Essential Diagnostics: A Key Element of Universal Health Coverage
Peer review under responsibility of the Dr. Sulaiman Al Habib Medical Services Group Company
- DOI
- 10.2991/dsahmj.k.190225.001How to use a DOI?
- Keywords
- Essential diagnostics; tests; global health; World Health Organization; Universal Health Coverage
- Abstract
Good primary care is an essential precondition for a decent healthcare system. In fact, primary health care is at the heart of Universal Health Coverage (UHC). UHC, in turn, is critical to achieve the sustainable development goals. While access to essential medicines is explicit in UHC, access to essential diagnostics has received little attention. In May 2018, the World Health Organization (WHO) published the first Essential Diagnostics List (EDL), and declared its commitment to give equal importance to diagnostic tests and essential medicines. The EDL has been positively received by a variety of stakeholders, including industry. The EDL offers countries a benchmark that they can use to measure and improve diagnostic services, and preliminary data from India show limited access to essential tests at the primary care level. Some countries, notably India, have already begun developing National EDLs (NEDLs). Hopefully, such national efforts will enable the implementation of EDLs, and improve access to diagnostics. It is time for Low- and Middle-Income Countries (LMICs) to not only increase coverage of health care, but also improve quality of care. Access to essential tests is the first key step in improving quality of care.
- Copyright
- © 2019 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Health is a human right. Yet, the sad reality is that, 40 years after the Alma Ata declaration “Health For All by 2000”, half the world is still without access to essential healthcare services. To address this healthcare crisis, all countries have committed to the Sustainable Development Goals by 2030, which include Universal Health Coverage (UHC) [1]. UHC involves financial risk protection, access to quality essential healthcare services and access to essential medicines and vaccines for all.
While everyone accepts the importance of essential medicines, there is little acknowledgement of the central importance of diagnosis – the first, critical step in any disease management [2]. Diagnostics influence about 70% of healthcare decisions, and yet only 3–5% of healthcare budgets goes into tests [3]. When health systems do not sufficiently plan for or fund diagnostic services, health workers have to rely on empirical and/or syndromic diagnoses, leading to both missed cases and unnecessary treatments [3].
Mystery (standardized) patient studies in India, including some by our team, have shown that primary care providers in both urban and rural areas make a correct diagnosis in less than 25% of patients who present with typical symptoms of angina, tuberculosis (TB), asthma, and pneumonia [4–7]. They have also shown excessive use of medicines, and little testing. Not surprisingly, India is the biggest consumer of antibiotics in the world [8].
As medicines become expensive, and antimicrobial resistance emerges, we simply cannot afford to consume medicines like candies. Investing in diagnostics improves effectiveness of treatment and reduces costs and unnecessary drug use. The scale-up of malaria rapid tests has led to a substantial decline in the use of antimalarial drugs. In contrast, not having good, easy-to-use tests for typhoid and TB have resulted in the emergence of extensively drug-resistant bacteria. This problem of inadequate testing and empirical therapy is not limited to infections. For example, nearly one in two Indians with diabetes are unaware that they have diabetes. Cancers are usually detected when they are advanced, resulting in poor survival rates.
Diagnostic testing also matters for public health. Every year, dozens of outbreaks occur all over the world without any clarity on what is causing them. A fever outbreak, for example, could be due to dengue, malaria, influenza, Chikungunya, or scrub typhus. Laboratory testing is the only way to know the underlying cause of illness. Ideally, treatment should follow diagnosis, and treatment should be tailored to the exact cause of illness. Sadly, as the above examples illustrate, this rarely happens. Lack of access to good, affordable testing is one reason why healthcare providers bypass the process of diagnosis.
