Comparison of Conventional Cyclophosphamide versus Fludarabine-Based Conditioning in High-Risk Aplastic Anemia Patients Undergoing Matched-Related Donor Transplantation
 , Qamar un Nisa Chaudhry1, Tariq Mehmood Satti1, Syed Kamran Mahmood1, Tariq Ghafoor1,
, Qamar un Nisa Chaudhry1, Tariq Mehmood Satti1, Syed Kamran Mahmood1, Tariq Ghafoor1,  , Ghassan Umair Shamshad1, Nighat Shahbaz1,
, Ghassan Umair Shamshad1, Nighat Shahbaz1,  , Mehreen Ali Khan1, Tariq Azam Khattak1, Jahanzeb Rehman1, Muhammad Farhan1, Saima Humayun1, Humera Haq1, Syeda Ammaara Anwaar Naqvi1, Faiz Anwer2,
, Mehreen Ali Khan1, Tariq Azam Khattak1, Jahanzeb Rehman1, Muhammad Farhan1, Saima Humayun1, Humera Haq1, Syeda Ammaara Anwaar Naqvi1, Faiz Anwer2,  , Humayoon Shafique Satti3, Parvez Ahmed4
, Humayoon Shafique Satti3, Parvez Ahmed4- DOI
- 10.2991/chi.d.200426.001How to use a DOI?
- Keywords
- Aplastic anemia; Cyclophosphamide; Fludarabine; Stem cell transplantation
- Abstract
Allogeneic stem cell transplant for high-risk aplastic anemia (AA) yields inferior results using conventional cyclophosphamide (CY)-based conditioning. The use of fludarabine (Flu)-based regimens has resulted in improved outcomes in high-risk patients. Limited data are available comparing these two conditioning regimens in such patients. We retrospectively analyzed 192 high-risk patients undergoing matched-related donor transplantation from July 2001 to December 2018. The median age was 19.5 (2–52) years. Patients were divided into 2 groups, Cy200 anti-thymocyte globulin (ATG)20 (Gp1 n = 79) or Flu120–150 Cy120–160 ATG20 (Gp2 n = 113). The risk of graft failure was significantly higher in Gp1, and the majority occurred in patients with >2 risk factors (p = 0.02). The incidence of grade II-IV acute graft versus host disease (GVHD) and chronic GVHD was not significantly different between the two groups. The overall survival (OS) of the study cohort was 81.3 %, disease-free survival (DFS) 76.6 % and GVHD-free relapse-free survival (GRFS) was 64.1%. DFS and GRFS were significantly higher in Gp2 as compared to Gp1: DFS 84.1% versus 68.4 % (p = 0.02), GRFS 77.9% versus 54.4% (p = 0.01), respectively. We conclude that Flu-based conditioning is associated with superior OS, DFS and GRFS as compared to the conventional Cy-based regimen in high-risk AA.
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Allogeneic hematopoietic stem cell transplant (HSCT) is the standard treatment for patients younger than 40 years with severe and very severe aplastic anemia (AA) who have a matched-related donor (MRD) [1]. A combination of cyclophosphamide (CY) at 200 mg/kg plus rabbit anti-thymocyte globulin (ATG) conditioning regimen, cyclosporine and methotrexate (MTX) for graft-versus host disease (GVHD) prophylaxis and bone marrow harvest (BMH) as stem cell source is considered preferable in MRD transplants [2]. Different studies have shown that increasing age, heavy pretransplant transfusion burden and prolonged disease duration before transplant are associated with inferior outcomes in AA patients [3,4]. In developing countries like Pakistan, the majority of patients have one or more of these risk factors at the time of transplant, and are thus considered high risk for graft failure (GF), GVHD and infectious complications posttransplant. Our institutional experience and different studies across the globe have suggested that fludarabine (FLU)-based conditioning regimens reduce GF, improve engraftment, reduce regimen-related toxicities and improve overall survival (OS) in these high-risk patients [5–8]. We recently published our single center experience using different FLU-based conditioning, and documented the efficacy of the different regimens used [8]. Despite favorable results with FLU-based conditioning [9,10], there is still no consensus on optimal regimen for these high-risk AA patients, and CY-ATG is still considered the standard of care for AA patients without giving due consideration to the presence of high-risk features. The purpose of this retrospective analysis is to compare the outcome of the two most frequently used regimens, CY-ATG versus FLU-CY-ATG, to identify the preferable conditioning regimen for high-risk AA patients.
2. MATERIALS AND METHODS
We retrospectively analyzed data from 241 AA patients who received MRD transplant using either conventional CY at 200 mg/kg and ATG at 20 mg/kg or FLU at 120–150 mg/m2, CY at 120–160 mg/kg and ATG at 20 mg/kg conditioning at the Armed Forces Bone Marrow Transplant Center/National Institute of Blood and Marrow Transplant (AFBMTC/NIBMT) from July 2001 to December 2018. Twenty-seven patients did not have any high-risk features and were excluded from the study. We identified 22 patients who had active infection at the time of transplantation and were taken to transplant as only available salvage therapy; these were also excluded from the study. Data analysis was done on 192 patients fulfilling the study inclusion and exclusion criteria. AA was defined as pancytopenia and hypocellular bone marrow in the absence of abnormal infiltrate or bone marrow fibrosis. We used Camitta et al.'s [11], and Bacigalupo et al.'s [12] criteria for grading the severity of AA. As per the AFBMTC/NIBMT defined criteria, patients were considered as high risk if they had 1 or more of the following features: Age ≥20 year, previous HSCT, time from diagnosis to transplant >3 months, >20 red cell transfusions or >50 platelet transfusions [8]. This score is currently being validated using a prospective trial at our center.
DNA-based low/intermediate resolution typing was done for HLA-class I and class II antigen matching. Recipients received grafts from 6/6 HLA-matched sibling or family donors (185 siblings, 6 parents, 1 nephew).
