How to Simplify the Evaluation of Newly Introduced Chemotherapeutic Interventions in Myeloma
 , Mary B. Drake2, Paul J. Kettle2, Tracey McGuigan2, Maeve Leahy3, Michael O’Dwyer4, Helen Enright5, Tanya O’Shea6,
, Mary B. Drake2, Paul J. Kettle2, Tracey McGuigan2, Maeve Leahy3, Michael O’Dwyer4, Helen Enright5, Tanya O’Shea6,  , Rakesh Popat7, Heather E. Oakervee8, Kwee Yong7, Jamie D. Cavenagh8, David A. Cairns9,
, Rakesh Popat7, Heather E. Oakervee8, Kwee Yong7, Jamie D. Cavenagh8, David A. Cairns9,  , Alberto Alvarez-Iglesias4, Gordon Cook9
, Alberto Alvarez-Iglesias4, Gordon Cook9- DOI
- 10.2991/chi.k.210201.001How to use a DOI?
- Keywords
- Myeloma; relapse; bortezomib; adriamycin; dexamethasone; response; PAD; Myeloma X
- Abstract
When the bortezomib [PS341], adriamycin and dexamethasone (PAD) regimen was first evaluated, the response rate in untreated patients was much superior to that elicited by conventional chemotherapeutic agents. We demonstrated the efficacy of PAD in relapsed or refractory patients by comparing the response rate obtained in 53 patients who received vincristine, adriamycin and dexamethasone (VAD) or equivalent regimen as induction therapy, using a comparative design in which each patient acted as their own control. Whereas 25 patients had a positive response to VAD, 37 patients had a response to PAD ≤ partial remission (PR) (p = 0.023). Using the more stringent response level of very good PR (VGPR) the results favored the PAD regimen very significantly (p = 0.006) (McNemars test). Similar results were seen using paired M-protein levels from individual patient comparisons. As the PAD regimen was subsequently adopted as the re-induction therapy in the British Society for Blood and Marrow Transplantation/United Kingdom Myeloma Forum Myeloma X (Intensive) trial, now concluded, we have retrospectively analyzed the findings from both studies. Comparison of response rates and adverse effects of patients having had previous autologous transplantation (Cohort 1) with the corresponding data from Myeloma X showed close correlation. These findings provide evidence that rapid results may be obtained in the evaluation of newly introduced, and potentially highly effective, anti-tumour agents by direct comparison to the response to the immediately preceding standard regimen, particularly in relatively resistant tumours.
- Copyright
- © 2021 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The efficacy of combining adriamycin and dexamethasone with the proteosome inhibitor bortezomib (original investigative name PS341) as induction therapy prior to autologous stem cell transplantation (ASCT) for previously untreated patients with multiple myeloma was clearly shown in two phase 2 studies [1,2]. Their observations were subsequently confirmed in a large multi-national randomized phase 3 clinical study [3].
Since the introduction of thalidomide and bortezomib to clinical practice at the start of this century the range of therapeutic options for patients with myeloma is growing exponentially, with no fewer than seven new drug approvals by the FDA since 2015 (Ixazomib [4], Daratumumab [5], Elotuzumab [6], Panobinostat [7], Isatuximab [8], Selinexor [9], and Belantamab [10]) with many more candidate therapeutic agents in the pipeline. All of these drugs are and will be expensive, on account of not only development cost but also the significant cost of undertaking large randomised clinical trials to demonstrate improved efficacy. Furthermore, the range of options for combination therapy will, of necessity, increase, giving rise to the problem of how to select most beneficial options for further investigation [11]. In addition, more new agents are currently under investigation, with a number of them showing sufficient promise as likely to achieve approval [12]. Recently, these trials have relied on comparisons of progression free survival (PFS) (often after two or more previous regimens) as their end point, in view of the difficulties in using overall survival, which can be significantly affected by downstream regimens. On occasion, such results have been used to grant accelerated regulatory approval [13]. As a result of a recent publication from the International Myeloma Working Group (IMWG), PFS after treatment of a first relapse (PFS2) has become a recognised endpoint for evaluation of newer agents in their initial assessment [14].
