Robust Selections of Various Hematopoietic Cell Fractions on the CliniMACS Plus Instrument
co-first authors
- DOI
- 10.2991/chi.d.190529.001How to use a DOI?
- Keywords
- CliniMACS; Cell selection; CD34; Cell purity; Cell recovery
- Abstract
Cell separation technologies play a vital role in the graft engineering of hematopoietic cellular fractions, particularly with the rapid expansion of the field of cellular therapeutics. The CliniMACS Plus Instrument (Miltenyi Biotec) utilizes immunomagnetic techniques to isolate hematopoietic progenitor cells (HPCs), T cells, NK cells, and monocytes. These products are ultimately used for HPC transplantation and for the manufacture of adoptive immunotherapies. We evaluated the viable cell recovery and cell purity of selections and depletions performed on the CliniMACS Plus over a 10-year period at our facility, specifically assessing for the isolation of CD34+, CD4+, CD3+/CD56+, CD4+/CD8+, and CD25+ cells. Additionally, patient- and instrument-related factors affecting these parameters were examined. Viable cell recovery ranged from 32.3 ± 10.2% to 65.4 ± 15.4%, and was the highest for CD34+ selections. Cell purity ranged from 86.3 ± 7.2% to 99.0 ± 1.1%, and was the highest for CD4+ selections. Undesired cell fractions demonstrated a range of 1.2 ± 0.45 to 5.1 ± 0.4 log reductions. Red cell depletions averaged 2.12 ± 0.68 logs, while platelets were reduced by an average of 4.01 ± 1.57 logs. Donor characteristics did not impact viable cell recovery or cell purity for CD34+ or CD4+ cell enrichments; however, these were affected by manufacturing variables, including tubing size, bead quantity, and whether preselection platelet washes were performed. Our data demonstrate the efficient recovery of hematopoietic cellular fractions on the CliniMACS Plus that may be optimized by adjusting manufacturing variables.
- Copyright
- © 2019 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The therapeutic value of individual cellular components of blood has been recognized since the 1950s, when the first developed blood cell separators paved the way for “component therapy” from whole blood, comprising segregated red cells, platelets, granulocytes, or plasma[1–3]. Cell separation technologies have since emerged as powerful tools in biological research, as well as in clinical graft engineering and regenerative medicine. In particular, cellular subfractions of blood and bone marrow, including CD34+ hematopoietic stem and progenitors, T-cell subsets, NK cells, and monocytes can be safely and consistently isolated and enriched for therapeutic use in hematopoietic stem cell transplantation, graft versus host disease (GvHD), and other conditions treated with immunotherapies and gene therapies.
A variety of cell separation methods is currently available for clinical use. These methods take advantage of cell characteristics such as adherence, density, and/or antibody binding [4]. Of the antibody binding methods, fluorescence activated cell sorting (FACS) and magnetic activated cell sorting (MACS) achieve high resolution sorting of cellular subsets based on surface antigens. In particular, magnetic isolation methods have become widely accepted for clinical use, owing to their efficient cell enrichment and/or reduction in closed, partially- or fully-automated, current good manufacturing practice (cGMP)-compliant instruments. The Isolex 300i Magnetic Cell Selection System was among the first instruments to receive FDA approval for autologous CD34+ cell-enrichment intended for hematopoietic reconstitution after myeloablative therapy. However, currently, the only clinically approved device is the CliniMACS Cell Isolation System, which employs a colloidal suspension of super-paramagnetic microbeads directly conjugated to a monoclonal anti-human antibody capable of binding to its corresponding antigen contained in bone marrow, peripheral blood, and umbilical cord blood products [5]. The attached cells are then separated from the rest of the product using a magnetic column. The isolated antibody and bead-coated cells (positive selection) or the “flow-through” fraction (negative selection) are used for further manufacture of cellular and immunotherapies.
The objective of our study was to examine selections and depletions of various hematopoietic cell fractions performed on the CliniMACS Plus instrument for early phase clinical trials at the National Institutes of Health (NIH). Specifically, we evaluated viable cell recovery (VCR) and cell purity (CP) of the enriched/depleted fractions, as well as cellular- and instrument-related factors affecting these parameters.
