Safety and Efficacy of Doxorubicin, Cyclophosphamide, Bortezomib, Dexamethasone and Lenalidomide Followed by Bortezomib Consolidation as First-Line Therapy in Patients with Newly Diagnosed Multiple Myeloma
- DOI
- 10.2991/chi.d.200116.001How to use a DOI?
- Keywords
- Multiple myeloma; Clinical trial; Hematology; First line therapy
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
Patients with multiple myeloma (MM) have experienced a significant improvement in treatment responses and survival outcomes for the past decades, after the introduction of novel agents [1,2]. However, this cancer remains incurable, and new combinations of drugs need continuous investigation in order to secure constant advancement in the clinical setting.
We conducted a phase-2 study to evaluate the safety and efficacy of a readily accessible 5-drug regimen consisting of doxorubicin, cyclophosphamide, bortezomib, dexamethasone and lenalidomide (ACVDL), followed by bortezomib consolidation, in patients with newly diagnosed symptomatic MM according to the International Myeloma Working Group (IMWG) criteria [3]. The study was based on previously reported experience with other treatment regimens including combinations of bortezomib, cyclophosphamide and dexamethasone (VCD), regimens containing lenalidomide, bortezomib and dexamethasone (RVD), and the intensive regimen of bortezomib, dexamethasone, cyclophosphamide and lenalidomide (VDCR) tested in the EVOLUTION study [4–7]. Furthermore, preclinical studies indicated that resistance to conventional chemotherapy including doxorubicin and melphalan might be overcome by concomitant treatment with bortezomib. Therefore, the combination and close timing of doxorubicin and bortezomib was part of the design of the ACVDL regimen [8,9].
The study was conducted as a single-center, investigator-initiated, non-randomized phase-2 trial enrolling both transplant-eligible and -ineligible patients with newly diagnosed MM. Patient characteristics are shown in Table 1. Patients were assigned to receive at least four 21-day cycles of ACVDL: doxorubicin 50 mg/m2 IV day 1, cyclophosphamide 750 mg/m2 intravenous (IV) day 1, bortezomib 1.3 mg/m2 IV days 2 and 9, dexamethasone 20 mg PO days 2, 3, 9 and 10 and lenalidomide 15 mg PO days 1 to 14. Transplant-eligible patients proceeded to standard high dose therapy (melphalan 200 mg/m2; HDT-ASCT) followed by autologous stem cell transplantation (ASCT). In the case of unsuccessful stem cell collection on standard granulocyte-colony stimulating factor (G-CSF), plerixafor was used. Patients ineligible to HDT-ASCT received additional four cycles of ACVDL. To resemble real world patients as much as possible, the trial allowed inclusion of patients with light chain amyloidosis (AL). In these patients, doxorubicin was omitted from the treatment scheme to minimize cardiac adverse events.
