School-based Cross-sectional Survey on Soil-transmitted Helminths in Rural Schools of Kogi East, Nigeria
- DOI
- 10.2991/dsahmj.k.200211.002How to use a DOI?
- Keywords
- Soil transmitted helminthes; prevalence; intensity; Kogi East; Nigeria
- Abstract
This study was undertaken to investigate the status of Soil-transmitted Helminths (STHs) in rural schools of Kogi East, Nigeria. The study was cross-sectional using stratified random cluster sampling procedure. Stool specimens were collected in a sterile specimen bottle from school pupils in five (5) randomly selected schools in each of the nine Local Government Areas (LGAs) (45 schools) of Kogi East to enable complete epidemiological survey, all schools selected were located within the rural areas of the LGAs. Collected samples were preserved in 10% formalin and examined for parasites using formal ether sedimentation technique. Structured questionnaires were administered to obtain information on the risk factors associated with STHs. Data were analysed using descriptive statistics and Chi-square test was used to test the relationship in prevalence of STHs according to categories of infections. The overall prevalence of STHs in Kogi East was 17.1% with Ascaris lumbricoides, hookworms and Strongyloides stercoralis having prevalence of 4.3%, 12.7% and 1.1% respectively. Hookworms’ infection was the most widespread in Kogi East. Omala LGA (29.6%) had the highest prevalence of STHs. No significant difference (p > 0.05) in prevalence between male (18.2%) and female (16.0%) pupils, and between the age groups of 5–8 years (16.6%) and 9–12 years (17.8%). STHs is endemic among rural pupils of Kogi East as observed in the prevalence of infection as well as the prevailing risk factors, preventive chemotherapy is warranted. Therefore, school-based deworming alongside health education programme should be extended to rural schools.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Soil-transmitted Helminths (STHs) are one the leading causes of global health problems especially in poorest and deprived communities where implementation and control are difficult to maintain. Soil-transmitted helminthiasis is caused by parasitic nematodes transmitted through contact with parasites eggs (Ascaris lumbricoides and Trichuris trichiura) or larvae (hookworms) and are responsible for more than 40% of worldwide morbidity from all tropical infections [1–3]. A recent report estimated that 2 billion people are infected with STHs worldwide, with 819, 439, and 439 million people infected with A. lumbricoides, T. trichiura, and hookworms, respectively [3,4]; about 610 million school-age children are at risk of infection with STHs [4].
Soil-transmitted helminthiasis is the most prevalent Neglected Tropical Diseases (NTDs) in Nigeria [1]. School-aged children in rural areas who lack access to clean water and sanitation infrastructures are the most affected [5]. Children infected with these parasites experience hampered cognitive and physical development that leads to significant negative educational and nutritional effects [6–8]. STH is prevalent in areas with favorable climatic and environmental conditions [9].
Neglected Tropical Diseases Roadmap, launched by the World Health Organization (WHO) and its partners [10], brought about escalated measures to control NTDs [11]. One of its consequences is the large-scale distribution of anthelminthic drugs commenced in many regions without prior diagnosis [12–16]. The WHO identifies three priority groups for deworming: preschool-age children, school-age children, and women of child-bearing age [17], with school-aged children topping the list [12]. Identification of areas with infections caused by one or more parasites in school-aged children will help increase efficiency in the integrated control of these parasites. Survey studies carried out at school level will help identify the distinctive factors associated with STH distribution at school level.
In Kogi State, there is a lack of information on the prevalence and risk factors associated with STHs. Such information—if available—will serve as a tool to guide the development of policies toward the control of STHs in school-aged children. Therefore, this study was carried to investigate the occurrence, intensity, and risk factors associated with STHs (A. lumbricoides, T. trichiura, and hookworms) in rural schools of Kogi East Senatorial District, Nigeria.
2. MATERIALS AND METHODS
2.1. Study Area
Kogi East Senatorial District is located in Kogi State, North Central Nigeria (Figure 1). It is a geographical region comprising nine Local Government Areas (LGAs). The district is located between latitude 6°32′33.8″N to 8°02′44.8″N and longitude 6°42′08.5″E to 7°51′50.3″E. The district occupies an area of 26,197 km2, and shares boundaries with six states of Nigeria. To the north, it shares boundaries with Nassarawa, to the west with Edo and Delta States, whereas to the east it is bounded by Benue, Anambra, and Enugu [18]. The population of the region as of 2006 was 1,479,144, and is projected to reach 1,996,700 by 2016 [19].