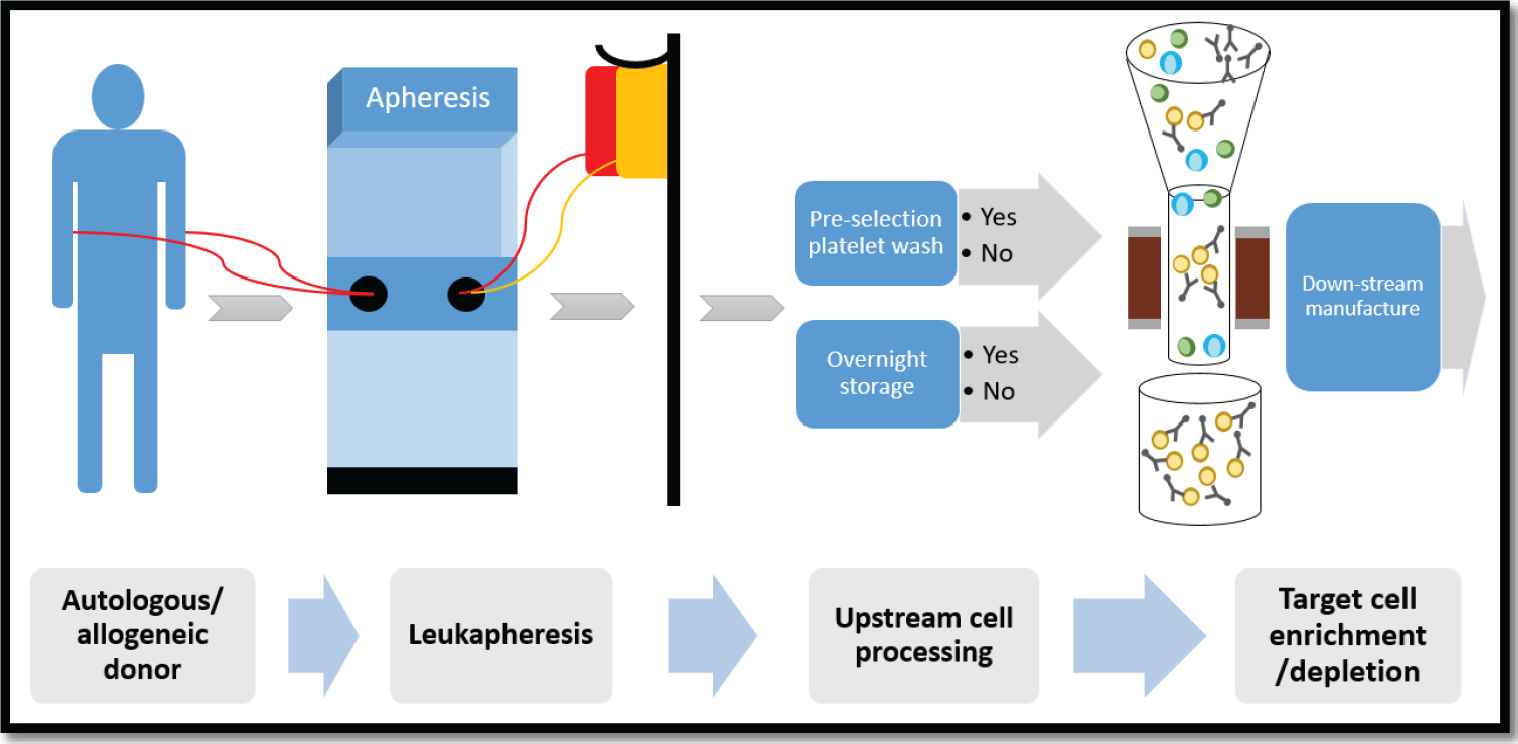
Cell enrichment/depletion schema using the CliniMACS Plus Instrument. G-CSF mobilized hematopoietic progenitor cells (HPCs) and/or nonmobilized mononuclear cell (MNC) fractions were collected by apheresis from autologous or allogeneic donors. Per protocol specifications, the apheresis product underwent a platelet reduction wash and/or overnight storage, followed by target cell enrichment/depletion on the CliniMACS Plus Instrument. The desired cell fraction was subsequently used for downstream manufacturing.
2. METHODS
2.1. Study Population
Data were collected from selections performed on the CliniMACS Plus Instrument (Miltenyi Biotec, Bergisch Gladbach, Germany) at the NIH Center for Cellular Engineering from January 1, 2008 to July 31, 2018. A total of 1221 records from 50 institutional clinical protocols were reviewed. After discarding records with incomplete or nonanalyzable data, 985 records were identified from 44 clinical trials. Thirty four (77.3%) of these trials with 669 (67.9%) allogeneic or autologous peripheral blood hematopoietic progenitor cell (HPC) products underwent CD34+ cell enrichment. The CD34+ cell enriched fraction was used in (graft manipulated) hematopoietic stem cell transplants or in nonclinical research studies. The remaining 316 (32.1%) products underwent (i) CD4 selection (posttransplant GVHD preventive cell therapy), (ii) CD3 depletion/CD56 enrichment (NK cell therapy for hematologic and solid organ malignancies), (iii) CD4/CD8 enrichment for CAR T-cell production, or (iv) CD25+ suppressor T-cell depletion for autologous lymphocyte infusions to treat solid organ malignancies.
2.2. Cell Enrichment and Depletion
Our cell enrichment/depletion schema using the CliniMACS Plus is shown in Figure 1. Briefly, peripheral blood mononuclear cells or HPC were collected by apheresis and processed fresh, in their entirety. A complete blood count was performed on the apheresis product prior to processing to determine the number of target cells in the product. Some of the apheresis concentrates were washed to reduce their platelet content. The cells of interest were separated and isolated using the Miltenyi CliniMACS Plus Instrument and a closed system disposable kit. All apheresis concentrates were incubated with MicroBeads (monoclonal antibody conjugated to super-paramagnetic particles) and washed again after incubation to remove excess reagent. A variable number of MicroBead vials, 1 to 4, were used depending on the quantity of nucleated cells and target cells that were in the apheresis concentrate. Based on the quantity of cells present and the number of MicroBeads used, a small or large instrument disposable tube set was used. The number of MicroBead vials and the size of the tube set used was determined using an algorithm provided by Miltenyi. In some cases, the cells were selected on the day they were collected. However, in other cases, particularly when the apheresis collection process was completed late in the day, the cells were held overnight at 4°C and were processed the following day.