| All Patients n = 35 | ASCT Group n = 21 | Non-ASCT Group n = 14 | |
|---|---|---|---|
| Median age, years (range) | 64 (49–81) | 61 (49–69) | 71 (49–81) |
| Age > 75 years (%) | 4 (11%) | 0 (0%) | 4 (29%) |
| Gender: male, n (%) | 21 (60%) | 13 (62%) | 8 (57%) |
| Race: white, n (%) | 35 (100%) | 21 (100%) | 14 (100%) |
| Karnofsky Performance Status, n (%) | |||
| 100 | 6 (17%) | 5 (24%) | 1 (7%) |
| 90 | 12 (34%) | 7 (33%) | 5 (36%) |
| 80 | 7 (20%) | 5 (24%) | 2 (14%) |
| 70 | 7 (20%) | 3 (14%) | 4 (29%) |
| 60 | 3 (9%) | 1 (5%) | 2 (14%) |
| ISS stage at baseline, n (%) | |||
| 1 | 17 (49%) | 9 (43%) | 8 (57%) |
| 2 | 10 (29%) | 8 (38%) | 2 (14%) |
| 3 | 8 (23%) | 4 (19%) | 4 (29%) |
| R-ISS stage at baseline, n (%)a | |||
| 1 | 11 (31%) | 6 (29%) | 5 (36%) |
| 2 | 20 (57%) | 12 (57%) | 8 (57%) |
| 3 | 3 (9%) | 3 (14%) | 0 |
| MM subtype, n (%) | |||
| IgG | 15 (43%) | 11 (52%) | 4 (29%) |
| IgA | 8 (23%) | 3 (14%) | 5 (36%) |
| Light chain | 9 (26%) | 5 (24%) | 4 (29%) |
| Non-secretory | 3 (9%) | 2 (10%) | 1 (7%) |
| Biochemistry | |||
| Hemoglobin (µmol/L), median (range) | 6.9 (5.0–9.4) | 6.9 (5.0–8.5) | 7.0 (5.2–9.4) |
| β-2-microglobulin (nmol/L), median (range) | 269 (129–792) | 269 (129–792) | 272 (161–675) |
| Albumin (g/L), median (range) | 39 (27–49) | 39 (33–49) | 39 (27–48) |
| LDH above upper limit at baseline, n (%) | 9 (26%) | 7 (33%) | 2 (14%) |
| eGFR below 60 mL/min/1.73 m2 b, n (%) | 10 (29%) | 6 (29%) | 4 (29%) |
| FISH abnormalities, n (%)c | |||
| del 13q | 15 (43%) | 10 (48%) | 5 (36%) |
| del 17p | 4 (11%) | 3 (14%) | 1 (7%) |
| t(4;14) | 3 (9%) | 3 (14%) | 0 |
| t(11;14) | 7 (20%) | 3 (14%) | 4 (29%) |
| t(14;16) | 0 | 0 | 0 |
| High-risk FISH abnormalitiesd | 7 (20%) | 6 (29%) | 1 (7%) |
| Osteolytic lesions, n (%)e | |||
| None | 8 (23%) | 5 (24%) | 3 (21%) |
| Present | 27 (77%) | 16 (46%) | 11 (79%) |
ASCT: Autologous stem cell transplantation; ISS: International Staging System; R-ISS: Revised International Staging System; MM: Multiple myeloma; IgG: Immunoglobulin G; IgA: Immunoglobulin A; LDH: Lactate dehydrogenase; eGFR: estimated glomerular filtration rate; FISH: fluorescence in situ hybridization.
R-ISS: Data missing for one patient in the non-ASCT group.
eGFR by MDRD study equation.
FISH abnormalities: deletion 13q, deletion 17p, translocation (4;14), translocation (11;14) and translocation (14;16). Data are missing in two patients, one in each treatment group.
High-risk FISH abnormalities: deletion 17p and/or translocation (4;14) and/or translocation (14;16).
Osteolytic lesions: Either by conventional X-ray and/or computed tomography (CT).
Demographics and baseline characteristics of patients enrolled in the clinical phase 2 study (ACVDL).
Supportive and prophylactic treatment consisted of G-CSF administered from days 3 to 10, daily oral aciclovir 400 mg × 2 and daily oral aspirin 150 mg or equivalent. In case of residual disease after the end of therapy detected by either conventional assessment methods or allele-specific oligonucleotide polymerase chain reaction (ASO-qPCR, sensitivity of 1 × 10−5), patients were offered five 35-day cycles of consolidation therapy with subcutaneous bortezomib 1.6 mg/m2 days 1, 8, 15 and 22. The IMWG response criteria (2011) were used for response assessment. Evaluation of treatment responses took place after four cycles of ACVDL in all patients (interim assessment). A second response evaluation was carried out three months after HDT-ASCT or one month after the eighth cycle of ACVDL in HDT-ASCT-eligible and ineligible patients, respectively [10]. A third response evaluation took place after bortezomib consolidation. Patients were followed monthly for four years after the end of therapy.
