN presenting with systemic/BM disease. This important point argues in support of systemic therapy for all patients with BPDCN, regardless of the involved disease compartment at presentation [18].
Systemic disease
No RCT has been performed to date comparing the various induction regimens used to treat BPDCN. Regimens commonly used to treat NHL have been prescribed for BPDCN. For example, Feuillard et al. described encouraging responses in 23 BPDCN cases where the majority of patients (n = 12, 52%) received anthracycline chemotherapy as part of the regimen [5]. The authors described a complete remission (CR) rate of 86%, and 3 patients were able to proceed to an allo-HCT in CR1 [5]. The median time to relapse was 9 months with BM involvement seen in 73% of cases [5]. The 2-year OS was 25% [5]. More intensive regimens used as induction therapy in ALL and NHL, such as hyper-CVAD, have been reported to yield more encouraging results [29]. Pemmaraju et al. described the outcomes of 13 BPDCN patients, with a median age of 62 years, of whom 10 received hyper-CVAD [29]. Nine (90%) of these 10 patients achieved CR1 and the reported median OS for this group was 29 months [29].
AML induction regimens have also been prescribed in BPDCN. Pagano et al. reported the outcomes of a retrospective multicenter study involving 28 Italian centers, where 41 patients received AML- (n = 26, 63%) or ALL-induction therapy (n = 15, 37%) [6]. Six underwent allo-HCT. A CR was attained in 17 (41%) patients: 7 after AML-induction and 10 after ALL induction regimens [6]. Patients treated with an AML induction regimen had a median OS of 7.1 months, whereas this was 12.3 months in those treated with ALL-type chemotherapy (p = 0.02) [6]. Moreover, a survival advantage was observed in allo-HCT recipients when compared with those who were not allografted (22.7 vs. 7.1 months, p = 0.03) [6].
It is generally common practice to offer an allo-HCT as frontline consolidation in patients deemed eligible and fit enough to tolerate the procedure.
1.3.2. Hematopoietic cell transplantation
To date, no RCT has been ever conducted evaluating the role of autologous (auto) or allo-HCT vis-à-vis conventional chemotherapy in BPDCN. Data reporting on the efficacy of auto-HCT or allo-HCT are limited to case reports, retrospective single-institution evaluations, and multicenter or registry observational studies [16,23,30–34].
1.3.3. Allo-HCT
Allo-HCT provides the benefit of a disease-free hematopoietic stem cell graft from a healthy donor. This is a significant advantage when considering the high proclivity of BPDCN to involve the BM, and the lack of standardized methods to measure minimal residual disease (MRD) in this disease.
In 2012, the European Society for Blood and Marrow Transplantation (EBMT) reported on the outcomes of 34 (male = 21; 62%) patients, with a median age of 41 (range, 10–70) years, who received an allo-HCT with curative intent [32]. Skin involvement was the most common presentation (n = 26; 79%) [32]. The majority (n = 19; 56%) of patients were in CR1 at the time of allografting, and preparative allo-HCT regimens were predominantly myeloablative (MAC) (n = 25; 74%) [32]. The reported 3-year cumulative incidence of relapse, disease-free survival (DFS), OS, and non-relapse mortality (NRM) were 32%, 33%, 41%, and 30%, respectively [32]. The authors reported that being in CR1 (vs. >CR1) at the time of allografting yielded higher OS post-allografting (3-year OS: 52% vs. 29%, p = 0.057) [32]. Also, when the analysis was restricted to CR1 patients receiving MAC regimens, the resulting 3-year OS was 60% [32].
In 2015, the Japanese Society for Hematopoietic Cell Transplantation reported on the outcomes of 14 (male = 11; 79%) BPDCN patients, with a median age of 58 (range,17–64) years, who received an allo-HCT (MAC = 8; 57%), mostly in CR1 (n = 10; 71%) [33]. The reported 4-year progression-free survival (PFS) and OS were 60% and 69%, respectively, for patients allografted in CR1 [33]. Five patients relapsed after receiving a MAC (n = 2) or a reduced-intensity conditioning (RIC) (n = 3) allo-HCT [33]. Consistent with the aforementioned study [32], allo-HCT in CR1 was an important predictor of improved OS [33].