For cell enrichment, labeled cells are captured magnetically and nonlabeled cells are collected into the negative fraction bag. Captured (labeled) cells are released when the magnet is turned off and are collected into the collection bag. For cell depletion, labeled cells are retained by the magnet while nonlabeled cells are collected in the collection bag. Captured (labeled) cells are released when the magnet is turned off and are collected into the negative fraction bag. Depending on the type of enrichment and/or depletion, the CliniMACS software settings are programmed with information on the labeled cells to be processed, number of stages and volumes to process per stage, buffers needed, bag volumes of the cell collection bag, negative fraction bag and priming waste bag, and loading volume ranges based on the separation program, tubing set, total nucleated cells (TNC), number/frequency of target/labeled cells, and sample loading volume.
| Antigen Marker Used for Selection | CD34 | CD4 | CD3/56 | CD4/8 | CD25 | Total | |
|---|---|---|---|---|---|---|---|
| Selection method | Positive | Positive | Negative/Positive | Positive/Positive | Negative | ||
| N (%) | 669 (67.9) | 179 (18.2) | 79 (8.0) | 21 (2.1) | 37 (3.8) | 985 | |
| Donor category (n) | Allogeneic | 436 (65.2) | 179 (100) | 22 (27.8) | 0 (0) | 0 (0) | 637 |
| Autologous | 233 (34.8) | 0 (0) | 57 (72.2) | 21 (100) | 37 (100) | 364 | |
| Age (in years) | 34.7 ± 15.6 | 48.2 ± 13.4 | 42 ± 21.5 | 52.2 ± 24.4 | 17 ± 8.5 | ||
| Gender | Male | 438 (65.5) | 102 (57.0) | 58 (73.4) | 16 (76.2) | 27 (73.0) | 640 |
| Female | 231 (34.5) | 77 (43.0) | 21 (26.6) | 5 (23.8) | 10 (27.0) | 342 | |
| Race/ethnicity | White | 371 (55.5) | 120 (67.0) | 47 (59.5) | 15 (71.4) | 29 (78.4) | 582 |
| Black | 95 (14.2) | 15 (8.4) | 6 (7.6) | 0 (0) | 1 (2.7) | 117 | |
| Asian | 47 (7.0) | 6 (3.4) | 1 (1.3) | 2 (9.6) | 2 (5.4) | 58 | |
| Other | 156 (23.3) | 38 (21.2) | 25 (31.6) | 4 (19.0) | 5 (13.5) | 228 | |
| BMI | 26.0 ± 6.2 | 29.7 ± 6.2 | 25.3 ± 6.3 | 19.9 ± 4.4 | 20.9 ± 5.4 | ||
| Product type | HPC (A) | 667 | 0 | 0 | 0 | 0 | 667 |
| Lymph | 0 | 179 | 79 | 21 | 37 | 316 | |
| HPC (M) | 2 | 0 | 0 | 0 | 0 | 2 | |
| Years of selection | 11 (2008–2018) | 9 (2008–2016) | 8 (2008–2015) | 3 (2016–2018) | 4 (2008–2011) | ||
| Preselection wash (n) | Yes | 513 (76.7) | 107 (59.8) | 57 (72.2) | 21 (100) | 0 (0) | 698 |
| No | 156 (23.3) | 72 (40.2) | 22 (27.8) | 0 (0) | 37 (100) | 287 | |
| Held overnight (n) | Yes | 219 (32.7) | 46 (25.7) | 77 (97.5) | 18 (85.7) | 37 (100) | 397 |
| No | 450 (67.3) | 133 ((74.3) | 2 (2.5) | 3 (14.3) | 0 (0) | 588 | |
BMI: Body mass index; HPC(A): Hematopoietic progenitor cells, apheresis; HPC(M): Hematopoietic progenitor cells, marrow
Donor and cell selection characteristics.
2.3. Viable Cell Recovery and Cell Purity
Following processing, cell count and flow cytometric analysis on specified fractions are used to evaluate cell separation efficiency (purity) and cell recovery.
Viable post-selection cell recovery (%) was calculated using the formula:
2.4. Statistical Analysis
Data are presented as the mean ± 1 standard deviation. The Student's t-test was used to compare differences between two groups. Analyses comparing multiple groups was performed with one-way analysis of variance (ANOVA) or linear regression. All analyses were performed using Prism version 7.04 for Windows, GraphPad Software (La Jolla, California, USA), and a p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographics
Donor and cell selection characteristics are summarized in Table 1. The majority of selections were CD34+ cell enrichments (67.7%) performed on allogeneic mobilized peripheral blood stem cell donors. CD4 (18.2%), CD3/56 (8.0%), CD4/CD8 (2.1%), and CD25 (3.8%) selection/depletions were carried out on lymphocyte fractions. Prior to selection on the CliniMACS Plus magnetic column, the majority of products (70.9%) underwent a wash procedure and were processed immediately without overnight storage (59.7%).