Most patients with myeloma present well-defined and easily measured markers of disease activity (response and progression) in the form of a serum paraprotein or specific serum free light chain. Depth of response (DoR) is also well-categorised, and is also related to response duration [6], which is recognized to shorten with each successive relapse [15]. In this study, we show that advantage can be taken of these facts to develop analyses requiring relatively small numbers of patients who act as their own controls, thus reducing the size of the required cohort and, potentially, the costs. As the best outcomes in myeloma are usually obtained at induction [15], the achievement of response equivalent to or better than that obtained with initial therapy at diagnosis (VAD in this study; vincristine, adriamycin and dexamethasone) by the therapy being tested (in this instance PAD; bortezomib, adriamycin and dexamethasone) at relapse after VAD would be indicative of its superior effectiveness. Similarly, if a therapeutic PAD-based response could be obtained in patients initially refractory to VAD this would also indicate superior clinical effectiveness. Furthermore, using DoR and PFS2 as markers of therapeutic efficacy, very rapid signals of treatment effectiveness can be generated. In this report, we show the results of our phase 2 study showing the same effects seen with PAD therapy in subsequent larger studies in identical clinical circumstances. Obtaining positive signals in this way facilitates the selection of the most promising regimens with which to proceed to phase 3 trials, and gives a good estimate of the likely common side effects. With the advent of new tumor markers and highly accurate cross-sectional imaging for solid tumors, it is possible that this approach might be applied to other malignancies, facilitating the optimal design of future pivotal trials.
2. MATERIALS AND METHODS
Patients with relapsed myeloma requiring further treatment were eligible if they had previously received induction therapy with VAD or VAD like regimen [cyclophosphamide, vincristine, adriamycin and dexamethasone (C-VAD), cyclophosphamide, vincristine, adriamycin, melphalan and prednisolone (C-VAMP), idarubicin and dexamethasone (Z-DEX)] [16]. Patients previously given induction therapy with VAD (or VAD like regimen) were subsequently treated with PAD in three separate cohort: (1) Patients who had proceeded to ASCT and subsequently relapsed; (2) Patients not transplanted but now requiring further therapy and; (3) Patients refractory to VAD (given as initial therapy) and requiring further treatment. PAD therapy was administered as described by Oakervee et al. [1]. Patients in Cohorts 1 and 2 were permitted to have one additional line of therapy (but not a second transplant) prior to trial entry. Patients in Cohort 3 on being found refractory to VAD or a VAD like regimen proceeded to PAD without any intervening therapy. For the purposes of analyzing their response to PAD, their paraprotein level on starting PAD was used as baseline for assessing response to this regimen.
As patients had already received therapy containing an anthracycline and dexamethasone prior to being given PAD, detailed comparisons of responses (and the potential benefit of bortezomib) could be made using the data obtained from previous treatment compared to that with PAD therapy. In the primary analysis, responses were assessed by the European Blood and Marrow Transplant (EBMT) group criteria in use at that time [17] with the addition of very good partial remission (VGPR) (indicating a 90% paraprotein response), and a comparison of PAD and VAD made. The second end point compared best M-protein responses obtained with VAD and PAD therapy in individual patients. The % fall of the M-protein on VAD therapy was compared to the % fall in M-protein achieved by PAD treatment. Safety and toxicity data for each treatment cycle were collected using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 during routine clinical assessments at each centre (All patients were included in the analyses except one in group 1 who was oligosecretory; comparisons were made using remission status from bone marrow samples and this patient was not included in this comparison.).
Eligible patients had Eastern Cooperative Oncology Group (ECOG) status of 0–2, platelets <25 × 109/L, haemoglobin >80 g/L, and neutrophils >1.0 × 109/L with creatinine clearance >30 mL/min. In addition, patients were excluded if the ejection fraction (EF) [measured by echocardiography or multigated acquisition scan (MUGA)] was less than 40%, and therapy was to be stopped if the EF fell by more than 10% after any two cycles of therapy. Neuropathy of greater than grade 2 was also an exclusion criterion for treatment discontinuation. The study received appropriate ethical review at participating institutions and was conducted according to the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent. The trial was registered with the Irish Clinical Oncology Research Group number ICORG 05-01. Cohort 1 was used as the pilot study for the induction therapy given in the British Society for Blood and Marrow Transplantation/United Kingdom Myeloma Forum (BSBMT/UKMF) Myeloma X Relapse (Intensive) Trial, which investigated the efficacy of a further ASCT in patients relapsing after a >18 month treatment-free interval following a first ASCT [18]. The comparator group received oral cyclophosphamide weekly (400 mg/m2) for 12 weeks. The data obtained from this non-intensive arm have subsequently been used in this study for comparison with Cohort 1. Comparison was made of demographic matching, response rates and adverse effects.