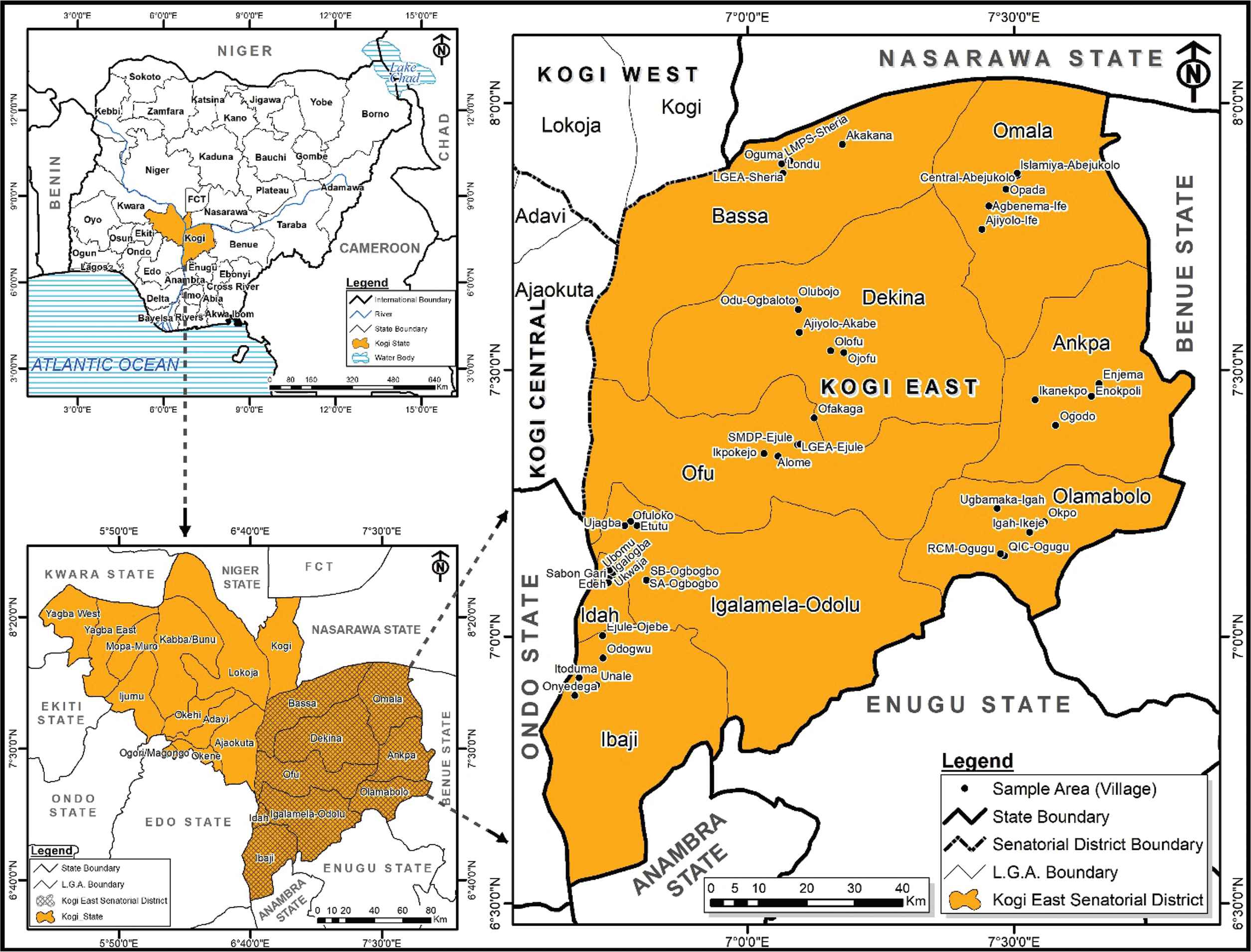
Sampling Villages in Kogi East Senatorial District, Nigeria. Source: Map Gallery, Geography Department, ABU Zaria.