3.2. Selection Characteristics
Post-selection, FACS analysis demonstrated CP in the range of 86.3 ± 7.2% to 99.0 ± 1.1% for the enriched cells. The highest average post-selection CP was for CD4 enrichment (99.0 ± 1.1%). For the protocol on regulatory T-cell depletion, the residual CD25-fraction post-depletion was 0.14 ± 0.09%. Subfraction purity for CD4 and CD8 positive selections performed in the same run were 50.4 ± 13.6% and 41.1 ± 13.3%, respectively. VCR or selection efficiency was in the range of 32.3 ± 10.2% to 65.4 ± 15.4%, and was the highest for CD34+ cell selections (Table 2, Figure 2).
| Selection Method | CD34 | CD4 | CD3/56 | CD4/8 | CD25a |
|---|---|---|---|---|---|
| Selection Type | Positive | Positive | Negative/Positive | Positive/Positive | Negative |
| N | 669 | 179 | 79 | 21 | 37 |
| Purity (%) | CD34: 94.9 ± 7.0 | CD4: 99.0 ± 1.1 | CD56: 86.3 ± 7.2 | CD3: 92.1 ± 3.8 CD4: 50.4 ± 13.6 CD8: 41.1 ± 13.3 |
__ |
| Viable cell recovery (%) | CD34: 65.4 ± 15.4 | CD4: 32.3 ± 10.2 | CD56: 34.3 ± 17.1 | CD3: 57.0 ± 16.7 CD4: 60.3 ± 18.4 CD8: 58.0 ± 17.0 |
CD25: 1.9 ± 1.1 |
| Log depletions | CD3: 5.1 ± 0.4 CD19: 3.8 ± 0.4 |
CD8: 3.29 ± 1 | CD3: 4.01 ± 0.39 | CD56: 1.2 ± 0.45 CD22: 2.17 ± 0.85 CD14: 1.33 ± 0.43 CD15: 1.76 ± 0.83 |
CD25: 1.78 ± 0.28 |
| Residual fractions post-depletion (%) | CD3: 0.06 ± 0.14 CD19: 0.44 ± 1.31 |
CD8: 0.04 ± 0.15 | CD3: 0.02 ± 0.05 | CD56: 2.46 ± 2.62 CD22: 0.56 ± 0.63 CD14: 1.61 ± 2.05 CD15: 0.03 ± 0.04 |
CD25: 0.14 ± 0.1 |
| Log red cell depletions | 2.31 ± 0.55 | 1.19 ± 0.61 | 2.16 ± 0.57 | 1.65 ± 0.56 | 0.65 ± 0.66 |
| Log platelet depletions | 3.79 ± 1.39 | 4.38 ± 1.83 | 5.16 ± 1.55 | 2.98 ± 1.34 | 1.13 ± 0.45 |
Negative selection process resulting in low post-selection CD25+ cell purity and viable cell recovery as expected.
Summary of the results of the cell selection procedure.
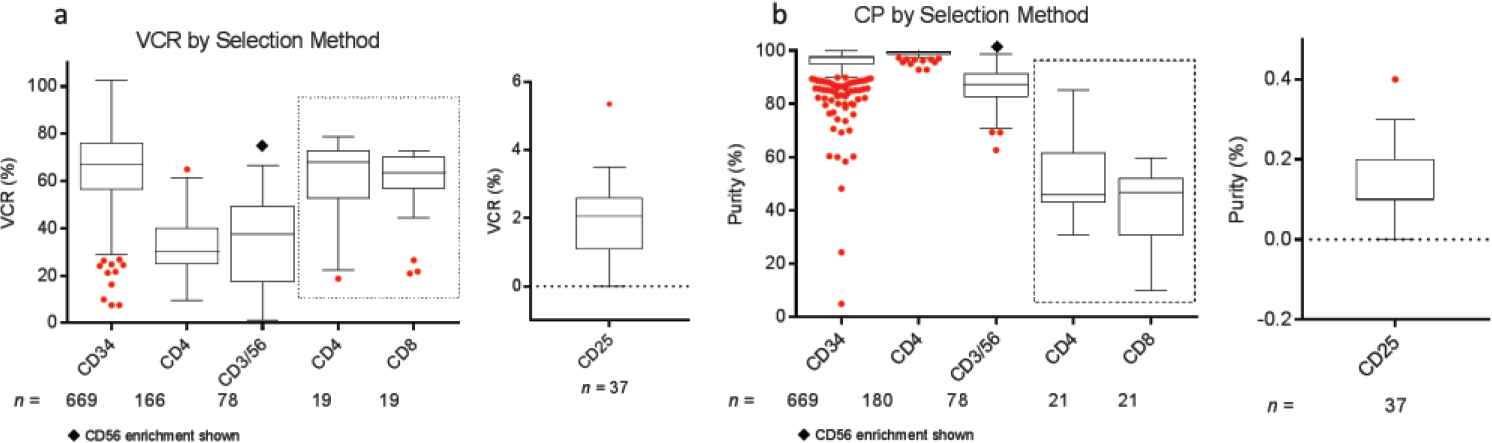
Comparison of (a) viable cell recovery (VCR) and (b) cell purity (CP) across various selection protocols over a period of 10 years. Cells were selected from mobilized peripheral blood hematopoietic progenitor cell (HPC) concentrates or from mononuclear cell (MNC) fractions. Box plots represent minimum, median, and maximum (1.5 times the interquartile range), with outliers shown as individual red dots. The CD4 and CD8 cell selection results shown in the dotted box represents combined CD4/CD8 cell selection. The VCR and CP for the negative selection of CD25+ cells are shown separately.