2. WORLD HEALTH ORGANIZATION ESSENTIAL DIAGNOSTICS LIST
In a path-breaking development, 40 years after publishing the first Essential Medicines List (EML), World Health Organization (WHO) published the first Essential Diagnostics List (EDL) in May 2018 [9]. This new list is expected to enhance the impact of the EML. After all, essential medicines require essential diagnostics. The WHO EDL defines essential diagnostics as those “that satisfy the priority health care needs of the population and are selected with due regard to disease prevalence and public health relevance, evidence of efficacy and accuracy, and comparative cost-effectiveness”, similar to the definition of essential medicines. Tests for the EDL were chosen from existing WHO guidance, priority medical devices lists, and the WHO Prequalification of In Vitro Diagnostics Program [10]. In its first version, the EDL contained 113 tests; of which, 58 were considered general laboratory tests and 55 disease-specific tests with public health importance covering human immunodeficiency virus (HIV), TB, malaria, hepatitis B and C, syphilis, and human papilloma virus.
The EDL identifies tests that should be present at different health system tiers: primary care facilities as well as facilities with clinical laboratories. In primary care, defined by WHO as settings where no or basic laboratories are available, general tests include urine dipstick, complete blood count, lactate, glucose, and microscopy (which can be used to diagnose a range of conditions such as anemia, malaria, TB, and parasites). Disease-specific testing in primary care includes rapid diagnostic tests to detect HIV, malaria, syphilis, and viral hepatitis. Figure 1 shows the tests included in the EDL for the primary care level. Figure 2 shows tests included in the EDL that are specific for infectious diseases [11].

Tests included in the WHO essential diagnostics list at the primary care level. HbA1c, glycated hemoglobin; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; LAMP, loop-mediated isothermal amplification; RDT, rapid diagnostic test.
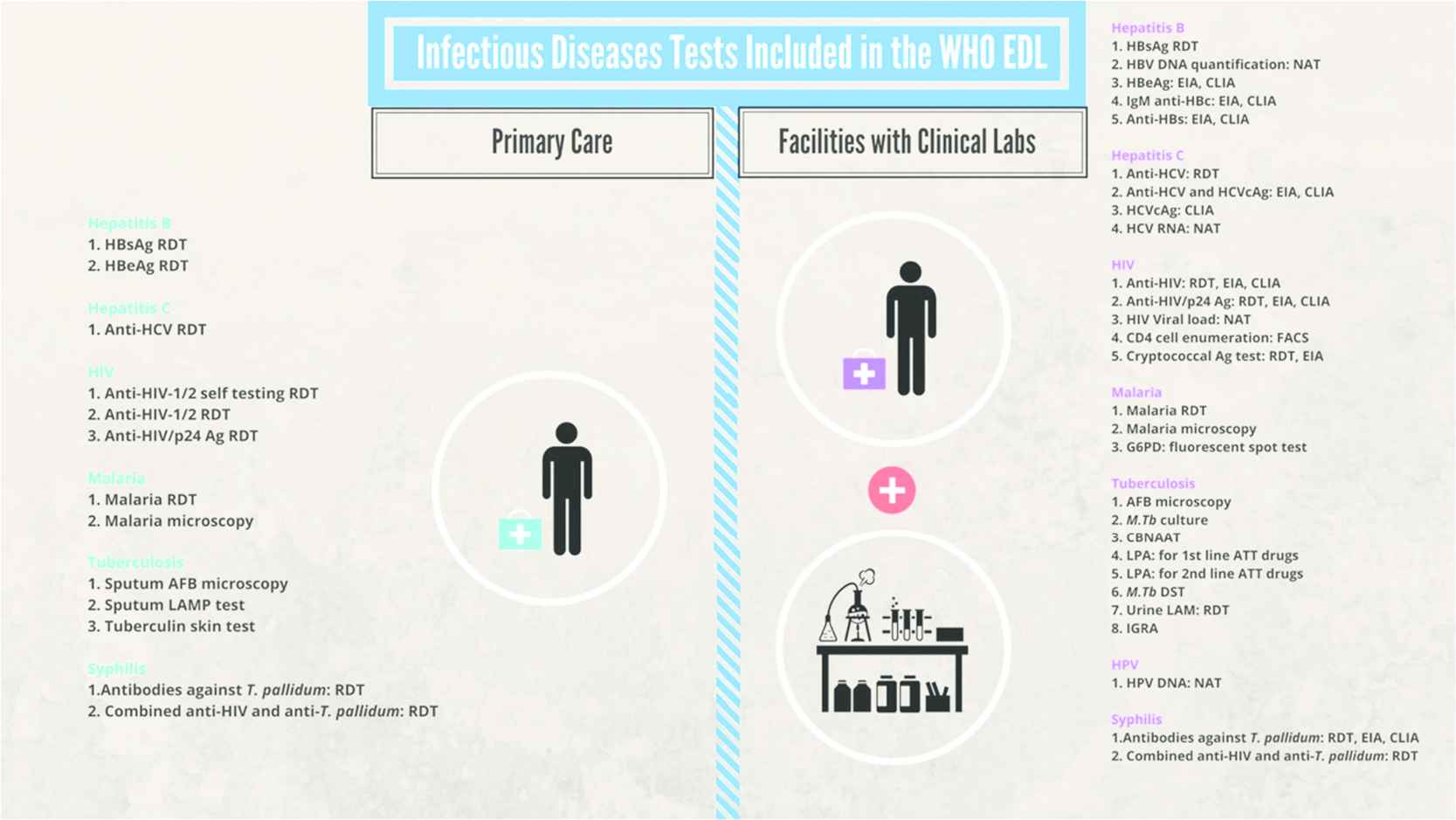
Infectious diseases tests included in the first edition of the WHO essential diagnostics list. AFB, acid fast bacilli; ATT, antituberculosis therapy; CBNAAT, cartridge-based nucleic acid amplification test; CLIA, chemiluminescence immunoassay; DST, drug susceptibility testing; EIA, enzyme immunoassay; FACS, fluorescence-activated cell sorting; G6PD, glucose 6 phosphate dehydrogenase; HIV, human immunodeficiency virus; HPV, human papillomavirus; IGRA, interferon gamma release assay; LAMP, loop-mediated isothermal amplification; LPA, line probe assay; M.Tb, Mycobacterium tuberculosis; NAT, nucleic acid amplification; RDT, rapid diagnostic test. Reproduced from Kohli et al. [11].