The 2017 Kharfan-Dabaja et al.'s report described the outcomes of 37 patients (male = 29; 78%), with a median age of 50 (range, 14–74) years, who received an allo-HCT (MAC = 20, RIC = 17) for BPDCN at a median of 7 months from diagnosis [16]. The majority (n = 28; 76%) were allografted in CR1. The authors described a 3-year PFS and OS of 55% and 58%, respectively, for all patients [16]. The 1-year cumulative incidence of relapse was 10% [16]. For patients allografted in CR1, the 3-year PFS and OS were 69% and 74%, respectively. Consistent with previously published studies [32,33], receiving an allo-HCT in CR1 (vs. not in CR1) was associated with significantly improved PFS and OS [16]. The regimen intensity did not appear to affect PFS or OS, within the limitation of a relatively small sample size [16]. These results support the efficacy of allo-HCT in BPDCN, particularly if the procedure is performed in CR1 [16,32,33].
Leclerc et al. published the outcomes of 43 adult BPDCN patients (male = 29; 67%), with a median age of 57 (range, 20–72) years, who received an allo-HCT (MAC = 14, RIC = 29) at one of 21 transplant centers throughout France [34]. Most of the cases were in CR1 (n = 34; 79%) at the time of the procedure [34]. The 2-year cumulative incidence of relapse, DFS, OS, and NRM were 26%, 45%, 52%, and 33%, respectively [34]. The OS was comparable after MAC or RIC allo-HCT [34]. These and other studies are summarized in Table 1 [16,23,32–34].
| Authors, Year Published [Ref] | Study Type | N | Median Age (Range), Years | Disease Status at Transplant, N (%) | Conditioning Regimen Intensity, N (%) | NRM | Relapse | Survival |
|---|---|---|---|---|---|---|---|---|
| Dalle et al. 2010 [23] | Retrospective, registry France | 9 | 38 (25–67) | NR | NR | NR | NR | Median = 21 (range, 12–77) months |
| Roos-Weil et al. 2013 [32] | Database registry EBMT | 34 | 41 (10–70) | CR1 = 19 (56%) >CR1 = 15 (44%) | MAC = 25 (74%) RIC = 9 (26%) | 30% (3-year) | 32% (3-year) | All pts DFS = 33% OS = 41% (3-year) |
| Aoki et al. 2015 [33] | Database registry Japan | 14 | 58 (17–64) | CR1 = 10 (71%) CR2 = 2 (14%) Refract = 2 (14%) | MAC = 8 (57%) RIC = 6 (43%) | 2 of 14 pts | 5 of 14 pts | All pts PFS = 48% OS = 53% (4-year) Pts in CR1 PFS = 60% OS = 69% (4-year) |
| Kharfan-Dabaja et al. 2017 [16] | Retrospective, multicenter North America | 37 | 50 (14–74) | CR1 = 28 (76%) CR2 = 4 (11%) CR3 = 1 (2%) PIF = 4 (11%) | MAC = 20 (54%) RIC = 17 (46%) | 10 of 37 pts | 2 of 37 pts | All pts PFS = 55% OS = 58% (3-year) Pts in CR1 PFS = 69% OS = 74% (3-year) |
| Leclerc et al. 2017 [34] | Retrospective, multicenter France | 43* | 57 (20–72) | CR1 = 34 (79%) CR2 = 5 (12%) No CR = 2 (5%) Unk = 2 (5%) | MAC = 14 (33%) RIC = 29 (67%) | 33% (2-year) | 26% (2-year) | All pts DFS = 45% OS = 52% (2-year) |
Abbreviations: N: Number of patients; NRM: Non-relapse mortality; NR: Not reported; EBMT: European Society for Blood and marrow Transplantation; CR: Complete remission; MAC: Myeloablative conditioning; RIC: Reduced intensity conditioning; Pts: Patients; DFS: Disease-free survival; OS: Overall survival; Refract: Refractory disease; PFS: Progression-free survival; PIF: Primary induction failure; Unk: Unknown.