The inclusion criteria were high-risk AA patients receiving MRD HSCT with either conventional CY200 ATG20 or Flu120–150 CY120–160 ATG20) conditioning; age >2 years, absence of severe infection at the time of transplant; ejection fraction >50%; serum creatinine <130 µmol/L; serum bilirubin <34 µmol/L; pulmonary functions >70% of predicted and informed written consent for recipient and donor. Exclusion criteria included high hematopoietic cell transplantation-comorbidity index (HCT-CI), paroxysmal nocturnal hemoglobinuria (PNH) clone with clinical evidence of hemolysis, presence of somatic abnormalities reflective of constitutional bone marrow failure [13], positive cytogenetic studies for chromosomal breakage, pregnancy, Karnofsky score <70% and patients lost to follow up. The study was approved by the Institute's Ethical Review Board and Research Department.
2.1. Transplantation and Supportive Care
The AFBMTC/NIBMT is the only purpose-built Transplant center of Pakistan, currently carrying out around 100 allogeneic HSCT per year. It complies with international guidelines on the protective environment for HSCT published in 2009 [14]. Patients were admitted to isolation rooms with laminar airflow and HEPA filters. Antiviral, antifungal, and Pneumocystis jirovecii (PJP) prophylaxis were administered to all transplant recipients. We used a preemptive strategy for cytomegalovirus (CMV) treatment: ganciclovir/valganciclovir was given if >2,000 CMV copies were detected, and continued until 2 consecutive PCR results were negative.
AA is the leading transplant indication at our center since 2001 [15]. Patients receiving conventional CY-ATG conditioning were labeled as group Gp1 and those receiving FLU-CY-ATG were labeled as Gp2. Stem cell sources included BMH alone, BMH combined with peripheral blood stem cell (PBSC) and PBSC alone. PBSC alone was used due to donor choice, major ABO mismatch, and disparity of age and weight between donor and recipient.
Cyclosporine alone and cyclosporine plus a short course of MTX (10 mg/m2 on day +1, 8 mg/m2 on day +4 and day +7) were used as GVHD prophylaxis. Whole blood and lineage-specific chimerism were used for posttransplant monitoring on days +28, +100 and +180, or as needed per clinical indication, e.g. drop in blood counts.
Neutrophil engraftment was defined as the first of three consecutive days with ANC >0.5 × 109/L and platelet engraftment as unsupported platelet count >20 × 109/L for seven days. Primary graft failure (PGF) was defined as the failure to achieve neutrophil engraftment by day +28 and secondary GF as persistent neutropenia (ANC <0.5 × 109/L) after initial engraftment. Acute GVHD (aGVHD) was diagnosed by the presence of skin rash, loose stools and jaundice early posttransplant (<100 days), confirmed with or without biopsy and graded according to the Glucksberg criteria [16]. Chronic GVHD (cGVHD), clinically diagnosed as per NIH criteria, was divided into limited and extensive disease [17]. The primary endpoint was OS. Secondary endpoints were day-100 transplant-related mortality (TRM), disease-free survival (DFS), GVHD-free relapse-free survival (GRFS), aGVHD, cGVHD and infectious complications. TRM was defined as death within 100 days due to any transplant-related cause other than disease relapse. OS was defined as the time from HSCT to death from any cause. DFS was calculated as survival in the absence of rejection, and GRFS included patients who were alive, free of GVHD and disease relapse on the last evaluation.
3. STATISTICAL ANALYSIS
Kaplan-Meier survival curves were used to estimate OS, DFS and GRFS. Patients were censored at the time of last follow-up and differences in survival were compared using the log rank test. We used univariate and multivariate Cox regression analysis to determine the significance of different variables as per conditioning regimen used, and their effect on survival. The chi-square test was used to compare transplant and patient-related categorical variables between conditioning regimens, while a Student's t-test or a Mann–Whitney U-test was used for continuous variables. The Fisher's exact test was used to confirm chi-square results when less than 5 observations were present in any group. We considered a p = 0.05 or less to be significant, and used SPSS version 23.0 (IBM; NY; USA) to complete our statistical analysis.
4. RESULTS
4.1. Patient Characteristics
As per inclusion and exclusion criteria, we identified 192 patients who had received either Cy200 ATG20 (Gp1 n = 79) or Flu120–150 Cy120–160 ATG20 (Gp2 n = 113) conditioning. The median age was 19.5 years (range 2–52 years). None of the patients >40 years received HSCT in Gp1 while 3 were transplanted in Gp2. The study cohort included 150 males and 42 females (3.5:1).
The median time from diagnosis to transplantation was 10 months and it ranged from 1.5 to 132 months. Nine patients (4.6%) received immunosuppression pre-HSCT. Cytogenetic records were available for 164 patients. Cytogenetic abnormalities were detected in only 4. These included trisomy 8 in 2 patients, del13q in 1 and del20q in 1. Two patients (1%) received rabbit ATG and cyclosporine, and seven (3.6%) received cyclosporine alone. None of the patients had a hematologic response to immunosuppression. Four patients (2%) received second HSCT for secondary GF. Three of these four had received CY at 200 mg/kg and ATG-F 20 mg/kg as first conditioning regimen, while 1 had initially received FLU at 150 mg/m2; CY at 300 mg/m2 and ATG at 20 mg/kg. A PNH clone was present in 14.6% of the cohort, but was clinically insignificant, without hemolysis, in all patients. Comparison of demographic characteristics of the study population as per conditioning regimen showed that patients in the Gp2 (FLU-based conditioning) were older (p = 0.01), had longer duration of disease from diagnosis to transplant (p = 0.024), received more red cell and platelet transfusions (p = 0.001 and <0.001, respectively). This group included all four patients who had failed previous HSCT (Figure 1). Patients' characteristics according to the type of conditioning regimen used are summarized in Table 1.