Depletion of undesired cell fractions resulted in a 1.2 ± 0.45 to 5.1 ± 0.4 log reduction in these cell types across various selection protocols as shown in Table 2. Post-depletion, the residual proportion of undesired cells ranged from 0.02 ± 0.05% to 2.46 ± 2.62%. Red cell and platelet depletions varied by selection type as well. On average, depletions of 2.12 ± 0.68 and 4.01 ± 1.57 logs were noted for red cells and platelets, respectively (Table 2, Figure S1).
3.3. Factors Affecting Selection Characteristics
For the two most frequently used selection types (CD34+ and CD4+ cell enrichments), donor- and cell selection/instrument-related parameters were evaluated for their effect on VCR and CP.
Donor demographic factors, including age, gender, race, and body mass index (BMI), did not impact VCR or purity of CD34+ (Figure S2) or CD4+ (data not shown) cell enrichment (p > 0.05). No correlations were seen between starting red blood cell, platelet, and neutrophil contents and VCR or purity (data not shown). Manufacturing factors that affected VCR and purity in CD34+ selections included tubing size, bead quantity used, preselection platelet wash, and CD34 counts per bead vial (p < 0.05) (Figure 3). Overnight storage marginally decreased CP for CD34+ cell selections, from 95.5 ± 6.65% to 93.8 ± 7.68% (p = 0.003). Ten-year trends in VCR were also evaluated for CD34+ and CD4+ enrichments. Marginal improvement in CD34+ VCR was noted over the years (p < 0.001). This was not the case for CD4+ cell recovery (p = 0.03) (Figure 4). These changes were not noted to correlate with changes in processing technologist or their cumulative experience.
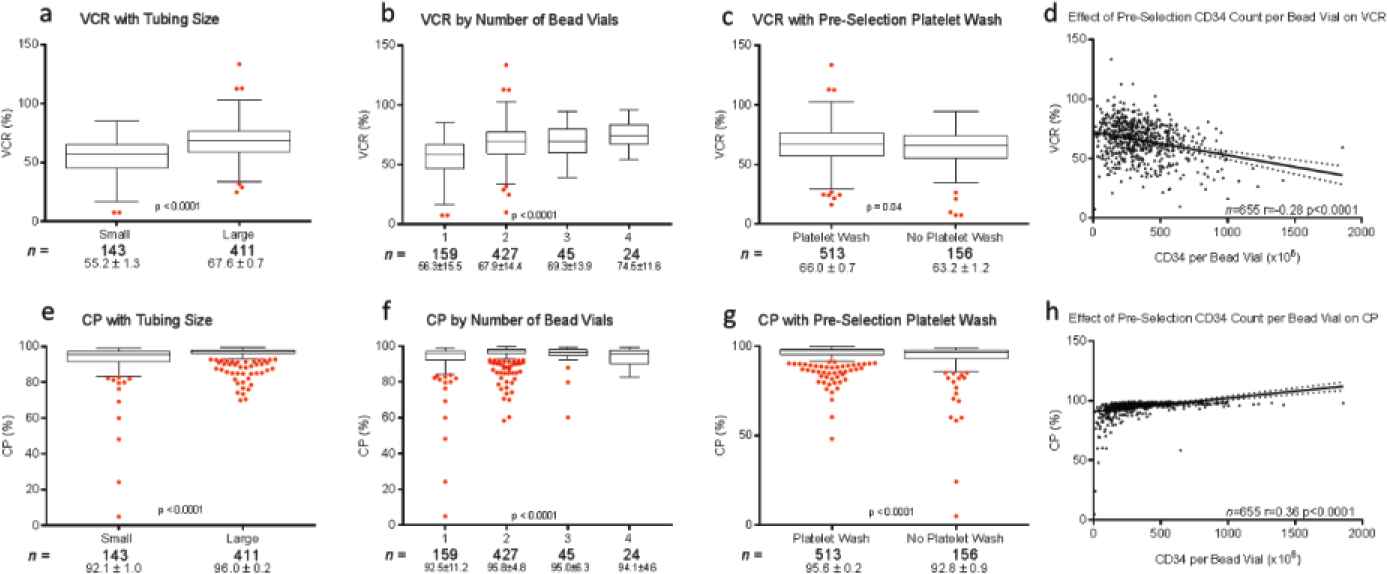
Effect of cell selection instrument tubing set size (a, e), number of MicroBead vials (b, f), preselection platelet wash (c, g) CD34+ cell count per bead vial (d, h) on viable cell recovery (VCR) (top row) and cell purity (CP) (bottom row) following CD34+ cell selections. Box plots represent minimum, median, and maximum (1.5 times the interquartile range), with outliers shown as individual red dots. CD34+ cell count per bead vial is plotted as a continuous variable with a linear regression analysis of its effect on CD34+ VCR and CP.
