Repeat Endoscopy Affects Patient Management in Gastrointestinal Graft-Versus-Host Disease
 , Umar Farooq1,
, Umar Farooq1,  , Burhan Yanes2, Margarida Magalhaes-Silverman1
, Burhan Yanes2, Margarida Magalhaes-Silverman1- DOI
- 10.2991/chi.d.200220.001How to use a DOI?
- Keywords
- Endoscopy; Gastrointestinal graft versus host disease; Hematopoietic; Stem cell transplantation
- Abstract
Graft versus host disease (GVHD) of the gut is associated with significant morbidity and mortality after allogeneic hematopoietic cell transplant (allo-HCT). No guidelines exist regarding repeat endoscopy after failure of first-line treatment with steroids. We aimed to study if repeat endoscopic biopsy can be helpful in these patients to guide treatment decisions. We retrospectively reviewed medical records of all patients who underwent repeat endoscopy for clinical suspicion of gastrointestinal (GI) GVHD after allo-HCT. Of the 318 patients, 24 underwent endoscopy twice after allo-HCT. At first endoscopy, 20 patients (80%) showed abnormal findings: 16 with GVHD alone, 1 with GVHD plus cytomegalovirus (CMV), and 3 with GVHD plus infectious colitis. On repeat endoscopy in these 20 patients with GVHD, 6 showed improvement leading to de-escalation of therapy, 8 showed worsening of GVHD including detection of CMV in 2 patients, and 2 had no histological changes. One patient with simultaneous GVHD and CMV diagnosed on first biopsy, displayed significant improvement leading to de-escalation of therapy. Three patients with GVHD along with infectious colitis on biopsy subsequently showed improvement on repeat biopsy leading to de-escalation of therapy. Among 4 patients with normal findings on first endoscopy, 3 had GVHD and 1 had epstein-barrvirus-associated post-transplant lymphoproliferative disorder (EBV-PTLD) on repeat procedures. This study supports the usefulness of repeat endoscopy in persistently symptomatic patients when there is no improvement after the initial treatment based on the results of the first endoscopy. Repeat endoscopy may guide therapy without significant complications.
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Allogeneic stem cell transplant (Allo-HCT) has become a standard therapeutic procedure in various hematological diseases and conditions of bone marrow failure [1–4]. Graft versus host disease (GVHD) remains a leading cause of non-relapse mortality (NRM) in Allo-HCT patients [5,6]. Depending upon the risk factors, the incidence of acute GVHD may range from 20% to 50%, and more than 50% of these patients are likely to suffer from gastrointestinal tract GVHD (GI GVHD) [7–9]. Among the various organs involved, GI GVHD may be the most difficult to treat [10]. GI complaints are commonly seen within the first 100 days following allo-HCT [11,12]. Intestinal GVHD manifests as nausea, anorexia, vomiting, high volume watery diarrhea, intestinal bleeding, or abdominal pain [7,13,14]. Other etiologies such as infection (CMV, EBV, Rotavirus, Adenovirus, Clostridium difficile, or Norovirus), mucosal damage due to conditioning therapy or drug toxicities, either alone or in addition to GVHD, can present with similar symptoms or mimic clinical findings of GI GVHD [15]. In clinical practice, it can be challenging to attribute these nonspecific symptoms to GVHD alone. Additional investigations, including endoscopic biopsy and pathological evaluation can help exclude other potential etiologies in the differential diagnosis. Histologically, the most important signs of GI GVHD are evidence of crypt losses and crypt cell apoptosis [16–18]. However, histological evaluation alone is not 100% sensitive to detect GI GVHD due to sampling error, patchiness of GVHD-related mucosal findings, and absence of early histological abnormalities [19].