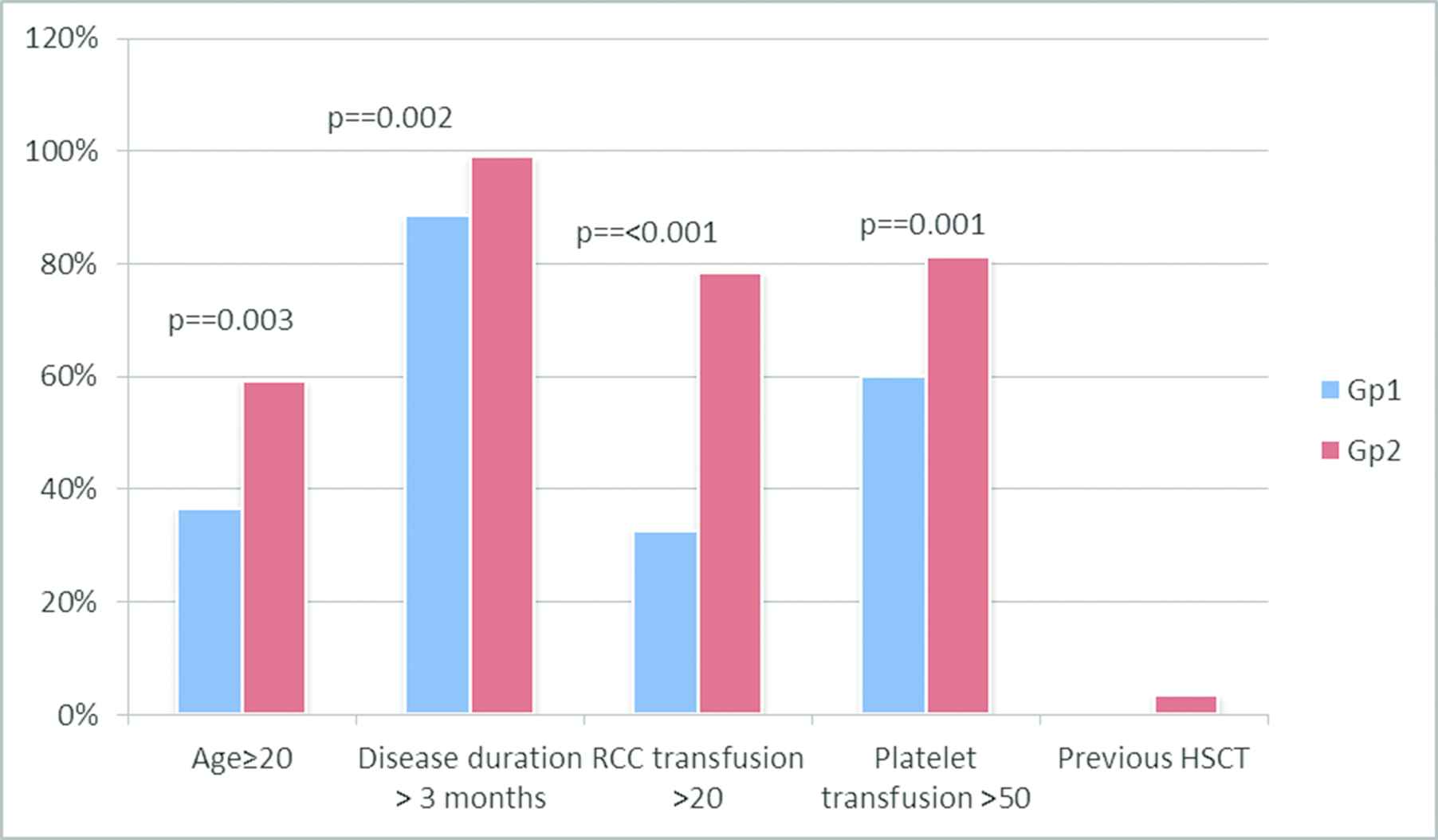
Comparison of the frequency of high-risk factors in patients receiving Gp1 and Gp2 conditioning. Patients in Gp2 had older age, prolonged disease duration prior to transplant, and had received higher number of RCC and platelet transfusions.
| Ser No | Patient Characteristics | Conditioning Regimen |
p | |
|---|---|---|---|---|
| Gp1a | Gp2a | |||
| 1. | Number of patients (n) | 79 | 113 | 0.44 |
| 2. | Gender male: female | 2.4:1 | 4.9:1 | 0.05 |
| 3. | Age years median (range) | 16 (2–38) | 21 (3–52) | 0.01 |
| 4. | Age groups n(%) | 0.01 | ||
| >2–10 years | 19 (24) | 11 (9.7) | ||
| 11–20 years | 34 (43.2) | 43 (38.3) | ||
| 21–30 years | 19 (24) | 48 (42.4) | ||
| 31–40 years | 7 (8.8) | 8 (7) | ||
| >40 years | 3 (2.6) | |||
| 5. | Prior ATG n(%) | 1 (50) | 1 (50%) | – |
| 6. | Prior cyclosporine | 4 (57.1) | 3 (42.9) | 0.52 |
| 7. | Time from diagnosis to transplant months, (range) | 6 (1.5–76) | 13 (2–132) | 0.024 |
| 8. | PNH clone (41 evaluable cases n (%) | – | 6 (14.6) | 0.392 |
| 9. | Previous HSCT; n (%) | – | 4 (3.5) | 0.145 |
| 10. | Serum ferritin ng/mL; median (range) | 1015 (169–3161) | 2840 (63–5400) | 0.042 |
| 11. | RCC transfused; median (range) | 20 (2–120) | 38 (1–200) | 0.001 |
| 12. | Platelets transfused; median (range) | 50 (3–400) | 111 (13–400) | <0.001 |
| 13. | Donor age; years (median: range) | 17 (3–45) | 22 (2–50) | 0.361 |
Gp1 and Gp2 denotes patients receiving Cy200/ATG20 and Flu120–150 Cy120 ATG20 respectively.
N: number; PNH: paroxysmal nocturnal hemoglobinuria; HSCT: hematopoietic stem cell transplant; RCC: red cell concentrate; NSAA: nonsevere aplastic anemia; SAA: severe aplastic anemia; VSAA: very severe aplastic anemia; UPN: unique patient number.
Demographic characteristics of study population.