Six cases were also reported in study by Roos-Weil et al. [32]
Selected studies of allo-HCT in BPDCN (N ≥ 8 patients).
A recently published systematic review/meta-analysis showed pooled OS rates of 67% when patients receive an allo-HCT for BPDCN in CR1 [35].
1.3.4. Auto-HCT
There is paucity of data on the role of auto-HCT in BPDCN [16,33]. A registry study of the Japanese Society for Hematopoietic Cell Transplantation reported on the outcomes of 11 patients (male = 9; 82%), with a median age of 57 (range,19–67) years, who received high-dose therapy followed by auto-HCT after a median of 6 (range, 2–7) months from original diagnosis [33]. Ten patients had skin involvement at initial presentation and in 8 of them the disease also involved the BM [33]. All 11 patients were in CR1 at the time of auto-HCT after having received an NHL- (n = 7) or an ALL-like (n = 4) induction regimen [33]. The authors reported an encouraging 4-year OS of 82% [33].
In contrast, a multicenter observational study from North America showed a dismal 1-year OS of 11% in 8 (male = 5; 63%), patients, with a median age of 67 (range, 45–72) years, who received an autograft in CR1 (n = 5), CR2 (n = 1), CR3 (n = 1), or in primary induction failure (n = 1) [16]. In keeping with the findings of the North American study [16], Reimer et al. also described poor outcomes after auto-HCT in 4 patients, with a median age of 29 (range, 23–51) years (CR1 = 3, PR = 1 at time of autografting) [30]. The authors reported 3 (75%) relapse-related deaths after 13 to 20 months from autografting [30]. Table 2 summarizes some of these studies [16,33].
| Authors, Year Published [Ref] | Study Type | N | Median Age (Range), Years | Disease Status at Transplant, N (%) | Conditioning Regimen, N (%) | Relapse | Survival |
|---|---|---|---|---|---|---|---|
| Aoki et al. 2015 [33] | Database registry Japan | 11 | 57 (19–67) | CR1 = 11 (100%) | Various | 2 of 11 patients | PFS = 73% OS = 82% (4-year) |
| Kharfan-Dabaja et al. 2017 [16] | Retrospective, multicenter North America | 8 | 67 (45–72) | CR1 = 5 (63%) CR2 = 1 (13%) CR3 = 1 (13%) PIF = 1 (13%) | BEAM = 8 (100%) | 3 of 8 patients | PFS = 11% OS = 11% (1-year) |
Abbreviations: N: Number of patients; CR: Complete remission; PFS: Progression-free survival; OS: Overall survival; PIF: Primary induction failure; BEAM: Carmustine, etoposide, cytarabine and melphalan.
Selected studies of auto-HCT in BPDCN (N ≥ 8 patients).
The small number of cases and the discordant outcomes among studies [16,30,33] limit our ability to recommend auto-HCT as a standard treatment approach for patients with BPDCN outside the context of a clinical trial.
1.3.5. Targeted therapies
SL-401
SL-401 has been granted “breakthrough therapy” designation by the United States Food and Drug Administration for the treatment of BPDCN. Pemmaraju et al. presented the results of a pivotal phase-2 study of SL-401 in 45 patients with BPDCN in the frontline as well as in the relapsed/refractory setting [10]. The authors showed encouraging ORR of 90%, and a median OS which had not been reached when 29 patients were treated with a dose of 12 mcg/kg in the frontline setting [10]. Of note, 13 (45%) of 29 patients were bridged to a HCT (allo-HCT = 10, auto-HCT = 3) [10].