With the classical presentation, a clinical diagnosis can readily be made, although some cases prove to be challenging [7]. There is paucity of data to guide the decision regarding repeat endoscopy in GI GVHD patients on high dose steroids and ongoing diarrhea. Determination of the yield of repeat endoscopy on in the assessment of GI GVHD may lead to better indications for the procedure, enhanced risk-benefit analysis by physicians and improved long-term outcomes. In this retrospective study, we reviewed allo-HCT patients who remained symptomatic despite getting treatment based on the first endoscopy and subsequently underwent the second endoscopy. We also documented the histological/microbiological changes in their repeat endoscopy and their impact on determining a change in GVHD management.
2. MATERIAL AND METHODS
2.1. Patients
Medical records from 318 patients who underwent allo-HCT at the University of Iowa from 2012 to 2016 were retrospectively reviewed. Adult patients who underwent at least two endoscopies for the clinical suspicion of GI GVHD were included in the current study. Demographic information, disease characteristics, HCT data, and histological findings during first and second endoscopies were extracted from medical records. We also noted any changes in disease management based on repeat endoscopy findings as well as any complications from the procedure. Patients with grades 3–4 thrombocytopenia at the time of the procedure were transfused to maintain platelet counts of 30,000 to 50,000/µL for the procedure. This retrospective study was approved by the institutional review board.
2.2. Endoscopy and Infectious Work Up
The most common indication for the first endoscopy was diarrhea followed by nausea/vomiting and anorexia. Patients were evaluated with upper GI endoscopy or both upper and lower GI endoscopy (sigmoidoscopy or colonoscopy) according to the presenting symptoms. All endoscopies (upper and lower) were performed at the tertiary care center with standard preparation. Infection workup was performed immediately in symptomatic patients and endoscopies were performed within 48 hours if GI GVHD was suspected. Infectious workup included stool enteric pathogenic panel, stool Clostridium difficile toxin assay, blood cultures, weekly CMV polymerase chain reaction on blood, and histopathological testing of biopsy samples for CMV.
2.3. Treatment Protocols
The standard front-line treatment for GI GVHD consisted of corticosteroid therapy at a dose of 1–2 mg/kg/day. Any ongoing therapy with calcineurin inhibitors/mycophenolate mofetil (MMF) was continued. Treatment was modified appropriately (escalated/de-escalated) based on response to first-line corticosteroid therapy. First-line therapy for C. difficile infection was oral vancomycin; ganciclovir/foscarnet was given for CMV disease. Decisions regarding the timing of starting treatment and endoscopies were made at the discretion of the treating physician.
2.4. GVHD Diagnosis and Grading System
GVHD was diagnosed based on clinical suspicion, endoscopic appearance of the gut and pathological evaluation of biopsy samples. Histological grading of intestinal GVHD was uniformly applied to biopsy samples (Table 1) [18].
For the purpose of this study, we relied on histological grading of GVHD.
| Grade | Histological Feature |
|---|---|
| 1 | Isolated apoptotic epithelial cells without crypt loss |
| 2 | Loss of isolated crypts without the loss of contiguous crypts |
| 3 | Loss of 2 or more contiguous crypts |
| 4 | Extensive crypt loss with mucosal denudation |
aGVHD, acute graft versus host disease.
3. RESULTS
Among the 318 patients who received allo-HCT between 2012 and 2016, 24 underwent GI endoscopy at least twice for suspicion of GVHD. Patient and allo-HCT characteristics are summarized in Table 2. First endoscopies were performed at a median interval of 81 days (range 24–158 days) post allo-HCT (10 patients had lower endoscopy only, 2 had upper endoscopy only, and 12 had both upper and lower endoscopy). The most common presenting symptom prompting endoscopy was diarrhea. A repeat endoscopy was performed at a median interval of 42 days (range 16–74 days) after the first (7 patients had lower endoscopy only, 3 had upper only, and 14 patients had both upper and lower endoscopy). The most common presenting symptom prompting repeat endoscopy was persistent diarrhea followed by hematochezia.