4.2. Transplant Characteristics and Engraftment
We started using FLU-based conditioning at our center in 2004, and then increased its use after encouraging results. Our experience of FLU with reduced dose CY (300 mg/m2) showed inferior results, due to a higher frequency of primary and secondary GF [8], so this regimen is no longer used for high-risk patients at our center. We used BMH alone as a stem cell source in 97 patients (50.5%), combined BMH and PBSC harvest in 82 (42.7%) and PBSC alone in 13 (6.8%) patients. Transplant characteristics and engraftment details are summarized in Table 2.
| Ser. No | Transplant Characteristics | Gp1 (Cy-ATG) | Gp2 (Flu-Cy-ATG) | p |
|---|---|---|---|---|
| n = 79 | n = 113 | |||
| 1. | Stem cell source | 0.316 | ||
| BM | 28 (35.4 %) | 69 (61%) | ||
| BM + PBSC | 39 (49.3%) | 43 (38%) | ||
| PBSC | 12 (15.3%) | 1 (1%) | ||
| 2. | GVHD prophylaxis n(%) | <.001 | ||
| Cyclosporine | 32 (40.5%) | 92 (81.4%) | ||
| Cyclosporine plus methotrexate | 47 (59.5%) | 21 (18.6%) | ||
| 3. | Nucleated Cell dose (108/kg) median (range) | 4.92 (4.81–8.6) | 5.04 (2.9–8.79) | 0.599 |
| 4. | CD34 dose: (106/kg) median (range) | 7.8 (3.22–14.58) | 5.9 (1.53–11.7) | 0.034 |
| 5. | Neutrophil engraftment days: median (range) | 12.5 (9–20) | 13 (10–19) | 0.282 |
| 6. | Platelet engraftment days: median (range) | 21 (14–34) | 23 (14–43) | 0.923 |
| 7. | Major ABO mismatch; n (%) | 18 (22.7) | 25 (22.3) | 0.53 |
| 8. | Gender mismatch donor–recipient (female to male); n (%) | 27 (34.1) | 49 (43.3) | 0.231 |
BM: bone marrow; PBSC: peripheral blood stem cell; GVHD: graft versus host disease; TMA: thrombotic microangioapthy; CMV: cytomegalovirus.
Transplant characteristic of study population.
4.3. Transplant Complications
Transplant complications in the study cohort are summarized in Table 3. Primary GF occurred in 8 (4.1%) and secondary GF in 10 (5.2%) patients. GF was more common in Gp1 (15.1%) as compared to Gp2 (5.2%) p = 0.03. The majority of GF (86.3%) occurred in patients with >2 risk factors (p = 0.02). The cumulative incidence of grade II-IV aGVHD was 8.1% (8.8% in Gp1 and 7% in Gp2; p = 0.597) for the difference. Thirty (15.6 %) patients developed cGVHD, limited in 13 (6.7%) and extensive in 17 (8.85%). Chronic GVHD was more common in Gp1 (p = 0.03). There was no effect of the type of GVHD prophylaxis used (cyclosporine alone or cyclosporine plus MTX) on the frequency and severity of acute or chronic GVHD in the whole study cohort (p = 0.951) and between either group (p = 0.34). The type of GVHD prophylaxis had no significant effect on the outcome. OS in cyclosporine alone and cyclosporine plus MTX was 78.1% and 78.3% in Gp 1(p = 0.163) and 85% and 83.7% in Gp2 (p = 0.671), respectively. Similarly, DFS was not different between the 2 groups (p = 0.391) (Figure 2).
| Ser no | Complication n (%) | Gp1 (n = 79) | Gp2 (n = 113) | p |
|---|---|---|---|---|
| 1. | Graft failure | 0.03 | ||