2.1. Statistical Methods
The primary outcome was to determine the response status (partial response, PR, or complete response, CR) to PAD therapy. Assuming that 25% of patients reaching CR or PR state would be unsatisfactory (null hypothesis) and that 50% of patients reaching this state would be satisfactory (alternative hypothesis) a single stage phase 2 design with 23 patients per group was required. All analyses were performed on an intention to treat basis. All statistical tests were at the two-sided p-value of 0.05. Patients were compared for their difference in response to previous VAD and to PAD therapy using McNemar’s test. Confidence intervals were produced using Wilson’s method. For the secondary analysis, difference in the M-protein level during the previous VAD and the PAD therapy was tested for significance using the paired t-test or with a non-parametric option if the variables were non-normal. PFS and overall survival (OS) were estimated using the Kaplan–Meier method [19]. In all time-to-event analyses, patients who had not experienced the event in question (progression or death) were censored on the last date seen. As only one patient in Cohort 1 had a second ASCT, this cohort was used for comparison with the non-intensive arm of the Myeloma X study, although it should be noted that the Myeloma X patients did receive “intensive” oral cyclophosphamide for up to 12 weeks.
3. RESULTS
The study opened in February 2006 and Cohort 1 was fully recruited by the end of February 2007. Cohort 3 was fully recruited by June 2009 and the study was closed with a total of seven patients recruited to Cohort 2. The characteristics of all 53 patients (by cohort) are shown in Table 1. Cytogenetic studies were not performed. Eight patients had received thalidomide-based therapy for their first relapse in Cohort 1. Following PAD therapy, 17 patients proceeded to transplant: one (second) transplant in Cohort 1, two in Cohort 2 and 14 in Cohort 3, including one matched and one mismatched allogenic transplant.
| Cohort | 1 | 2 | 3 |
|---|---|---|---|
| Number | 23 | 7 | 23 |
| Gender | |||
| Male/Female | 14/9 | 5/2 | 17/6 |
| Age | |||
| Median | 58 | 59 | 57 |
| Range | 41–70 | 50–72 | 35–70 |
| Type of myeloma | |||
| IgG | 12 | 2 | 15 |
| IgA | 6 | 5 | 6 |
| BJM | 4 | – | 1 |
| NS | 1 | – | – |
| Relapse status | |||
| First | 15 | 7 | Refractory |
| Second | 8 | 0 | – |
| Previous chemo | |||
| VAD | 13 | 5 | 15 |
| VAMP | 1 | 0 | 0 |
| C-VAMP | 6 | 2 | 0 |
| C-VAD | 0 | 0 | 2 |
| Z-DEX | 3 | 0 | 6 |
| Cycles of previous chemotherapy | |||
| Median | 5 | 4 | 4 |
| Range | 4–6 | 3–6 | 2–6 |
| Time from diagnosis to registration | 49 | 11 | 6 |
| Months | 20–179 | 4–83 | 2–34 |
| ISS at trial entry | |||
| 1 | 10 | 2 | 13 |
| 2 | 5 | 2 | 5 |
| 3 | 4 | 3 | 3 |
| Unknown | 4 | 0 | 2 |
| ECOG at trial entry | |||
| 0 | 8 | 0 | 9 |
| 1 | 15 | 4 | 9 |
| 2 | 3 | 2 | 4 |
| 3 | 1 | 1 | – |
| Cycles of PAD therapy | |||
| Median | 4 | 4 | 4 |
| Range | 2–6 | 3–4 | 2–6 |
BJM, Bence Jones myeloma; NS, Non Secretory myeloma.