| Age (median, range) | 55 years (18–72) |
| Gender (M/F) | 13/11 |
| Donor | |
| HLA matched related donor | 5 |
| HLA matched unrelated donor | 13 |
| HLA mismatched -unrelated donor | 5 |
| HLA mismatched cord blood | 1 |
| Diagnosis | |
| Acute lymphoblastic leukemia (ALL) | 4 |
| Acute myelogenous leukemia (AML) | 7 |
| Chronic myelogenous leukemia (CML) | 1 |
| Myelodysplastic/myeloproliferative diseases | 10 |
| Non-Hodgkin lymphoma (NHL) | 2 |
| GVHD Prophylaxis | |
| Tacrolimus, MMF | 2 |
| Tacrolimus, MMF, ATG | 5 |
| Tacrolimus, MTX, ATG | 10 |
| Tacrolimus, MTX | 7 |
MTX; Methotrexate; MMF: Mycophenolate mofetil; ATG: Antithymocyte globulin.
Baseline transplant characteristics.
At first endoscopy, 20 patients showed abnormal findings. These included 16 with GVHD alone, 1 with GVHD and CMV, and 3 with GVHD and non-CMV infectious colitis. Marked improvement was observed in histology on repeat endoscopy in 10 out of 20 patients, despite persistent symptoms. Among these 10 patients, 2 with grade III GVHD, 2 with grade IV GVHD, and 6 with either grade I-II GVHD alone or in combination with other infections, repeat endoscopy showed histological improvement leading to treatment de-escalation. Significant worsening of histological findings of GVHD or emergence of infection was observed in 7 patients on repeat endoscopy (4 had worsening of GVHD on histological grading and 3 were found to have CMV infection). These findings led to escalation of therapy via the addition of an anti-viral drug or of a second-line agent for the treatment of GVHD. In 3 patients with Grade IV GVHD on the first endoscopy, the histological findings were unchanged on the second procedure (Figure 1; Table 3).
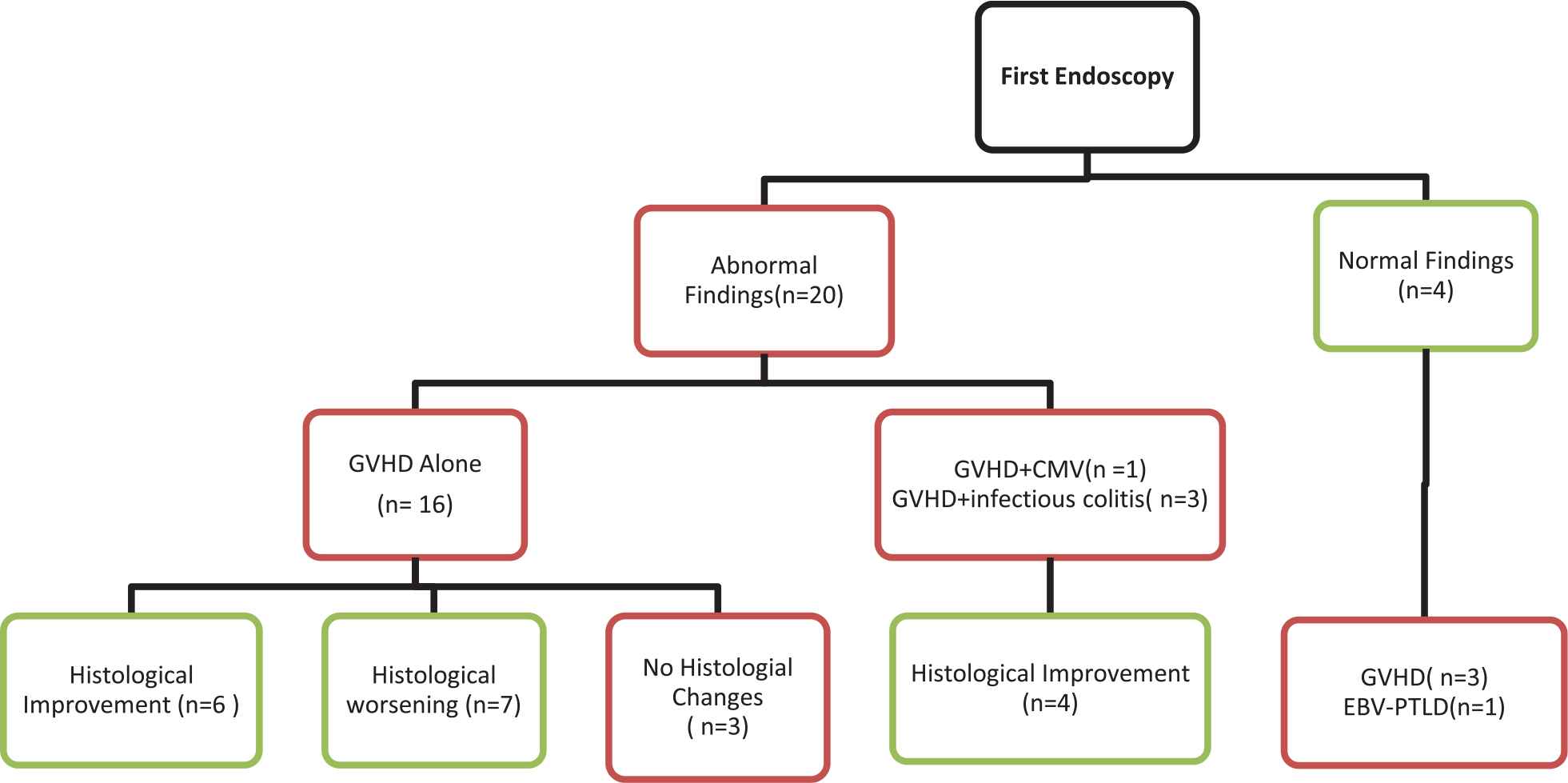
Histological changes during first and repeat endoscopy.
| First Endoscopy |
Second Endoscopy |
Changes Observed |
||||
|---|---|---|---|---|---|---|
| Pts | Type of Endoscopy | Histological Finding | Type of Endoscopy | Histological Findings | Change in Pathology Findings | Change in GVHD Management |
| 1 | Sigmoidoscopy | GVHD Grade 1 | Upper and lower endoscopy | No GVHD | Yes | De-escalation |
| 2 | Sigmoidoscopy | No GVHD | Upper and lower endoscopy | GVHD Grade 4 | Yes | Escalation |
| 3 | Upper and Lower Endoscopy | GVHD Grade 4 | Upper and lower endoscopy | GVHD Grade 4 | No | Continued management |
| 4 | Upper and Lower Endoscopy | GVHD Grade 3 | Upper and lower endoscopy | GVHD Grade 3 + CMV | Yes | Escalation with addition of anti-viral |