| Primary | 4 (5) | 4 (3.5) | ||
| Secondary | 8 (10.1) | 2 (1.7) | ||
| 2. | Acute GVHD Grade II-IV | 7 (8.8) | 8 (7) | 0.59 |
| 3. | Chronic GVHD | 17 (21.5) | 13 (11.5) | 0.03 |
| 4. | TMA | 2 (2.5) | 7 (6.1) | <0.001 |
| 5. | CMV reactivation (10 evaluable patients in Gp1 and 41 evaluable patients in Gp2) | 4 (40) | 14 (34) | 0.23 |
| 6. | Tuberculosis reactivation | 5 (6.3) | 13 (11.5) | 0.134 |
| 7. | Serum sickness | 2 (2.5) | 3 (2.6) | 0.419 |
| 8. | Erythrocytosis | 3 (3.7) | 10 (8.8) | 0.137 |
| 9. | Hemorrhagic cystitis | 15 (18.9) | 9 (7.9) | 0.03 |
GVHD: Graft versus host disease; TMA: thrombotic microangiopathy; CMV: cytomegalovirus.
Comparison of transplant complications.
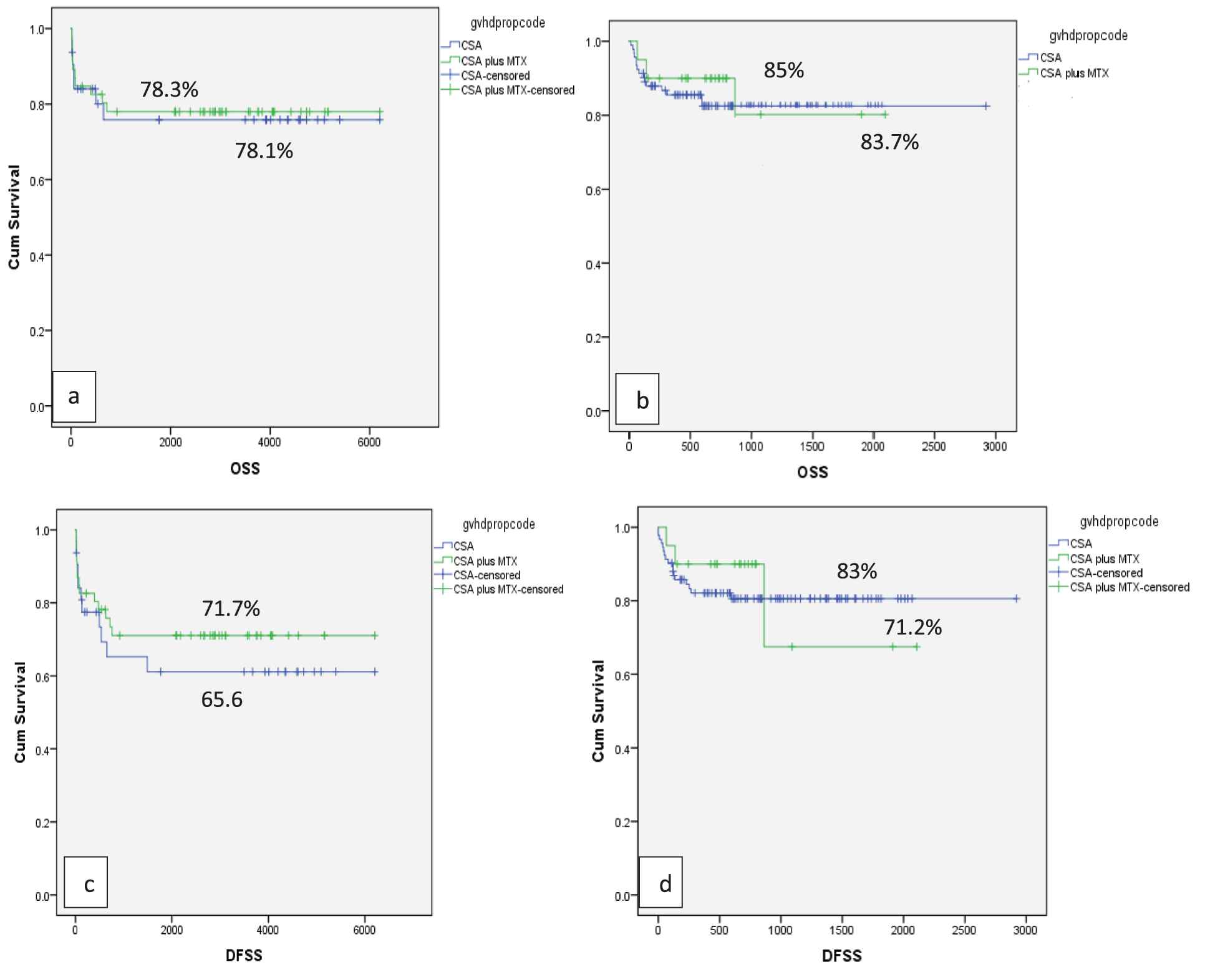
Effect of GVHD prophylaxis on OS and DFS (a) In Gp1: OS with Cyclosporine (blue line) alone and Cyclosporine plus MTX (green line) was 78.1% and 78.3% respectively with p = 0.163 (b) In Gp2: OS with Cyclosporine alone (blue line) and Cyclosporine plus MTX (green line) was 85% and 83.7% respectively with p = 0.671 (c) In Gp1: DFS 65.6% in Cyclosporine alone (blue line) while 71.7 % in Cyclosporine plus MTX (green line), p = 0.165 (d) In Gp2: DFS 83% in Cyclosporine alone (blue line) while 71.2 % in Cyclosporine plus MTX (green line), p = 0.06. OS: overall survival; DFS: disease-free survival; MTX: methotrexate; GRFS: graft versus host disease free relapse free survival.
Hemorrhagic cystitis (HC) was seen in 12.5% of patients. Patients receiving the Gp1 conditioning had a significantly higher frequency of HC (18.9 %) as compared to Gp2 (7.9 %) p = 0.03. In patients with HC, BKV reactivation was detected by PCR in four patients (2.7%), being 3 in Gp1 and 1 in Gp2. Transplant-associated thrombotic microangiopathy (was more common in Gp2 (p ≤ 0.001). CMV reactivation was seen in 4 out of 10 patients in Gp1 (40%) and 14 out of 41 evaluable patients in Gp2 (34%). Reactivation of tuberculosis occurred in eighteen patients (9.3%) and the risk was not different between the 2 groups (p = 0.134).
Erythrocytosis was an unusual complication seen in 13 patients (6.7%), at a median of 12 months after transplant. Ten patients (8.8%) belonged to Gp2 while 3 (3.7%) belonged to Gp1 (p = 0.137).
5. SURVIVAL
The median follow-up of entire study group was 36 (7–206) months, being 39 months (13–206) in Gp1 and 33 (7–165) in Gp2 (p = 0.391). The day-100 TRM was significantly higher in patients receiving the Gp1 (17.7%), as compared to the Gp2 conditioning (7.9%; p = 0.04). The OS of the study cohort was 81.3 %, DFS was 76.6 % and GRFS was 64.1%.
When patients were stratified into two groups, patients receiving Gp2 conditioning had a nonsignificant higher probability of OS as compared to Gp1 conditioning: 85.8 % versus 77.2% (p = 0.15), respectively. DFS and GRFS were significantly higher in Gp2 as compared to Gp1: DFS of 84.1% versus 68.4 % (p = 0.02), GRFS of 77.9% versus 54.4% (p = 0.01), respectively (Figure 3).