Baseline characteristics of patients with relapsed (Cohorts 1 and 2) or refractory (Cohort 3) myeloma
3.1. Response Data
3.1.1. EBMT response (modified) findings
The best response to the PAD chemotherapy in each cohort are shown in Table 2. Thirty seven of the 53 patients achieved a response of ≥PR (70%), therefore not only exceeding the level required (25%) to avoid a negative result, but also reaching the target indicating a satisfactory result (50%). Table 3a shows there are 18 discordant pairs in favor of PAD treatment, compared to only six discordant pairs in favor of VAD, suggesting a positive effect in favor of the PAD chemotherapy (Exact McNemar test; p = 0.023). When the more stringent response level of ≥VGPR (>90% reduction of paraprotein) was used in a similar comparison (Table 3a), the analysis showed an even stronger result in favour of the PAD combination (p = 0.006). When Cohorts 1 and 3 were analysed using a cut off of ≤PR versus PR or greater and also ≤VGPR versus VGPR or greater, the McNemar’s exact probability test gave results of p = 0.727, p = 0.070, p < 0.001 and p = 0.250 respectively (Table 3b and 3c).
| CR | PR | MR | NC | PD | |
|---|---|---|---|---|---|
| Cohort 1 | 8 (35%) | 10 (43%) | 3 (13%) | – | 2 (9%) |
| Cohort 2 | 1 (14%) | 4 (57%) | 1 (14%) | – | 1 (14%) |
| Cohort 3 | 2 (9%) | 12 (52%) | 6 (26%) | 2 (9%) | 1 (4%) |
MR, minimal response; NC, no change; PD, progressive disease.
Best overall response to PAD
| Response to PAD | ||||
|---|---|---|---|---|
| Response to VAD | <PR | PR or greater | Total | p-values |
| <PR | 10 | 18 | 28 | 0.023 |
| PR or greater | 6 | 19 | 25 | |
| Total | 16 | 37 | 53 | |
| <VGPR | 36 | 11 | 47 | 0.006 |
| <VGPR or greater | 1 | 5 | 6 | |
| Total | 37 | 16 | 53 | |
Overall comparison of response to VAD and PAD: exact McNemar test
| Response to PAD | ||||
|---|---|---|---|---|
| Response to VAD | <PR | PR or greater | Total | p-values |
| <PR | 0 | 3 | 3 | 0.727 |
| PR or greater | 5 | 15 | 20 | |
| Total | 5 | 18 | 23 | |
| <VGPR | 12 | 7 | 19 | 0.0703 |
| <VGPR or greater | 1 | 3 | 4 | |
| Total | 13 | 10 | 23 | |
Comparison of response to VAD and PAD Cohort 1: exact McNemar test
| Response to PAD | ||||
|---|---|---|---|---|
| Response to VAD | <PR | PR or greater | Total | p-values |
| <PR | 9 | 14 | 23 | <0.001 |
| PR or greater | 0 | 0 | 0 | |
| Total | 9 | 14 | 23 | |
| <VGPR | 20 | 3 | 23 | 0.250 |
| <VGPR or greater | 0 | 0 | 0 | |
| Total | 20 | 3 | 23 | |
Comparison of response to VAD and PAD Cohort 3: exact McNemar test
3.1.2. M-protein levels
The percentage change in M-protein levels achieved by VAD and VAD-like therapy was compared to the percentage decrease in M-protein levels achieved by PAD therapy. Figure 1 illustrates the results for all patients. In Cohort 1 the percentage median decrease (±SD) in paraprotein for VAD was 63% (±25) % and for PAD 79% (±29) % (p = 0.233), clearly not an inferior result. In Cohort 3 the median decrease in M-protein with VAD was 19% (±44) % and when these patients received PAD it was 60% (±26) % (p = 0.0002).