| 5 | Upper and Lower Endoscopy | GVHD Grade 4 | Upper and lower endoscopy | GVHD Grade 4 | No | Continued Management |
| 6 | Colonoscopy | GVHD Grade 2 | Colonoscopy | GVHD Grade 4 | Yes | Escalation |
| 7 | Colonoscopy | GVHD Grade 4 | Colonoscopy | GVHD Grade 1 | Yes | De-escalation |
| 8 | Upper and Lower Endoscopy | GVHD Grade 2 | Colonoscopy | GVHD Grade 1 + CMV | Yes | Escalation with addition of anti-viral |
| 9 | Upper Endoscopy | GVHD Grade 2 | Upper endoscopy | No GVHD | Yes | De-escalation |
| 10 | Upper and Lower Endoscopy | GVHD Grade 2 | Upper and lower endoscopy | GVHD Grade 3 | Yes | Escalation |
| 11 | Upper and Lower Endoscopy | GVHD Grade 1 + CMV | Colonoscopy | GVHD Grade 1 | Yes | De-escalation |
| 12 | Colonoscopy | GVHD Grade 3 | Colonoscopy | GVHD Grade 4 | Yes | Escalation |
| 13 | Sigmoidoscopy | No GVHD | Upper and lower endoscopy | GVHD Grade 3 | Yes | Escalation |
| 14 | Upper and Lower Endoscopy | GVHD Grade 2 | Upper and lower endoscopy | GVHD Grade 3 | Yes | Escalation |
| 15 | Upper and Lower Endoscopy | GVHD Grade 3 | Upper and lower endoscopy | GVHD Grade 1 | Yes | De-escalation |
| 16 | Sigmoidoscopy | GVHD Grade 4 | Upper and lower endoscopy | GVHD Grade 2 | Yes | De-escalation |
| 17 | Upper Endoscopy | GVHD Grade 1 + infectious Colitis | Upper endoscopy | No GVHD | Yes | De-escalation |
| 18 | Colonoscopy | No GVHD | Upper and lower endoscopy | GVHD Grade 2 | Yes | Escalation |
| 19 | Upper and Lower Endoscopy | GVHD Grade 3 | Colonoscopy | No GVHD | Yes | De-escalation |
| 20 | Upper and Lower Endoscopy | GVHD Grade 4 | Upper and lower endoscopy | GVHD Grade 4 | No | Continued Management |
| 21 | Upper and Lower Endoscopy | GVHD Grade 4 | Colonoscopy | GVHD Grade 4 + CMV | Yes | Escalation |
| 22 | Upper and Lower Endoscopy | No GVHD | Upper endoscopy | EBV-PTLD | Yes | Escalation |
| 23 | Colonoscopy | GVHD Grade 1 + infectious Colitis | Upper and lower endoscopy | GVHD Grade 1 | Yes | De-escalation |
| 24 | Colonoscopy | GVHD Grade 1 + infectious Colitis | Upper and lower endoscopy | No GVHD | Yes | De-escalation |
GVHD: Graft versus Host Disease; CMV: Cytomegalovirus; PTLD: Post-transplant lymphoproliferative disorders.
Endoscopic and histological findings of the first and second endoscopies.
Four patients had normal endoscopic findings on their first endoscopy (2 sigmoidoscopies, 1 colonoscopy, and 1 upper and lower endoscopy). On repeat colonoscopy, one patient was found to have Grade I-II to GVHD, two showed grade III-IV GVHD, and one was found to have EBV-lymphoproliferative disorders (Figure 1; Table 3).