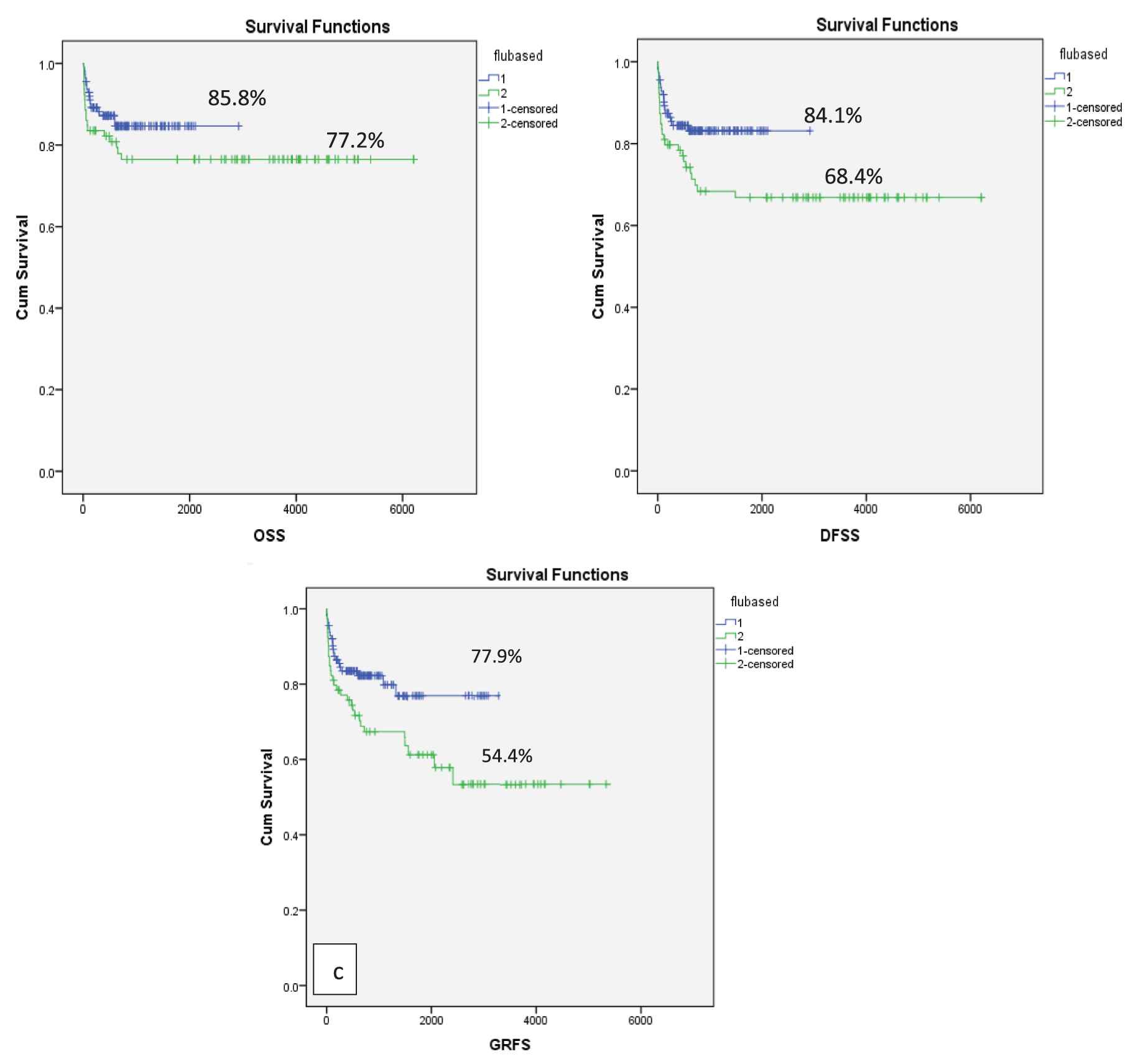
OS, DFS and GRFS as per conditioning regimen Gp1 and Gp2(a) OS of 77.2% in Gp1 and 85.8% in Gp2, p = 0.15 (b) DFS of 68.4 % in Gp1 and 84.1% in Gp2 (p = 0.02) (c) GRFS of 54.4% in Gp1 and 77.9% in Gp2, p = 0.01. OS: Overall survival; Flu: Fludarabine; Gp: group; GRFS: graft versus host disease relapse-free survival.
The FLU-based conditioning was associated with a better outcome than that of to the conventional CY + ATG regimen, when compared for different stem cell sources. The OS among Gp1 (CY + ATG) and Gp2 (FLU-CY-ATG) was 78.6% versus 82.4% for BMH, 74.4% versus 93% for BMH + PBSC and 83.3% versus 100% in the case of PBSC (only 1 patient in Gp 2 received PBSC and is alive) (p = 0.054). The DFS in Gp1 versus Gp2 was 64.3% versus 79.4% (BMH), 71.8% versus 93% (BMH + PBSC) and 66.7% versus 100% for PBSC only (p = 0.006).
Overall, 36 patients died after transplantation, the most common cause of death being GF (5.2 %; n = 10) and infection (5.2; n = 10). Six patients died of primary and 4 of secondary GF. Causes of death per conditioning regimen are summarized in Table 4.
| Ser no | Complication n (%) | Gp1 | Gp2 |
|---|---|---|---|
| 1. | Graft failure | ||
| Primary | 3 (3.7) | 3 (3.5) | |
| Secondary | 7 (8.8) | 1 (0.7) | |
| 3. | GVHD | 2 (2.5) | 2 (2.5) |
| 4. | TMA | 1 (1.2) | 4 (3.5) |
| 5. | Infection | 6 (7.5) | 4 (3.5) |
| 6. | Secondary malignancy | 1 (1.2) | – |
| 7. | Hepatic failure | 1 (1.2) | 1 (0.8) |
| 8. | Bleeding | 1 (1.2) | 2 (2.5) |
| 9. | TRALI | 1 (1.2) | – |
GVHD: Graft versus host disease; TMA: thrombotic microangiopathy; CMV: cytomegalovirus.
Cause of death.
When data were split into three quartiles (2001–2008; 2009–2014; 2015–2019) patients transplanted with FLU-based conditioning had superior OS, DFS, GRFS in all quartiles after adjusting for co-variates, indicating that the benefit of FLU remained consistent over long-time periods in high-risk patients. The majority of patients (67%) had >2 risk factors in our study; therefore, we investigated if the type of conditioning regimen influenced the survival in this individual cohort. The OS and DFS were significantly better in patients with ≤2 risk factors (p ≤ 0.001). The use of FLU-based conditioning led to better OS and DFS in patients with >2 risk factors as compared to the CY-ATG conditioning (Figure 4).