1.3.6. Investigational therapies
B-cell lymphoma 2 (BCL-2) inhibitors
BCL-2 overexpression has been noted in many subtypes of leukemia, resulting in the oral small molecule inhibitor, venetoclax, gaining approval for treatment of chronic lymphocytic leukemia [36]. Further investigation of this agent led to the understanding that BCL-2 inhibition is effective in myeloid malignancies, particularly AML [37]. In a recent study, it was shown that BPDCN cells are also highly dependent on the antiapoptotic protein BCL-2 [38]. Montero et al. demonstrated that BPDCN is sensitive to venetoclax, as measured by direct cytotoxicity, apoptosis assays, and dynamic BH3 profiling [38]. Moreover, the authors reported significant disease response in 2 patients with relapsed/refractory BPDCN who received venetoclax off-label [38]. Combinations of venetoclax with hypomethylating agents have also been observed to have clinical activity in BPDCN [39]. The efficacy of venetoclax in skin-only BPDCN relapse has been described in a case report [40]. A phase-1 study evaluating the role of venetoclax in BPDCN is being planned (ClinicalTrials.gov Identifier: NCT03485547).
Chimeric antigen receptor T-cell therapy
Cai et al. reported in vitro cytotoxic activity of UCART123 cells, which represent genetically modified allogeneic T-cells (from healthy volunteer donors) containing an anti-CD123 CAR (CD123 scFv-41BB-CD3z) and a RQR8 depletion ligand that confers susceptibility to rituximab [41]. UCART123 caused BPDCN-specific killing with antigen-specific T-cell degranulation and robust levels of IFN-gamma production [41]. Moreover, UCART123 eradicated BPDCN in a xenograft derived from a patient with relapsed BPDCN in NSG-SGM3 mice [41]. An ongoing phase-1 study at City of Hope is evaluating antitumor activity and safety of a CD123-specific CAR with a truncated EGFR following lymphodepletion in CD123+ relapsed or refractory AML or persistent/recurrent BPDCN (ClinicalTrials.gov Identifier: NCT02159495).
Hypomethylating therapy
Whole-exome sequencing of 3 BPDCN cases showed that the 2 most frequent mutations occurred in the TET2 (36%) and ASXL1 (32%) genes [42]. Patients with mutations in proteins in the methylation pathways had worse OS rates vis-à-vis those without mutations [42]. Laribi et al. reported encouraging skin response and hematologic stability in 2 patients (ages 78 and 81) with BPDCN and myelodysplastic syndrome after receiving azacytidine [43]. Three additional elder patients, 75–80 years of age, treated with azacytidine with or without localized RT exhibited PFS of 6, 7, and 24 months, respectively [44].
Bromodomain inhibition
The role of MYC and other pathways which may be suppressed by bromodomain inhibition remains unclear [45]. Emadali et al. demonstrated that haploinsufficiency for the gene encoding the glucocorticoid receptor in BPDCN may be particularly amenable to bromodomain inhibition in preclinical models [46]. Moreover, Ceribelli et al. showed that TCF-4 may constitute one of the major regulators of normal and aberrant pDC in BPDCN, and that this target may lead to targeted strategies with bromodomain inhibition [47]. Another interesting element to the evolution of this new area is the finding that aberrant MYC expression may be a somewhat common perturbation in BPDCN, which further lends to the study of bromodomain inhibition as a future targeted therapy program to be investigated in this disease [48].
Bortezomib
The NFkB pathway has been previously shown to be important in BPDCN and a possible target for clinical activity [49]. A recent study by Phillipe et al., based on preclinical work, suggests that bortezomib, either as single-agent or in combination with cytotoxic chemotherapy, may represent another new direction for clinical trial investigation in BPDCN [50].
2. DISCUSSION
Allo-HCT yields durable remissions in BPDCN, especially when offered in the frontline consolidation setting in CR1. Published data show 3- to 4-year PFS ranging from 69% to 74% when patients are allografted in CR1 [16,33]. Unfortunately, disease relapse still remains a concern occurring in up to one-third of cases based on a registry study [32]. This suggests that additional treatment strategies are needed to help improve outcomes. These strategies may include considering post-allograft consolidation/maintenance therapy in patients deemed at high(er) risk of relapse, such as those allografted with residual disease or beyond CR1, or for those who fail to achieve or maintain a CR after allografting. Future phase 1/2 multicenter studies evaluating the feasibility and safety of therapies such as SL-401 or venetoclax in the post-allograft consolidation/maintenance setting, alone or in combination with donor lymphocyte infusion, should be considered.