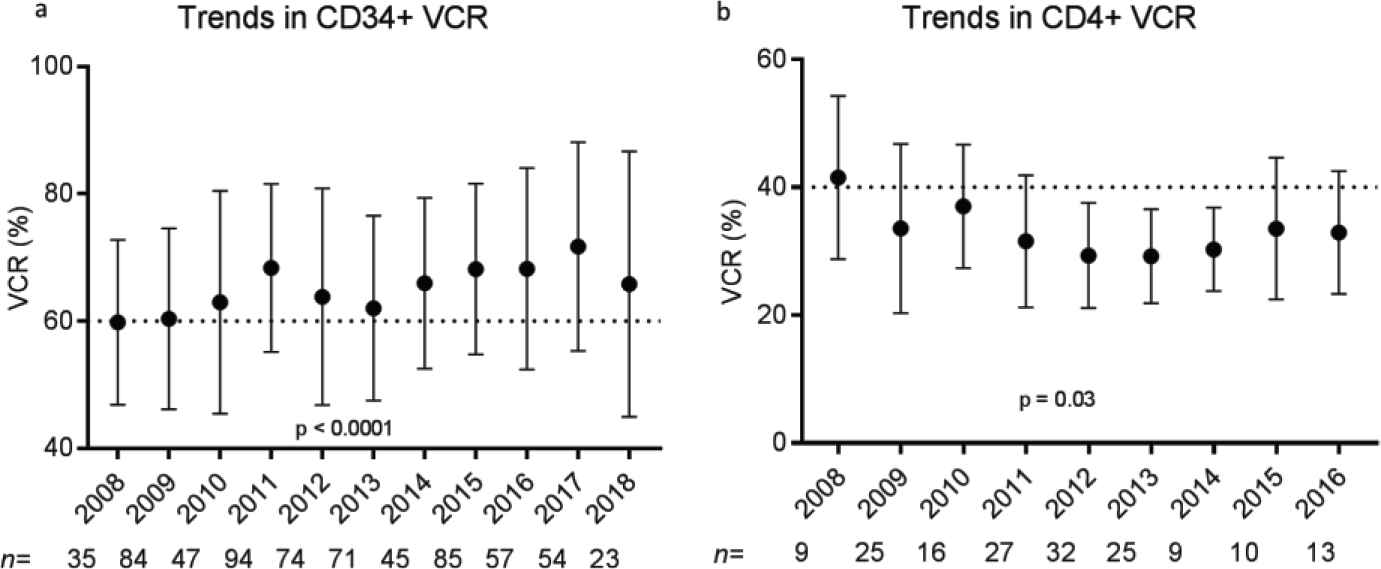
Trends in (a) CD34+ viable cell recovery (VCR) and (b) CD4+ viable cell recovery (VCR) over a 10-year time frame. Plots demonstrate mean VCR (%) and standard deviations for each year of selection performed. p Values were calculated for the multiple comparisons test (analysis of variance [ANOVA]).
4. DISCUSSION
Graft engineering by immunomagnetic selection of hematopoietic cellular subtypes can have significant impact on clinical outcomes in patients receiving HPC transplantation [6,7], CAR T-cells [8], and other cellular therapies [9,10]. At our cell processing facility, the CliniMACS Plus has been used for this purpose for more than 10 years in over 50 investigational new drug protocols. In prior studies, we and others have found that the CliniMACS Plus immunomagnetic selection method has shown superior CP and VCR compared with other technologies, particularly with regard to CD34+ cell selection from mobilized peripheral blood [5,11]. Since then, this technique has been successfully applied to CD34+ cell selections on bone marrow collections and umbilical cord blood, as well as on cryopreserved and thawed cells [12–15]. Further, other cell subtypes, including monocytes, NK cells and T-lymphocytes selected on the instrument have also been utilized in early phase clinical studies [8,16–18]. As the only FDA approved cell isolation system for clinical use, it is critical to periodically assess the efficacy of the instrument for various selections at each institution.
We undertook a large systematic study to evaluate selections on the CliniMACS Plus instrument performed at our facility over a 10-year period. As anticipated, VCR and CP post-selection varied by antibody-bead conjugate, with VCR being highest for CD34+ selections, and CP being highest for CD4+ selections. This was consistent with data from previous studies evaluating these selections individually [19]. Our results also indicated near complete depletion of undesired cell fractions, platelets, and red blood cells, with an average depletion of 3 logs.
We also evaluated factors affecting selection efficiency. Previous studies have demonstrated significant variability in mobilized CD34+ cell collections by race, gender, age, and BMI [20]. Contrary to our hypothesis, none of the donor demographics factors impacted selections on the Miltenyi CliniMACS Plus instrument. Donor type (autologous versus allogeneic) did not affect selection efficiency either. However, several manufacturing parameters were noted to impact VCR and purity in the two largest and analyzable groups, the CD34+ and the CD4+ selections. Regardless of cell concentration, larger tubing size positively impacted VCR and purity, likely due to increased surface area for antibody-bead-cell conjugation in the larger tubing set. Likewise, increasing the number of MicroBead vials positively impacted VCR and purity.