Every patient who received at least one dose of treatment was included in the intention-to-treat (ITT) population for statistical analysis. Dichotomous variables were compared using Fischer's exact test. All tests were two-sided and p-values <0.05 were regarded as significant. Statistical analysis was performed using STATA software version 15.0 (StataCorp, College Station, Texas).
From 2011 to 2014, a total of 35 patients with newly diagnosed symptomatic MM were enrolled in the study. Three of these patients had AL. The median age was 64 years (range 49–81). Four patients left the study prior to interim assessment (2 due to prolonged neutropenia, 1 due to the onset of depression after loss of his spouse and 1 due to refractory disease). Two transplant-eligible patients left the study between the interim assessment and stem cell collection due to progressive disease (PD). The median number of collected CD34-positive cells was 4.6 × 106/kg (range 3.7–9.2) with a median viability of 97%. Nine (50%) patients received plerixafor in addition to the basic G-CSF stimulation regimen. One patient failed stem cell collection, despite the use of plerixafor and two collection attempts. The regimen of this patient was subsequently modified to include additional four cycles of ACVD without lenalidomide. Nineteen patients received consolidation therapy with bortezomib.
The overall response rate at the end of therapy was 80% with 28 patients from the ITT population achieving partial response (PR) or better. Sixteen patients (46%) achieved a very good partial response (VGPR) or better and 13 (37%) achieved complete response (CR) or better. Table 2 separately shows the response rates of HDT-ASCT-eligible and ineligible patients. Of note, the transplant-ineligible patients tended to have the best response rates (VGPR or better and CR or better), but, the differences were not significant. The mean cumulative dose of each drug in the ACVDL combination (delivered dose/planned dose × 100%) was: doxorubicin 75%, cyclophosphamide 97%, bortezomib 78%, dexamethasone 76% and lenalidomide 82%. We evaluated the effects of each drug on the response rates of patients who, as a minimum, underwent the interim assessment (n = 31). We found that patients who achieved VGPR or better (prior to HDT-ASCT or after eight cycles of therapy) had received significantly higher cumulative dosages of lenalidomide (p = 0.02), and had a trend towards exposure to higher cumulative dosages of bortezomib (p = 0.09). Nineteen patients received consolidation therapy with bortezomib. Responses deepened in four patients (1 SD → CR; 3 PR → VGPR), and remained unchanged in 12. In three patients, PD was detected during or just after the end of consolidation therapy (1 sCR → PD, 1 CR → PD and 1 PR → PD).
| Response | Interim (After 4 Cycles of ACVDL) |
End of Therapy (3 Months after HDT-ASCT or 1 Month after the Eighth Cycle of ACVDL) |
||
|---|---|---|---|---|
| HDT-ASCT n = 21 | Non-ASCT n = 14 | HDT-ASCT n = 21 | Non-ASCT n = 14 | |
| PD | 1 (5%) | 0 | 2 (10%) | 0 |
| SD | 5 (24%) | 1 (7%) | 1 (5%) | 0 |
| ORR (PR or better) | 14 (67%) | 10 (71%) | 17 (81%) | 11 (79%) |
| PR | 6 (29%) | 3 (21%) | 9 (43%) | 3 (21%) |
| VGPR | 4 (19%) | 2 (14%) | 2 (10%) | 1 (7%) |
| CR | 3 (14%) | 0 | 1 (5%) | 1 (7%) |
| sCR | 1 (5%) | 5 (36%) | 3 (14%) | 5 (36%) |
| mCR | 0 | 0 | 2 (10%) | 1 (7%) |
| Withdrawn | 1 (5%) | 3 (21%) | 1 (5%) | 3 (21%) |
| VGPR or better | 8 (38%) | 7 (50%) | 8 (38%) | 8 (57%) |
| CR or better | 4 (19%) | 5 (36%) | 6 (29%) | 7 (50%) |
ACVDL: doxorubicin, cyclophosphamide, bortezomib, dexamethasone and lenalidomide; HDT-ASCT: high-dose therapy with autologous stem cell transplantation; PD: progressive disease; SD: stable disease; PR: partial response; VGPR: very good partial response; CR: complete response; sCR: stringent complete response; mCR: molecular complete response; ORR: overall response rate.