Widespread availability of next generation sequencing (NGS) is helping better prognosticate treatment outcomes in various hematologic malignancies, and will likely prove to be valuable in BPDCN. Emerging data show that BPDCN with gene mutations in the methylation pathways have worse OS [42]. Accordingly, future studies incorporating data on the presence (or lack thereof) of somatic gene mutations may help tailor more personalized treatment strategies which target specific mutations, or a more intensive treatment approach in general. NGS may also be potentially evaluated as a tool to assess MRD in BPDCN, as it appears that the majority of patients, regardless of marrow presentation at baseline, harbor molecular mutations, most commonly in TET2, and in others such as TP53, ASXL1, RAS mutations [42,51,52].
The nature of the published data limits our ability to determine the significance of the ablative intensity of the preparative regimen in relation to posttransplant survival outcomes at this time, and this will be an active area in our field in the coming years. Three of the studies summarized in Table 1 illustrate a preferential use of MAC regimens [16,32,33] and one study favored using RIC regimens [34]. While it would be ideal to conduct a RCT to answer this question, it is highly unlikely that such study would ever be conducted, due to the rarity of this disease. In our opinion, a MAC regimen could be considered in patients deemed capable of tolerating higher ablative doses of chemotherapy or chemoradiotherapy, while a RIC regimen ought to be reserved for patients who are perceived as too frail due to advanced age or the presence of comorbidities.
Success of Chimeric antigen receptor T (CAR-T) cell therapy in ALL [53] and in diffuse large B-cell NHL [54] has awakened interest in exploring this treatment in BPDCN. Studies of CAR-T in BPDCN have already moved from the bench to the bedside and are currently accruing.