World Health Organization has already begun the process of expanding the list to other areas through a yearly call for submissions of additional categories of tests. Through these calls it will also be possible to submit corrections, clarifications, or even deletions. The WHO has appointed a Strategic Group of Experts on In Vitro Diagnostics (SAGE IVD) to advice on the development of the EDL. The second edition of the EDL is expected to be published in Spring 2019 [10].
3. RATIONALE FOR AN EDL
There are many benefits for countries that adopt the EDL [12]. These include improving patient care, helping detect outbreaks, increasing affordability of tests, reducing out-of-pocket expenses for tests, reducing antibiotic abuse, improving regulation and quality of diagnostic tests, strengthening accreditation and quality of laboratories, improving supply chains, and guiding the research and development of new diagnostic tools [3,12]. The EDL has been favorably received by all key stakeholders, including industry. A recent survey of industry representatives showed that the diagnostics industry largely welcomes the WHO EDL initiative and agrees that it will help improve access to diagnostics in Low- and Middle-Income Countries (LMICs) [13]. Industry members, however, did express concerns about the use of EDL as a price capping tool, and the need for the document to be updated frequently to ensure uptake of new tools [13].
4. USING EDL AS A TOOL FOR ESTIMATING ACCESS TO TESTING
The EDL offers countries a benchmark that they can use to measure and improve their diagnostic services. In our recent pilot study, we used the WHO EDL to assess availability of essential tests at the primary care level in the Indian public sector [14]. For this pilot, we deliberately chose three districts in three states in India, in north, south and central zones. Within each district, we randomly selected 20% of all the Primary Health Centers (PHCs). Each PHC was visited by a researcher with a checklist to assess availability of diagnostics. Twenty-one PHCs in Tumkur, 13 in Fatehpur, and six in Wardha were assessed between December 2017 and March 2018. Our results Figure 3 show that, while all three districts had major gaps in test availability, we found huge variations across the districts/states, with Wardha faring relatively better, and Fatehpur faring worst.