Treatment response at interim and at end of therapy for the ASCT group and the non-ASCT group.
There were no treatment-emergent deaths. The most common grade 3 to 4 adverse events were neutropenia (60%), infections (43%), thrombocytopenia (26%) and anemia (23%). Two patients developed congestive heart failure after seven and eight cycles of ACVDL, respectively. One patient experienced progressive multifocal leukoencephalopathy after the eighth cycle of ACVDL. One patient was diagnosed with myelodysplastic syndrome during follow-up. Three patients discontinued consolidation therapy due to adverse events (fatigue, diarrhea and peripheral neuropathy, respectively). The most common grade 3 to 4 adverse events during consolidation were infection (15.8%) and fever (10.5%). A total of 49 serious grade 3 to 4 adverse events occurred during the entire course of therapy; the most common were infection (n = 22) and febrile neutropenia (n = 8). Adverse events are listed in Table 3.
| Event—No. of Patients (%) | All Patients (n = 35) |
Transplant-Eligible Patients (n = 21) |
Transplant-Ineligible Patients (n = 14) |
|||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | Any Grade | Grade 3–4 | |
| Common hematologic adverse events: | ||||||
| Anemia | 35 (100%) | 8 (23%) | 21 (100%) | 4 (19%) | 14 (100%) | 4 (29%) |
| Neutropenia | 24 (69%) | 21 (60%) | 15 (71%) | 12 (57%) | 9 (64%) | 9 (64%) |
| Thrombocytopenia | 11 (31%) | 9 (26%) | 4 (19%) | 4 (19%) | 7 (50%) | 5 (36%) |
| Non-hematologic adverse events: | ||||||
| Infection | 27 (77%) | 15 (43%) | 15 (71%) | 9 (43%) | 12 (86%) | 6 (43%) |
| Skin symptoms | 20 (57%) | 2 (6%) | 10 (48%) | 0 | 10 (71%) | 2 (14%) |
| Fever | 19 (54%) | 0 | 13 (62%) | 0 | 6 (43%) | 0 |
| Diarrhea | 14 (40%) | 3 (9%) | 8 (38%) | 3 (14%) | 6 (43%) | 0 |
| Edema limbs | 14 (40%) | 0 | 8 (38%) | 0 | 6 (43%) | 0 |
| Nausea | 12 (34%) | 3 (9%) | 8 (38%) | 2 (10%) | 4 (29%) | 1 (7%) |
| Peripheral sensory neuropathy | 10 (29%) | 1 (3%) | 5 (24%) | 1 (5%) | 5 (36%) | 0 |
| Constipation | 11 (31%) | 1 (3%) | 6 (29%) | 1 (5%) | 5 (36%) | 0 |
| Febrile neutropenia | 7 (20%) | 7 (20%) | 3 (14%) | 3 (14%) | 4 (29%) | 4 (29%) |
| Thromboembolic event | 5 (14%) | 1 (3%) | 3 (14%) | 0 | 2 (14%) | 1 (7%) |
| Fatigue | 2 (6%) | 0 | 2 (10%) | 0 | 0 | 0 |
| Treatment-related congestive heart disease | 2 (6%) | 1 (3%) | 0 | 0 | 2 (14%) | 1 (7%) |
| Treatment-related secondary malignancy | 1 (3%) | 1 (3%) | 0 | 0 | 1 (7%) | 1 (7%) |
Adverse events were registered in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Treatment related adverse events during ACVDL-therapy (including adverse events during HDT-ASCT in the transplant-eligible group).