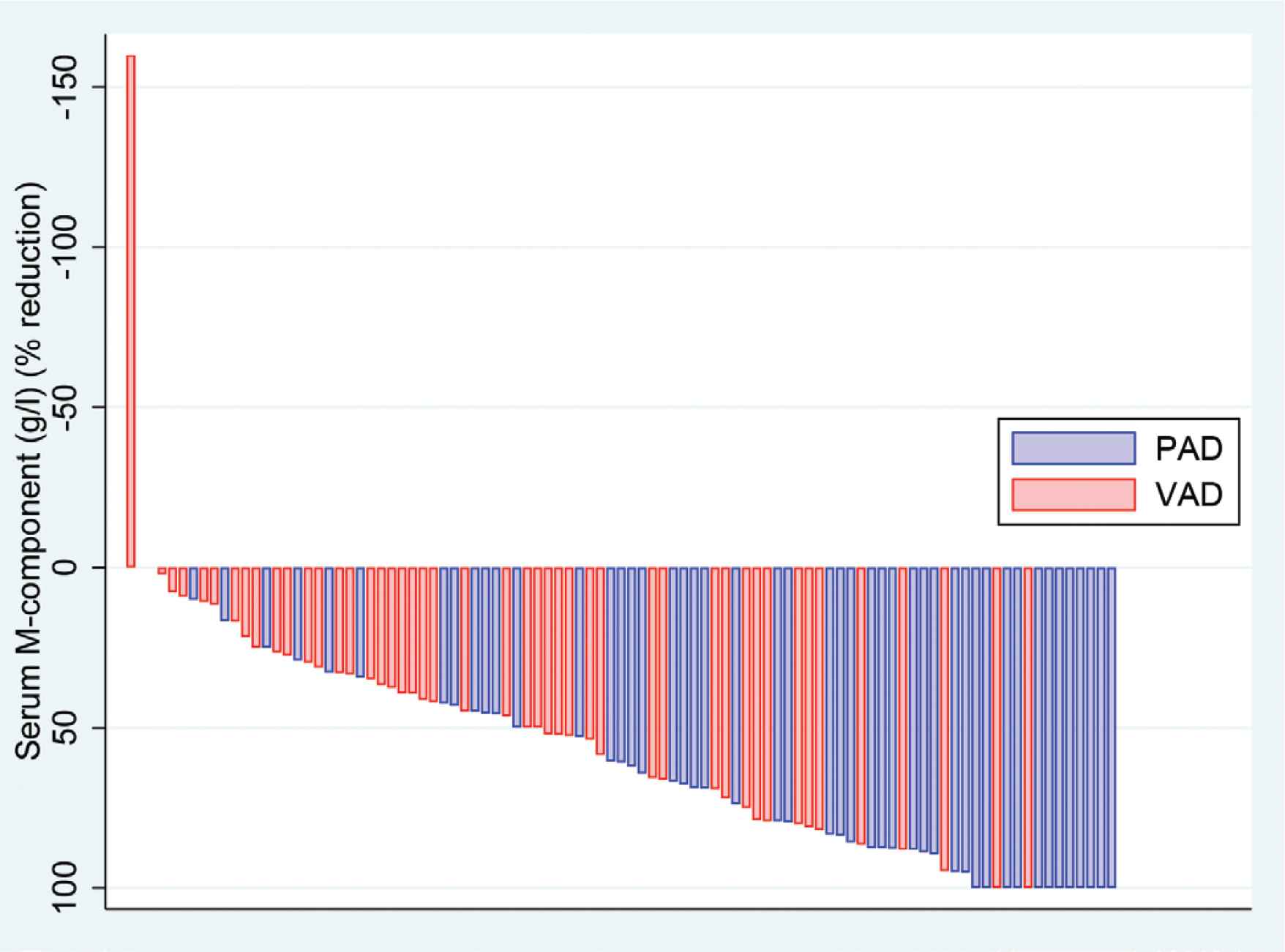
“Waterfall plot” for percentage of change in paraprotein levels following VAD and PAD chemotherapy (Two patients in Cohort 3 progressed on VAD).
3.1.3. Progression free survival and overall survival
Figure 2a and 2b shows Kaplan–Meier plots of the PFS and OS for Cohorts 1–3. The PFS was 10, 4 and 25 months, respectively, following PAD therapy (with no additional therapy given except for those patients proceeding to transplantation (n = 1, 2 and 14 respectively). The median OS was 35, 63 and 76 months, respectively.