In our study, treatment was modified based on repeat endoscopy in 21 out of 24 patients. No significant complications of repeated procedures were noted.
4. DISCUSSION
We report here the findings of repeat endoscopy in patients with persistently symptomatic GI GVHD after adequate frontline treatment and whether these findings led to treatment modification in these patients. This study adds to the evidence that repeat endoscopy can guide treatment decisions in selected patients. The response evaluation to treatment in GI GVHD is currently limited to improvement in clinical symptoms, primarily improvement in diarrhea volume. The clinical improvement may lag despite improvement in colonic GVHD or risk of additional infections.
For the past two decades, various groups have investigated potential biomarkers to augment early and more accurate diagnosis along with risk stratification of GVHD patients. Two of the most informative biomarkers with the greatest relevance to GI GVHD are suppression of tumorigenicity 2 (ST2) and regenerating islet derived protein 3α (REG3α). [20,21]. Despite these encouraging early advancements in the pathophysiology of GI GVHD, inconsistent results have been reported about the outcomes of GVHD based on these biomarkers [22]. This inconsistency may be due to the heterogeneity of patients receiving various treatments as well as to variations in study protocols [22]. Recently, The Mount Sinai Acute GVHD International Consortium (MAGIC) combined ST2 and REG3α in the model known as MAGIC algorithm probability (MAP) to predict long-term outcomes in GI GVHD [23,24]. Since each of these biomarkers reflects a distinct aspect of GI GVHD pathology, their combination can potentially quantify the extent of crypt damage throughout the intestine. MAP measured at one week of steroid treatment can predict day-28 response, overall survival (OS) and NRM. Recent data are promising in showing that, when measured at four weeks of therapy, the MAP predicts both NRM and OS better than the clinical response to treatment. Major limitations in that study was that the common GVHD prophylaxis regimens of posttransplant cyclophosphamide, tacrolimus/sirolimus and T-cell depletion were used in very few of the patients, and the study did not demonstrate that therapeutic decisions based on biomarker probabilities can change the outcome for patients with GVHD [23].