Preselection platelet reduction washing improved CP for CD34 and CD4 selections. Platelet wash improved VCR for CD34, but not for CD4. We hypothesize that this was likely because G-CSF stimulated (activated) platelets resulted in nonspecific bead binding and interference in the selection of G-CSF-mobilized CD34+ cells. CD34+ CP was slightly, but significantly higher for samples processed on the same day, possibly due to continued cell differentiation in samples held overnight. This difference was, however, not clinically significant. VCR improved over the years for CD34+ but not for CD4+ cell selections. The cause for these changes was likely multifactorial. Studies are ongoing to evaluate the effect of cell selection variations on downstream manufacturing steps and clinical outcomes.
Recently, the fully automated CliniMACS Prodigy system, which can be used for both cell selection and culture, has been shown to compare favorably with the CliniMACS Plus Instrument in regards to the CD34+ cell selection process [21,22]. As a completely closed system process, that device offers the potential for graft engineering in health care facilities without ISO grade clean rooms. However, marginally better selection efficiencies and lower operational costs seen with the semi-automated CliniMACS Plus may still result in its continued use in early phase studies.
In conclusion, the clinically approved CliniMACS Plus Instrument has been successfully used to perform various cell enrichments and depletions for graft engineering in early phase cell therapies. VCR, final CP, as well as depletions of undesired cells vary by selection type. However, most selection methods result in acceptable cell recoveries and purity, with about 3 log depletions of the undesired fractions. Manufacturing parameters including preselection wash steps, tubing size, and number of selection beads impact VCR and CP. Donor demographics have no impact on selection efficiency. While the CliniMACS Plus is the sole semi-automated selection instrument available for clinical use, institutions may benefit from performing periodic assessments of selection efficiencies across various protocols performed on this instrument.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