At the time of data cut-off on the 11th November 2019, the median (interquartile range) follow-up time was 90.4 (83.5; 94.2) months. At this time point 18 patients had died and 17 were alive. The median overall survival (OS) was 83.6 (45.4; not reached) months, Figure 1A. In the HDT-ASCT group, the eight-year survival was 76.2% and the 25th percentile was not reached. In the HDT-ASCT ineligible group, the median OS was 56.9 (36.9; 74.9) months. The median progression-free survival (PFS) was 26.2 (17.5; 35.0) months with one patient still in remission after ACVDL treatment (consisting of ACVDL and HDT-ASCT), four patients having started a second line of therapy without a progression event, and 30 having experienced progression on second line therapy or death, Figure 1B. In the HDT-ASCT group, the median PFS was 26.6 (18.9; 40.7) months. In the HDT-ASCT ineligible group, the median PFS was 23.1 (15.4; 31.2) months. Thirty-one patients started a second line of therapy. The median progression-free survival 2 (PFS2) was 40.9 (30.3; NR) months with five patients still in remission in their second line of therapy, 20 having experienced progression or death, and six having started a third line of therapy without a progression event, Figure 1C. In the HDT-ASCT group, the median PFS2 was 84.8 (33.4; NR) months, and in the HDT-ASCT ineligible group, it was 30.3 (22.0; 40.3) months.
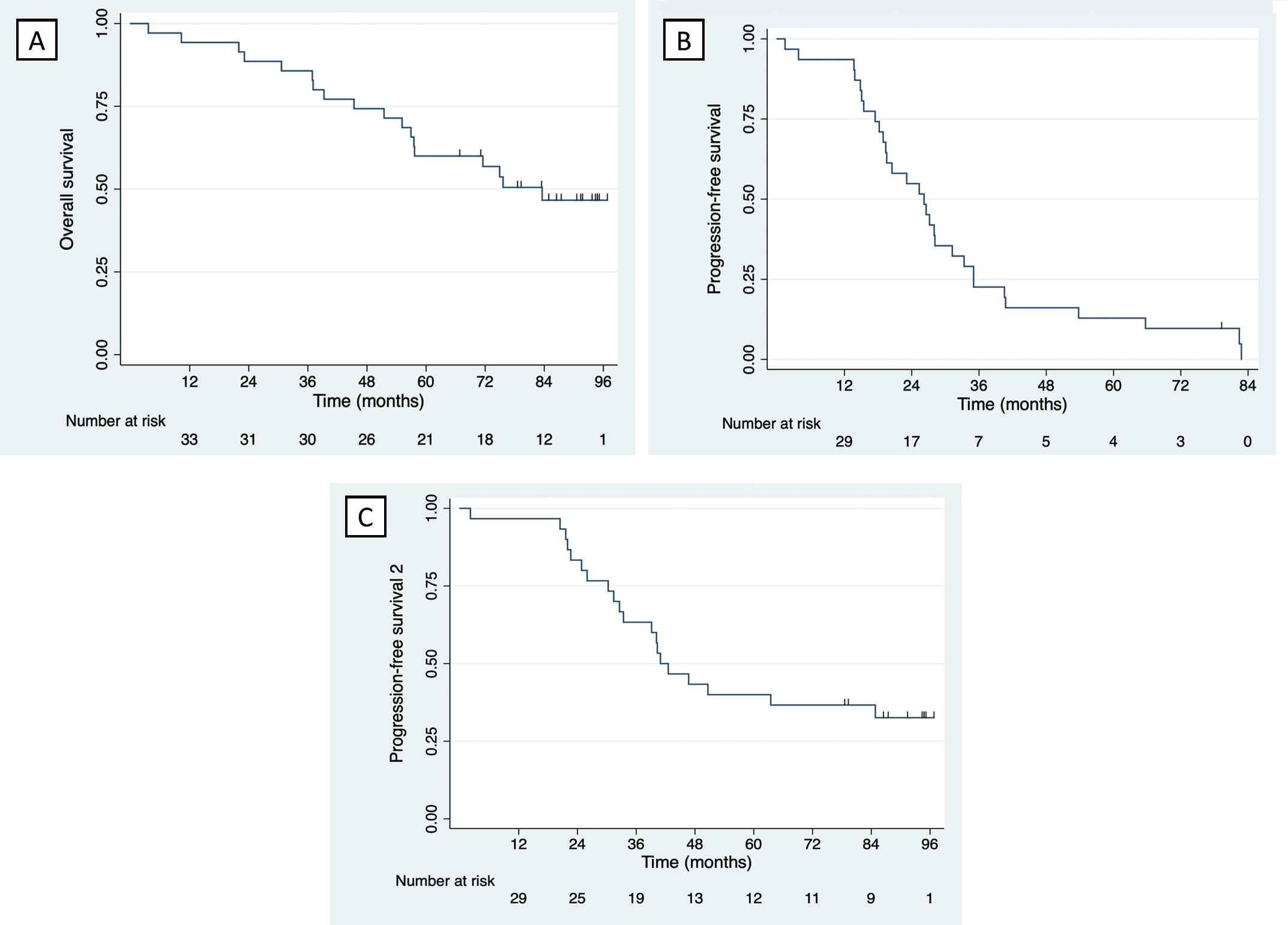
(A) Kaplan-Meyer curve illustrating overall survival for the intention to treat population. (B) Kaplan-Meyer curve illustrating progression-free survival for the intention to treat population. (C) Kaplan-Meyer curve illustrating progression-free survival 2 for the intention to treat population.
In conclusion, this phase-2 study is the first protocol to have prospectively evaluated the 5-drug combination of doxorubicin, cyclophosphamide, bortezomib, dexamethasone and lenalidomide as an induction and first-line regimen in newly diagnosed patients with MM. The ACVDL regimen was feasible and tolerable; however, the hematologic toxicities occurred at a higher rate than expected. The administration of doxorubicin and cyclophosphamide on day 1 in addition to the combination of lenalidomide and bortezomib resulted in a substantial number of neutropenic adverse events, which ultimately led to dose-reductions of lenalidomide and bortezomib. We suspect that the lower exposure to these novel agents had negative impact on the efficacy of the regimen.