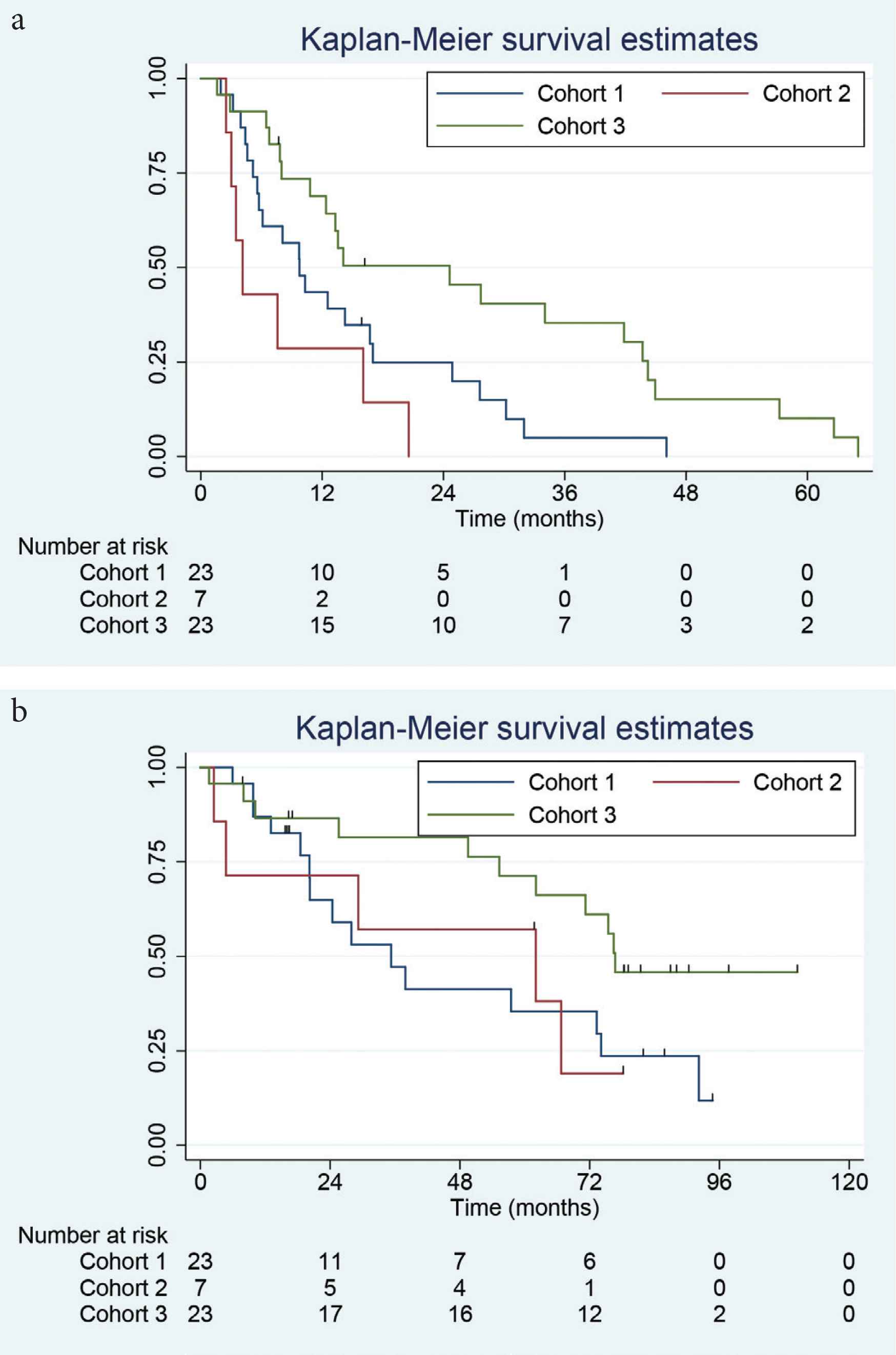
Kaplan–Meier plots of overall survival (a, upper plot) and progression-free survival (b, lower plot) for Cohorts 1–3 following PAD chemotherapy.
3.1.4. Adverse effects
Severe (Grade 3 and 4) cytopenias were the principal side effects noted, with Grade 3 and 4 thrombocytopenia in eight, four and three patients experiencing this at least once in Cohorts 1–3, respectively, while Grade 3 and 4 neutropenia was noted in seven, three and seven patients. While Grade 1/2 peripheral (sensory) neuropathy was observed in up to 40% of patients, the number experiencing Grade 3 and 4 was limited (Cohort 1 n = 3, Cohort 2 n = 1 and Cohort 3 n = 3). Fatigue was also commonly noted, but the incidence of Grade 3/4 was much lower with one, one and two in each of the three cohorts. One patient developed fatal pseudomembraneous colitis with neutropenia, pseudomonal sepsis and died.