AUTHORS' CONTRIBUTIONS
S.R.P., O.L.R, and D.F.S. performed conceptualization of this work; K.L., T.B., S.Y., and A.R. abstracted data and conducted formal analysis; S.R.P., S.H., D.F., R.W.C., M.B., J.B., A.L., C.M., and N.S. were involved in patient care. S.R.P., O.L.R, and D.F.S. wrote the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the staff and faculty at the Dowling Apheresis Clinic and the Center for Cellular Engineering, Department of Transfusion Medicine, Clinical Center. This work was supported (in part) by the Intramural Research Program of the National Institutes of Health, Clinical Center.
SUPPLEMENTARY FIGURES
Comparison of (a) platelet and (b) red cell log depletions following cell selection. The cells were selected from mobilized peripheral blood hematopoietic progenitor cell (HPC) concentrates or from mononuclear cell (MNC) fractions. Box plots represent minimum, median, and maximum (1.5 times the interquartile range), with outliers shown as individual red dots.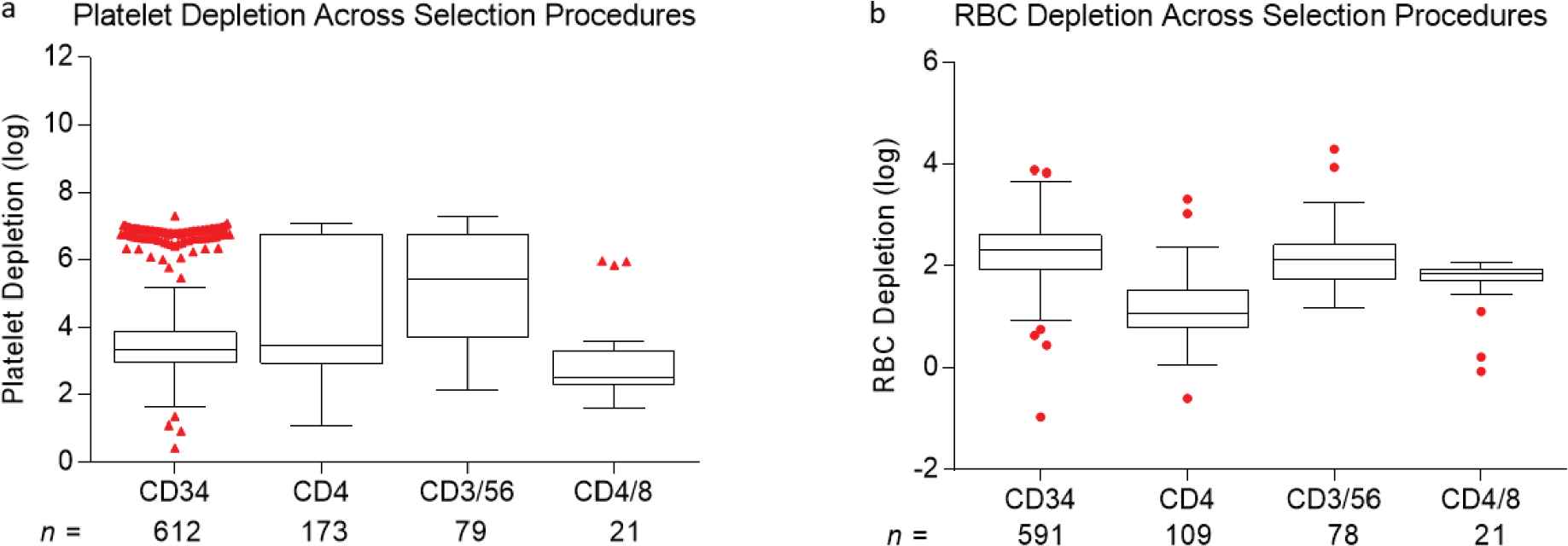
Comparison of (a) platelet and (b) red cell log depletions following cell selection. The cells were selected from mobilized peripheral blood hematopoietic progenitor cell (HPC) concentrates or from mononuclear cell (MNC) fractions. Box plots represent minimum, median, and maximum (1.5 times the interquartile range), with outliers shown as individual red dots.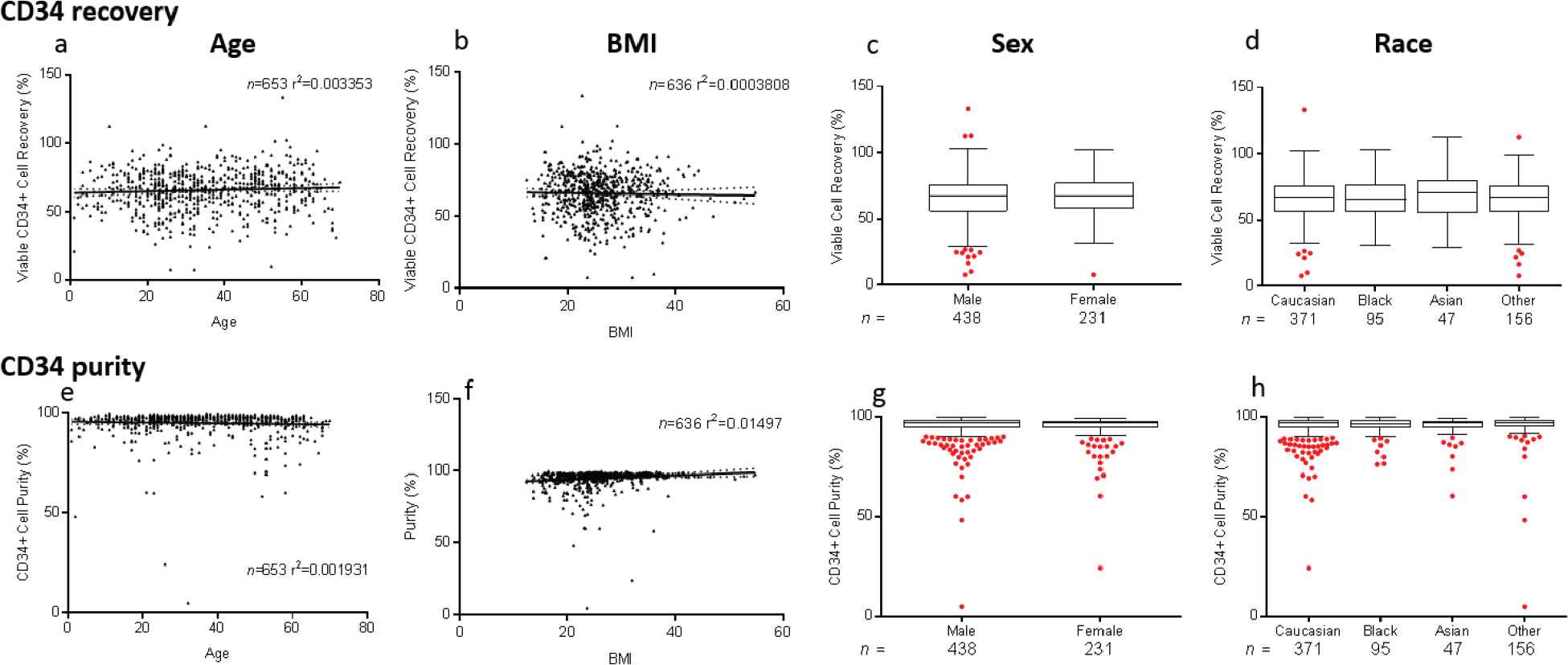
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Sandhya R. Panch AU - Opal L. Reddy AU - Katherine Li AU - Thejaswi Bikkani AU - Anusha Rao AU - Swathi Yarlagadda AU - Steven Highfill AU - Daniel Fowler AU - Richard W. Childs AU - Minocher Battiwalla AU - John Barrett AU - Andre Larochelle AU - Crystal Mackall AU - Nirali Shah AU - David F. Stroncek PY - 2019 DA - 2019/09/01 TI - Robust Selections of Various Hematopoietic Cell Fractions on the CliniMACS Plus Instrument JO - Clinical Hematology International SP - 161 EP - 167 VL - 1 IS - 3 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.190529.001 DO - 10.2991/chi.d.190529.001 ID - Panch2019 ER -