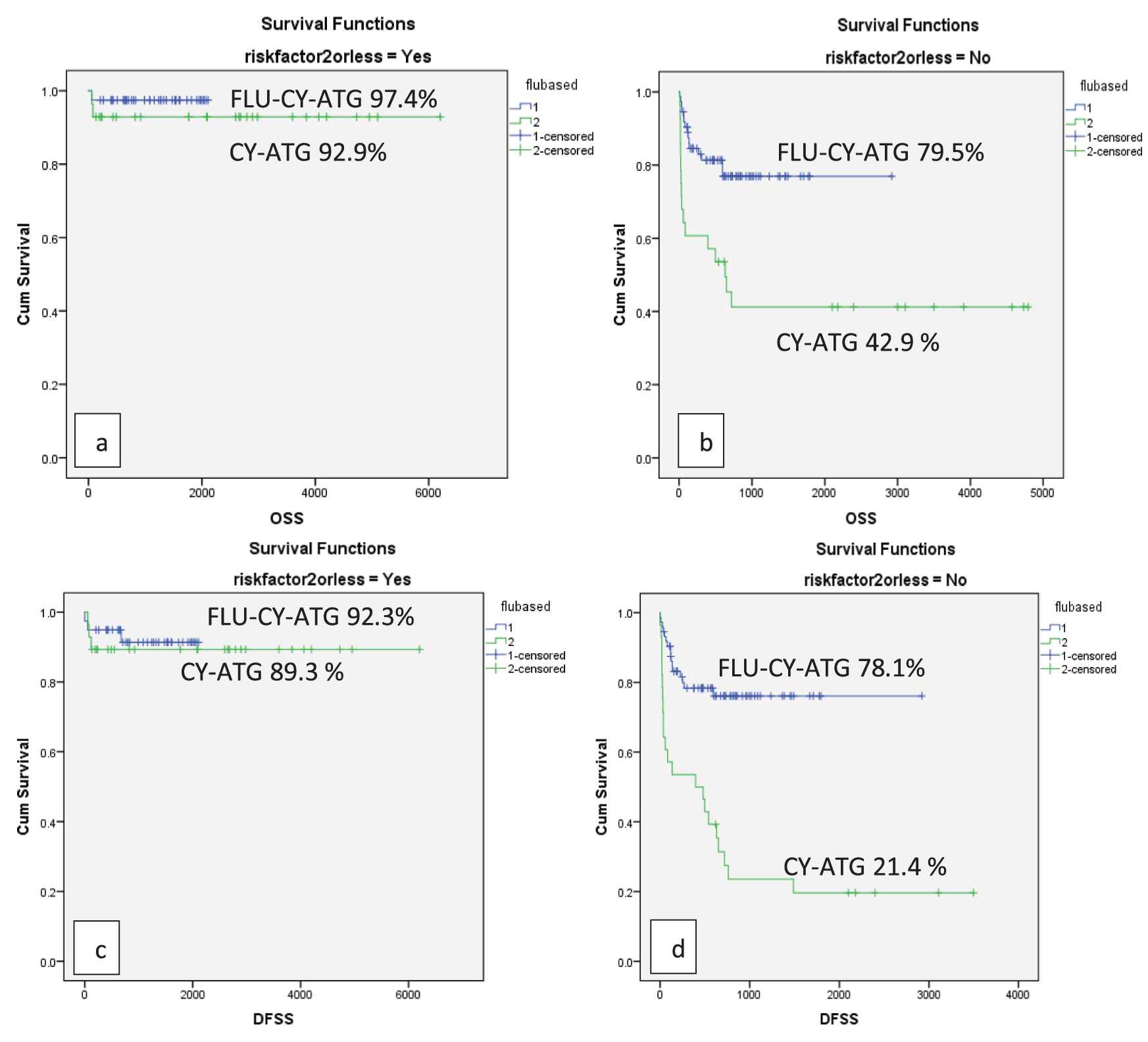
OS and DFS as per number of high-risk features in Gp1 and Gp2(a) OS of 92.9% in patients with ≤2 risk factors in Gp1 and 97.4% in Gp2 (p = 0.002) (b) OS of 42.9% in patients with >2 risk factors in Gp1 and 79.5% in Gp2 (p ≤ 0.001) (c) DFS of 89.3% in patients with ≤2 risk factors in Gp1 and 92.3% in Gp2 (p ≤ 0.001) (d) DFS of 21.4 % in patients with >2 risk factors in Gp1 and 78.1% in Gp2 (p ≤ 0.001).
We performed a Cox regression analysis on OS, DFS and GRFS using patient age, transfusion of RCC and platelets, disease duration before transplant and conditioning regimen as associated factors. This analysis revealed that all 3 parameters remained significantly superior in the FLU-based conditioning regimen, even after correcting for all the above factors (p = 0.001).
6. DISCUSSION
MRD transplant is the treatment of choice for younger patients with severe and very severe AA. Being a nonmalignant disorder, the goal of transplantation in AA is to achieve long-term GRFS [18]. In Western countries, early diagnosis and transplantation, better supportive care, use of CY + ATG conditioning, BMH as stem cell source and use of cyclosporine plus MTX for GVHD prophylaxis has resulted in significant reduction in GF, GVHD, improvement in OS and GRFS [19,20], with documented engraftment rates of 95% and OS nearing 90% [21].
On the contrary, the management of AA in developing countries is challenging. The prevalence of AA is higher as compared to the Western populations [22] and, yet, there is paucity of information regarding mutational profile and disease pathogenesis in this region [23]. Delay in diagnosis and referral, long waiting time before transplant, lack of finances, use of non-leuko-depleted blood products, family donations and recurrent infections pre-transplantation result in allo-immunization, GF, GVHD and increased risk of infectious and noninfectious complications. The presence of these risk factors leads to the inferior overall outcomes of conventional transplantation strategies in these high-risk patients [4,24,25].
The CIBMTR data on HLA-matched HSCT for severe AA showed a 5-year survival of 85% for patients <20 years and 64% for those >20 years using CY-ATG conditioning and PBSC harvest [26]. The EBMT data on 1,275 patients identified age≥20 years, time from diagnosis to transplant >114 days, absence of ATG in conditioning and use of conditioning other than CY200 mg/kg as negative predictors of survival, with an OS of 64% in patients with 3–4 negative predictors [4]. Saunders et al. reported that rejection rates among patients with or without prior transfusion were 22% and 10%, respectively [27]. Data from Japanese, Indian and Mexican studies suggested that >20 transfusions prior to transplantation were independently associated with high rates of GF, even after incorporation of ATG in the conditioning [6,28,29]. Lee et al. documented that higher pre-transplant transfusion (>32 RCC) is associated with increased TRM and reduced OS [3].
Different conditioning regimens were employed over the last 2 decades in an attempt to reduce GF, GVHD, TRM and improve outcome in these high-risk patients. However, most studies have remained limited to case series or single institutional data with limited number of patients and lack of consensus definition for high-risk AA [6,10,28,30].