Although combining intensive chemotherapy with the most effective anti-myeloma drugs of the time seemed plausible when this trial was designed, other new anti-myeloma drugs and drug combinations which are now available have had a greater impact on the treatment of MM. Quadruplet regimens consisting of proteasome inhibitors, immunomodulatory drugs and dexamethasone with the addition of CD38 monoclonal antibodies have recently been tested in the upfront treatment of MM and have achieved unprecedented depths of response [11]. In the light of these developments, the ACVDL regimen cannot be recommended as standard treatment for patients with newly diagnosed MM. If used as salvage therapy, the toxicity profile of ACVDL should be considered.
CONFLICT OF INTEREST
None of the authors have conflicts of interest to report.
AUTHORS' CONTRIBUTIONS
Conception and design of the trial: TP. Acquisition of data: KTA, MH, AGS, ES and TP. Analysis of data: KTA, MH, AGS, TO, PCH, NP and GK. Interpretation of data: KTA, MH, PCH and TP. All authors have critically revised the work and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Janssen-Cilag for the financial support of the clinical protocol. KTA received funding from the local Research Council at Lillebælt Hospital and from the foundation: “Overlæge Jørgen Werner Schous og hustru Else Marie Schou, født Wonge's, fond.”
REFERENCES
Cite this article
TY - JOUR AU - Kristian T. Andersen AU - Maja Hinge AU - Agoston Gyula Szabo AU - Erik Segel AU - Tina Ormstrup AU - Paw C. Holdgaard AU - Niels Pallisgaard AU - Gitte Kerndrup AU - Torben Plesner PY - 2020 DA - 2020/01/26 TI - Safety and Efficacy of Doxorubicin, Cyclophosphamide, Bortezomib, Dexamethasone and Lenalidomide Followed by Bortezomib Consolidation as First-Line Therapy in Patients with Newly Diagnosed Multiple Myeloma JO - Clinical Hematology International SP - 35 EP - 39 VL - 2 IS - 1 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.200116.001 DO - 10.2991/chi.d.200116.001 ID - Andersen2020 ER -