3. RECOMMENDATIONS
Given the historically poor outcomes and short OS in most patients with BPDCN, allo-HCT is recommended as frontline consolidation in patients who achieve a CR and are deemed eligible for the procedure, as this remains the only proven long-term curative strategy at the present time (Fig. 1). Patients with BPDCN should be promptly referred to transplant centers to confirm their candidacy for allo-HCT and to initiate the process of identifying a suitable HLA-compatible donor. The potential benefit of maintenance therapy after allo-HCT is an important research question which should be studied in prospective clinical trials. Patients who are deemed ineligible for an allo-HCT due to lack of response to treatment and/or poor performance status should be considered for enrollment in clinical trials (Fig. 1).
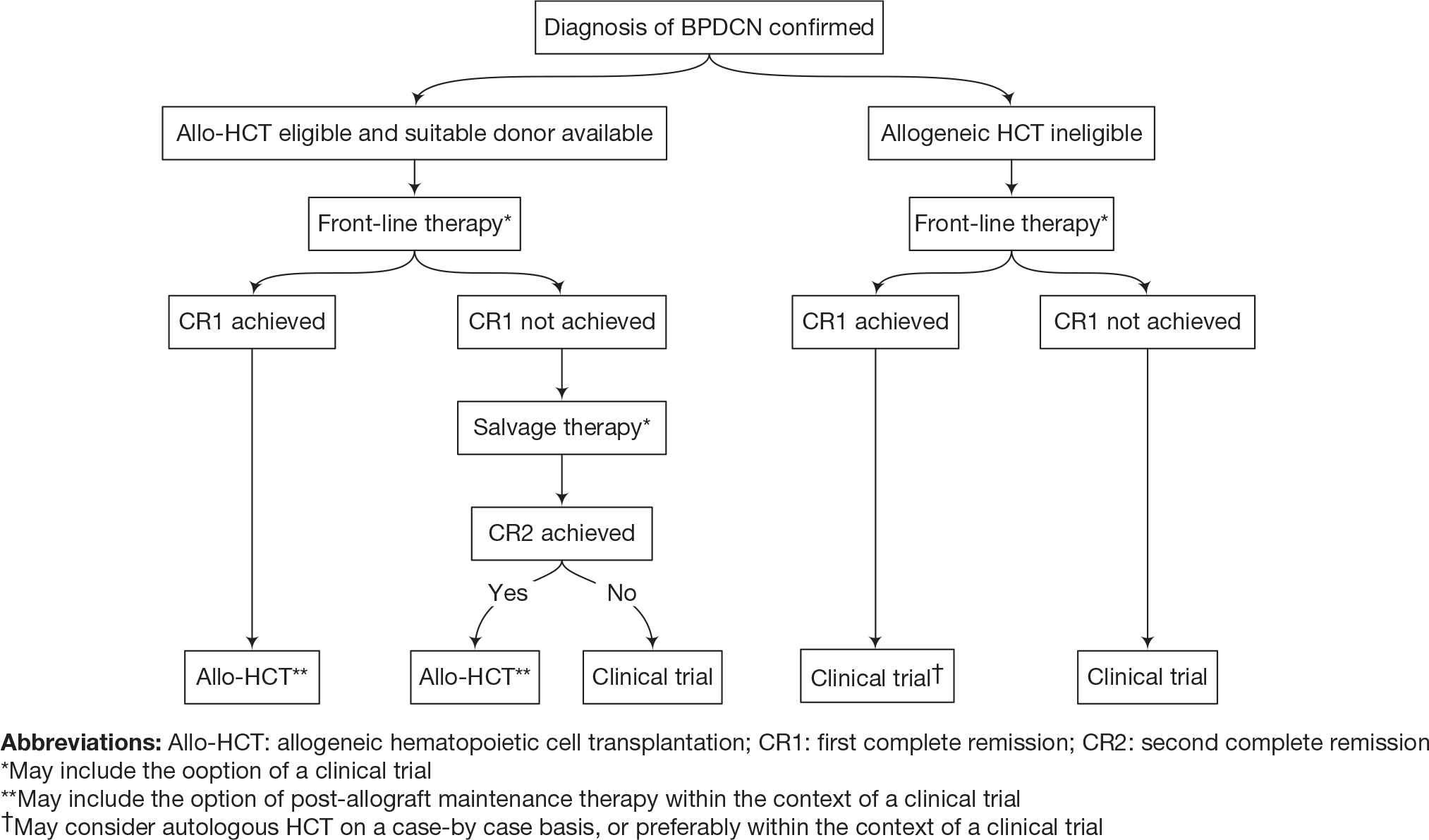
Recommendations.
CONFLICT OF INTERESTS
Mohamed A. Kharfan-Dabaja: Member of the speaker's bureau for Alexion Pharmaceuticals, Seattle Genetics and Incyte; Naveen Pemmaraju: Research funding/consulting: Stemline, Cellectis, Affymetrix, abbvie, Plexxikon. Mohamad Mohty: Consultancy for Servier
AUTHORS' CONTRIBUTIONS
Mohamed A. Kharfan-Dabaja: Contributed to the design and writing of this manuscript; Naveen Pemmaraju: Contributed to the design and writing of this manuscript; Mohamad Mohty: Contributed to the design and writing of this manuscript.
REFERENCES
Cite this article
TY - JOUR AU - Mohamed A. Kharfan-Dabaja AU - Naveen Pemmaraju AU - Mohamad Mohty PY - 2019 DA - 2019/03/18 TI - Therapeutic Approaches for Blastic Plasmacytoid Dendritic Cell Neoplasm: Allogeneic Hematopoietic Cell Transplantation and Novel Therapies JO - Clinical Hematology International SP - 2 EP - 9 VL - 1 IS - 1 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.190218.001 DO - 10.2991/chi.d.190218.001 ID - Kharfan-Dabaja2019 ER -