FLU is a purine analog with potent immunosuppressive and lympho-depleting properties. When used in combination with CY, it works synergistically by inhibiting alkylation-induced DNA repair. It is well tolerated at older ages, has low cost and has proven to be beneficial in reducing GF in high-risk AA patients [30]. The toxicities of CY at the doses used in CY-ATG conditioning regimens are substantial, especially in high-risk patients who are already allo-immunized and have a longer duration of illness, with recurrent infections. Using FLU-CY-ATG conditioning allows a reduction in doses of CY without compromising the success of engraftment and, at the same time, reducing toxicities. Retrospective data from EBMT also suggested favorable outcomes with FLU-based regimens in older patients [7]. Early studies reported inferior OS in transfused patients as compared to those untransfused, due to graft rejection [31].
A retrospective analysis from three Indian Centers documented GF of 3.3% using FLU-based conditioning. However, the study included both high-risk and non-high-risk cases [32]. GF in our study was 9.7%, being higher in patients receiving CY-ATG (15.1%) as compared to those treated with FLU-CY-ATG (5.2%). These results are lower than the rates previously reported with CY-ATG [33]. In our study, the use of FLU-based conditioning was associated with low toxicity and 100-day mortality, as compared to the CY-ATG group (7.9% versus 17.7%), p = 0.04. Similar results were documented by George et al. [32]. A randomized trial comparing FLU-CY-ATG with CY-ATG in severe AA and hypoplastic myelodysplastic syndrome (MDS) documented GF rates of 13.4% versus 16.8%, respectively and OS of (85.6% versus 77.7%; p = 0.407). That study, however, did not report on DFS and GRFS, included fewer patients, included alternative donor transplants and patients with hypoplastic MDS. Nonetheless, results with FLU-CY-ATG showed a nonsignificant OS and lesser regimen-related toxicities [34]. In our study, OS and DFS were significantly better in patients with ≤2 risk factors as compared to those with >2 risk factors (p ≤ 0.001). In the latter group, the OS (76.7%) and DFS (75.3%) were significantly better in patients receiving FLU-based conditioning as compared to OS (42.9%) and DFS (21.4%) in those receiving CY + ATG (p ≤ 0.001), thus emphasizing that for patients with increased number of risk factors, FLU-CY-ATG remains the optimal choice.
In our study, PBSC was more used in Gp1 patients, because these were transplanted between 2002 and 2006, when there was a trend to use PBSC to facilitate early engraftment and reduce infection-related complications [35]. Previous studies had shown that the use of PBSC reduces the risk of graft rejection in multiply transfused patients [36]. Many centers in developing countries continue to use PBSC as the preferred graft source in AA, because the early and sustained engraftment achieved with these stem cells helps to salvage patients with ongoing infection, and reduces graft rejection [32].
Cyclosporine alone was used for GVHD prophylaxis in 124 (64.5%) and cyclosporine plus MTX in 68 (35.4%) cases. The latter combination was used more frequently in patients receiving the Gp1 conditioning CY-ATG (59.5%) as compared to 18.6% in Gp2 (p ≤ 0.001). Patients in Gp2 were older, highly immunized, had a longer duration of disease and were at a higher risk of GVHD as compared to Gp2. However, the use of cyclosporine plus MTX was not superior to cyclosporine alone in preventing acute or chronic GVHD (p = 0.951). The type of GVHD prophylaxis had no significant effect on improving OS or GRFS. This may be due to the mucosal injury caused by MTX, leading to increased expression of damage-associated molecular patterns (DAMP), which are a known trigger for GVHD [37,38]. More studies are needed to validate this finding, and the use of FLU was not associated with increased infectious complications. George et al. reported the usefulness of FLU-Cy conditioning in patients with fungal infections, documenting an OS of 61.2% [39].
We acknowledge that there are a few limitations to the study. It was a retrospective analysis and patients were not randomized. The FLU-based regimen was used for high-risk patients, who were older, heavily transfused and had a longer duration of disease. Despite these biases, patients with Gp2 conditioning had better OS, DFS and GRFS, indicating that the survival could have been better in Gp2 if a more homogenous patient selection was done. The study by Qamar et al. [8] and the current one provide further evidence that FLU-based conditioning provides favorable outcomes in high-risk AA patients undergoing MRD. Furthermore, the AFBMTC defined high-risk criteria that can be used to develop risk adopted approach for selection of conditioning regimens in AA patients.
7. CONCLUSION
FLU-based conditioning is better tolerated with lower TRM and is associated with lower rates of rejection, better OS, DFS and GRFS as compared to conventional CY-based conditioning. The survival benefit is more pronounced in patients with more than 2 risk factors. Bone marrow as a source of stem cells and cyclosporine alone as GVHD prophylaxis are the preferable options. A randomized control trial of FLU-based versus conventional CY-containing conditioning would be helpful in establishing a standard of care regimen in high-risk AA patients.
CONFLICT OF INTEREST
This manuscript contains original research not previously published or submitted for publication elsewhere while under consideration. The authors declare no conflict of interest with this manuscript.
Funding Statement
The authors have no financial conflict with the subject matter or materials discussed in the manuscript.
AUTHORS' CONTRIBUTIONS
IR, CQN and AP designed the study and contributed equally to this article. All authors contributed to data collection, analysis, literature review and reference citation.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Raheel Iftikhar AU - Qamar un Nisa Chaudhry AU - Tariq Mehmood Satti AU - Syed Kamran Mahmood AU - Tariq Ghafoor AU - Ghassan Umair Shamshad AU - Nighat Shahbaz AU - Mehreen Ali Khan AU - Tariq Azam Khattak AU - Jahanzeb Rehman AU - Muhammad Farhan AU - Saima Humayun AU - Humera Haq AU - Syeda Ammaara Anwaar Naqvi AU - Faiz Anwer AU - Humayoon Shafique Satti AU - Parvez Ahmed PY - 2020 DA - 2020/05/18 TI - Comparison of Conventional Cyclophosphamide versus Fludarabine-Based Conditioning in High-Risk Aplastic Anemia Patients Undergoing Matched-Related Donor Transplantation JO - Clinical Hematology International SP - 82 EP - 91 VL - 2 IS - 2 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.200426.001 DO - 10.2991/chi.d.200426.001 ID - Iftikhar2020 ER -