There is an urgent need for novel biomarkers to monitor GVHD progression and to guide therapies for GI GVHD, as well as to identify the emergence of infection in these patients. The latter can confound the clinical symptoms attributed to GI GVHD, potentially leading to worse clinical outcomes. To date, biomarkers do not replace the need to repeat endoscopy, but should be used as a complementary modality to risk-stratify patients who are not responding to systemic therapy, and to help tailor GVHD treatment. In persistently symptomatic patients with GI GVHD despite receiving first line therapy, repeat endoscopy has been shown to be useful to evaluate the stage of disease and its response to the therapy, and to exclude superimposed or alternative diagnosis. A study by Martinez et al. reported that 71% of persistently symptomatic GI GVHD patients had different histological findings on repeat endoscopy, which led to changes in therapy in 77% of such patients [25]. Similarly, in another study, approximately one-quarter of the patients who did not respond to first-line GVHD treatment were found to have cytomegalovirus infection upon repeating the endoscopy [26]. We found similar results in our current study upon repeat endoscope. The overall rate of change in management was 87.5% after the second endoscopy. All patients with normal first endoscopy had abnormal histology on a repeat procedure resulting in management changes.
The current study provides preliminary data suggesting that repeat endoscopy can be of value, in the evaluation of GI GVHD patients with persistent symptoms after first-line treatment. Limitations of our data are their provenance from a single center, the lack of a control group, their historical nature of collection, and the small patient population. Additionally, the lack of a protocol may have contributed to patient and provider related bias to the study. Patient-related factors such as the severity of disease or clinical course may have dictated the need to repeat endoscopy. Similarly, some physicians may be more inclined towards repeating procedures. Since GI GVHD is a patchy disease, there may be some variability in tissue sampling as well.
In conclusion, repeat endoscopy findings such as improvement or emergence of an infection, or an etiology other than GVHD that was not evident by other standard of care investigations can clearly impact treatment decisions. It is less clear if change in treatment based on histological grading discovered on repeat endoscopy can improve GI GVHD outcomes, and this needs to be prospectively evaluated in a clinical trial.
CONFLICT OF INTEREST
The authors have no relevant competing interests to disclose.
AUTHORS' CONTRIBUTIONS
ES, UF, and MMS took direct care of patients, designed the study, and analyzed the data. ES wrote the manuscript. UF, BY, and MMS reviewed the manuscript.
Funding Statement
The authors have no financial conflict with the subject matter or materials discussed in the manuscript.
ACKNOWLEDGMENTS
Special thanks to Sarah Mott at the Department of Biostatistics at the University of Iowa Hospitals and Clinics to help us evaluate data and statistics. The authors gratefully thank Dr. Madiha Fyyaz at Miami Valley Hospital for help with the figure and tables.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Ehsan Shabbir AU - Umar Farooq AU - Burhan Yanes AU - Margarida Magalhaes-Silverman PY - 2020 DA - 2020/02/27 TI - Repeat Endoscopy Affects Patient Management in Gastrointestinal Graft-Versus-Host Disease JO - Clinical Hematology International SP - 69 EP - 73 VL - 2 IS - 2 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.200220.001 DO - 10.2991/chi.d.200220.001 ID - Shabbir2020 ER -