3.1.5. Comparison of cohort 1 and the non-intensive arm of Myeloma X
Comparison of characteristics and side effect profile of patients in Cohort 1 and the non-intensive arm of Myeloma X are shown in Table 4, where it can be seen that patients are well matched (taking into account that eight patients in Cohort 1 received thalidomide as a second therapy prior to trial entry). There is a striking concordance between the response rate (≥PR and ≥VGPR) and PFS of the two studies. The difference in OS may be related to the fact that, for patients relapsing in Cohort 1, lenalidomide would not have been available, except in a limited number of clinical trials, whereas lenalidomide (or pomalidomide) was widely available to patients relapsing in Myeloma X [20]. The side effect profile was also similar (Table 4).
| PAD Cohort 1 | Myeloma X non-intensive arm | |
|---|---|---|
| Age (years) | 60 | 61 |
| Median time from diagnosis to first relapse (months) | 47 | 39 |
| Range (months) | 20–179 | 18–150 |
| PR or better post PAD (%) | 79 | 78 |
| PFS (months) | 10 | 11 |
| OS (months) | 35 | 52 |
| Serious adverse events (%) | 47 | 45 |
| Anaemia (%) | 3 | 7 |
| Neutropenia (%) | 22 | 42 |
| Thrombocytopenia (%) | 39 | 52 |
| Infection (%) | 4 | 9 |
| Nausea (%) | 13 | 7 |
| Vomiting (%) | 4 | 3 |
| Diarrhoea (%) | 0 | 5 |
| Sensory neuropathy (%) | 4 | 10 |
Comparison of PAD Cohort 1 and Myeloma X non-intensive arm
4. DISCUSSION
Poor uptake of clinical trials by cancer treating institutions, sub-optimal enrolment and failure to complete recruitment are all barriers to advancing treatment of cancer patients [21]. A simple trial design requiring relatively small numbers of patients and capable of being carried out on multiple sites offers a potential solution to the recruitment of sufficient numbers of participants (patients), and the speed with which the study can be concluded, allowing recruitment to large phase 3 trials to be concentrated on studies which have the potential to change clinical practice for the better. This study acted as a feasibility study for the Cancer Research UK Myeloma X, which was for patients in first relapse [18,22], and assessed two therapeutic approaches by comparing PFS following the start of second line therapy (PFS2). Results from this major study were incorporated into the IMWG recommendations for the management of relapsed multiple myeloma [23].
Response and depth of response are convenient and widely used markers of therapeutic efficacy in myeloma. In this study, we show that a very demanding target in terms of achieving a depth of response at least as good as that achieved by the patients’ induction therapy on first line treatment has been clearly achieved by the substitution of the trial medication (in this instance, bortezomib for vincristine) in the now outdated VAD regimen for patients in Cohort 1. This also appears true for the small number of patients in Cohort 2 and, particularly, for Cohort 3, in which all patients initially failed to achieve at least PR. Note that the responses to PAD were calculated using the paraprotein level observed at the time of commencing PAD therapy and not that at diagnosis, again raising the bar very high.
Comparison of the characteristics of Cohort 1 in the present study and patients entered into Myeloma X shows that, while the mean age of the PAD Cohort 1 is 3 years lower than Myeloma X and contains a slightly higher proportion of women, there is broad similarity between the isotypes, International Staging System (ISS) stage and time to progression after first ASCT of the two groups. After PAD induction for all patients in Myeloma X followed by peripheral blood stem cell harvesting (if necessary) to support a second ASCT, patients were randomized to a second dose melphalan at 200 mg/m2, ASCT or to 12 weeks intensive (400 mg/m2) cyclophosphamide therapy. This latter group experienced a PFS of 11 months, a figure very similar to the control arm in other randomised controlled clinical trials of patients in first relapse [7,24]. As can be seen (Table 2) in this study, when patients received up to six cycles of PAD therapy (median 4) versus four (median 3) cycles of the same therapy plus consolidation (now seen as relatively ineffective, but commonly used at the time of the Myeloma X study design), the duration of PFS in the PAD study was an accurate forecast of PFS in the Myeloma X trial. Importantly, the safety signals noticed in our study also correlated well with Myeloma X (excluding those clearly related to the experimental ASCT) arm. The difference in OS may reflect the use of salvage ASCT as third line therapy in Myeloma X along with the greater availability of lenalidomide and other agents in the later timeframe of Myeloma X [20].