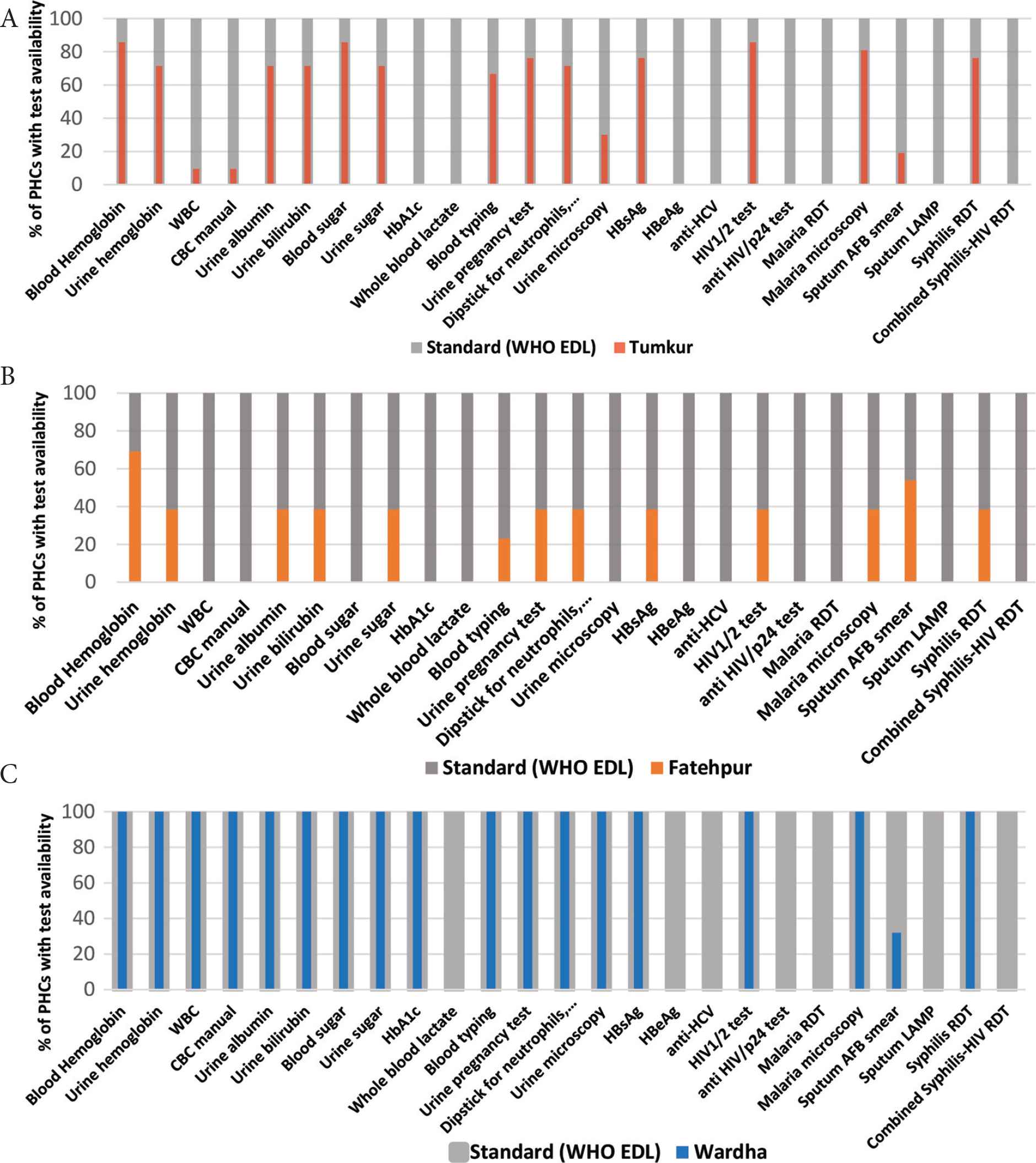
Results from pilot study on availability of essential diagnostic tests at the primary healthcare level in India, in Fatehpur (A), Tumkur (B) and Wardha (C) Districts. AFB, acid-fast bacilli; anti-HCV, antibodies to hepatitis C virus; CBC, complete blood count; HbA1c, glycated hemoglobin; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; LAMP, loop-mediated isothermal amplification test; RDT, rapid diagnostic test; WBC, white blood cells. Reproduced from Kohli et al. [14].