We have thus demonstrated a simple trial design in which a highly efficacious drug is substituted for a less effective agent in an established regimen. However, the results from Cohort 3 suggest that use of a modified (or indeed a totally new regimen) directly after demonstration of (relative) failure of the first regimen may be capable of sending an even stronger signal of the new regimen’s efficacy. Failure to respond to first line therapy (even VAD) is a clear sign of resistant disease in myeloma, and is associated with a poorer prognosis. Immediately switching to a more effective regimen is appropriate, allowing most of the transplant-eligible patients in Cohort 3 to proceed to ASCT. While the superiority of bortezomib over vincristine is hardly a surprise, we suggest this style of trial design is capable of producing clear signs of efficacy with small numbers of patients and correspondingly reduced costs in a short timeframe. Cohort 1 was filled within 12 months of opening in a small number of centres and was first reported 30 months later at the Haematology Association of Ireland Annual meeting and a few months later at the IMWG meeting of 2009 [25]. Larger clinical trial groups would clearly be capable of improving on this recruitment period. Although response rates, PFS2 and depth of response have all been used in obtaining regulatory approval, the aim of this manuscript was to look at designing sequential trials more efficiently rather than using this method for regulatory approval per se. We seek to streamline the process to randomized clinical trials by identifying the most promising drugs in rigorous phase 2 trials. Therefore, we suggest that new promising agents may be usefully studied in comparison with previous therapy to economically and promptly assess efficacy and facilitate timely and meaningful phase 3 trial development and clinical use.
5. CONCLUSION
Substituting bortezomib for vincristine in the VAD regimen (PAD) proved therapeutically effective in patients with myeloma already treated with VAD, using a simple trial design with small numbers of patients. The data obtained from this small study appeared to accurately predict the response rates and common side effect profile in the larger BSBMT/UKMF Myeloma X study. This approach appears capable of giving an accelerated estimate of the efficacy of a novel regimen, and could be of benefit in selecting the most promising new anti-cancer chemotherapeutic agents for large (and expensive) phase 3 clinical trials.
COMPETING INTERESTS
GC and TCMM Research support: Janssen, Celgene, Chugai-Pharma Consultancy: Janssen, Celgene, Chugai-Pharma.
JC Ad Board and Speaker’s Fees for Jansenn and Celgene.
AUTHORS’ CONTRIBUTION
The study was designed by TCMM. TCMM and GC analysed the data. TCMM wrote the article with contributions from MBD, PJK, HE, TO’S, RP, HEO, KY and GC. All authors obtained the data except AA-I who performed the statistical analysis, DAC who provided the Myeloma X data (with GC) and TO’S who was responsible for study co-ordination. All authors reviewed the final article and gave final approval.
Data collection was performed by ICORG (their first multinational study) and data analysis was by the Health Research Board, NUI Galway (AA-I).
FUNDING
The study was supported by an unrestricted educational grant from Janssen together with supplies of study drug (bortezomib).
ACKNOWLEDGMENTS
The study was designed by TCM Morris and co-ordinated through the All Ireland Clinical Oncology Research Group (now Cancer Trials Ireland). The final analysis was performed by the HRB Clinical Research Facility, National University of Ireland, Galway. We thank the study investigators, coordinators, nurses, research staff and patients and their families for their contributions to this study.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
All patients gave written informed consent. The study was approved by the Irish Clinical Oncology Research Group (now Clinical Trials Ireland) protocol number ICORG 05-01 and institutional review boards of the participating centres and was undertaken according to the Declaration of Helsinki and the principles of Good Clinical Practice as espoused in the Medicines for Human Use (Clinical Trials) Regulations.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Treen Carson Michael Morris AU - Mary B. Drake AU - Paul J. Kettle AU - Tracey McGuigan AU - Maeve Leahy AU - Michael O’Dwyer AU - Helen Enright AU - Tanya O’Shea AU - Rakesh Popat AU - Heather E. Oakervee AU - Kwee Yong AU - Jamie D. Cavenagh AU - David A. Cairns AU - Alberto Alvarez-Iglesias AU - Gordon Cook PY - 2021 DA - 2021/02/08 TI - How to Simplify the Evaluation of Newly Introduced Chemotherapeutic Interventions in Myeloma JO - Clinical Hematology International SP - 27 EP - 33 VL - 3 IS - 1 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.210201.001 DO - 10.2991/chi.k.210201.001 ID - Morris2021 ER -