Some of the tests listed in WHO EDL, such as, blood lactate, hepatitis B e-antigen, anti-hepatitis C virus, malaria rapid diagnostic tests, sputum loop-mediated isothermal amplification TB test, anti-HIV/p24 rapid test, and combined test for syphilis and HIV were not available in any district. With the other tests, availability varied a lot. For example, PHCs in both Tumkur and Fatehpur had limited or no availability of blood counts and hemoglobin A1c tests, while these tests were available in the Wardha District. Maharashtra State (which covers Wardha District) uses a public–private partnership model in which diagnostic testing is outsourced to a private laboratory.
Other studies have also shown similar gaps in other LMICs [15]. For example, pathology service coverage in sub-Saharan Africa is approximately one-tenth of that in high-income countries [16]. Even simple tests are often unavailable: blood glucose meters and urine strips were available in only 18–61% of facilities across Mali, Mozambique, and Zambia [17]. Another study of 10 countries found that only 2% of healthcare facilities had the eight diagnostic tests (hemoglobin, blood glucose, malaria test, urine dipstick for protein and sugar, HIV and syphilis test, and pregnancy test) included in WHO basic service readiness index [18].
5. COUNTRY ADOPTION AND IMPLEMENTATION
While the WHO EDL is a welcome development, the list, by itself, will not have an impact, unless countries adopt and adapt the WHO list, develop their own national lists, and put in place mechanisms to improve access to tests on the EDL. Most countries have national lists of essential medicines. Something similar is needed for diagnostics.
Recently, the Indian Government released a draft of their National EDL (NEDL), tailored to the Indian context, developed by Indian experts [19]. The draft EDL, which has been put out for public consultation, consists of 130 general laboratory tests required for routine patient care and for diagnosis of a wide array of both communicable and noncommunicable diseases. The list includes 26 disease-specific tests for diagnosis of diseases like malaria, dengue, Chikungunya, filariasis, scrub typhus, Japanese encephalitis, TB, HIV, and hepatitis. The list covers primary and secondary care levels of the Indian public healthcare delivery system. The NEDL in India, hopefully, will form the basis of providing essential primary care services across the country, and greatly improve quality of care. The NEDL can be used to impose price controls, streamline supply chain, and ensure better access to a variety of important tests.
However, lists alone will not change the reality. LMICs, including India, will have to invest in strengthening public laboratories, contract private laboratories as required, and make sure quality-assured testing services are freely available at all facilities. To address the weak laboratory capacity in LMICs, four key barriers must be tackled: insufficient human resources or workforce capacity, inadequate education and training, inadequate infrastructure, and insufficient quality, standards, and accreditation [20].
6. QUALITY DIAGNOSIS IS NECESSARY FOR HIGH-QUALITY HEALTHCARE
A recent Lancet Commission on High Quality Health Systems estimated that more than 8 million people per year in LMICs die from conditions that should be treatable by the health system [21]. About 60% of deaths from conditions amenable to health care are due to poor-quality care, whereas the remaining deaths result from non-utilization of the health system [21]. So, poor-quality care is now a bigger barrier to reducing mortality than insufficient access to medical care. It is time for LMICs to not only increase coverage of health care, but also improve quality of care. Access to essential tests is the first key step in improving quality of care.
CONFLICTS OF INTEREST
Madhukar Pai has no financial or industry conflicts to disclose. He is a member of the WHO SAGE IVD Committee that developed the EDL.
ACKNOWLEDGMENTS
We are grateful to Paulami Sen for assistance with graphics. This review draws heavily on editorials we have previously published on essential diagnostics.
REFERENCES
Cite this article
TY - JOUR AU - Madhukar Pai AU - Mikashmi Kohli PY - 2019 DA - 2019/03/28 TI - Essential Diagnostics: A Key Element of Universal Health Coverage JO - Dr. Sulaiman Al Habib Medical Journal SP - 3 EP - 7 VL - 1 IS - 1-2 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.190225.001 DO - 10.2991/dsahmj.k.190225.001 ID - Pai2019 ER -