2.2. Ethical Approval
Ethical clearance was obtained from the Research Ethics Committee, Kogi State Ministry of Health (KSMoH), Lokoja (reference number MOH/KGS/1376/1/82). Permission was obtained from the State Universal Basic Education Board (SUBEB), Lokoja, with reference number KG/SUBEB/GEN/04/‘T’, which was conveyed to the Education Secretaries of the nine LGAs and the headmasters/headmistresses of the schools.
2.3. Inclusion and Exclusion Criteria
Children attending schools in rural communities of Kogi state with ages ranging from 5 to 14 years were included in this study. Preschool-aged children (<5 years) and children older than 14 years attending rural schools in Kogi state were excluded from this study.
2.4. School Mobilization and Sensitization
Advocacy visits were paid to the Honourable Commissioner for Health, and this was preceded by letters from the KSMoH and also the SUBEB to the Education Secretaries of the Local Government Education Authorities (LGEAs).
2.5. Study Design
The study was carried out between January and May in 2018. District-wide mapping for STH infections was conducted in all nine LGAs of Kogi East Senatorial District (Ankpa, Bassa, Dekina, Ibaji, Idah, Igalamela/Odolu, Ofu, Olamaboro, and Omala LGAs) in a coordinated manner using Federal Ministry of Health protocol (this protocol recommended a minimum of 100 samples per LGA, i.e., for every homogenous setting) on integrated epidemiological mapping and baseline survey for STHs [1], which is also in line with the WHO [20] National Protocol Framework. Randomized selection of schools was done followed by a randomized systematic selection of children in the schools to be surveyed. Enrolled school-age children were targeted from the surveyed schools.
2.6. Sample Size
A total of 100 pupils were sampled in each LGA according to the WHO 2010 [20] national protocol framework. This study sampled 36 pupils per school, that is, 36 pupils in five schools (180 samples) in nine LGAs, which gave a sample size of 1620. However, with the minimum sample size of 100 pupils per LGA, the minimum sample size of 900 was reached.
2.7. Statement of Consent from Participants
Written consents were obtained from the guardians/parents of study participants, informing them of their rights and granting permission for their children to participate in the study.
2.8. Selection of Participating Schools and Children
In all selected LGAs, five schools were randomly selected from different rural communities of the LGAs, that is, a total of 45 schools were sampled. A sampling frame developed was used for selection of pupils in each selected school. A total of 36 pupils of both sexes, aged 5–16 years (from class one to class six), from each school were selected on pro-rata basis.
2.9. Sample Collection and Parasitological Examination
Stool samples were collected from selected schoolchildren using sterile specimen bottles. Each child in the study was given a sterile specimen bottle to take home after they were given instructions/explanations on how sample collection should be carried out. A single fecal sample was collected from each child and preserved using 10% formalin. Stool samples were taken to the laboratory of the Department of Animal and Environmental Biology, Kogi State University, Anyigba, for parasitological examination using the formal ether sedimentation technique [21].
In a suitable container, 1 g of stool sample was mixed thoroughly with 10 ml saline solution to form an emulsion, which was then filtered through fine-mesh gauze into a conical centrifuge tube. Suspension was centrifuged at Relative Centrifuge Force (RCF) of 600 g (about 2000 rpm) for about 10 min, yielding about 0.5 ml of sediment. After the supernatant was discarded, the sediment was washed with 10 ml saline solution and then recentrifuged. This was done repeatedly until the supernatant became clear. After the last wash, supernatant was discarded and 10 ml of 10% formalin was added, mixed, and then the mixture was allowed to stand for 5 min to effect fixation. About 2 ml ethyl acetate was added; then the tube was stoppered and vigorously mixed. The mixture was centrifuged at 450 g RCF (about 1500 rpm) for 10 min, which gave four results: a top layer of ethyl acetate, plug of debris, layer of formalin, and sediment. Plug of debris from the side of the tube was removed using the applicator stick, and the top three layers were carefully discarded. With a pipette, the remaining sediment was mixed with the small amount of fluid, and a drop each was transferred to a drop of saline and iodine on a glass side, covered with coverslip and examined microscopically for the presence of parasitic forms.
2.10. Administration of Questionnaire
A structured questionnaire was administered to the school headmaster or headmistress to collect information on demographic data and information on potential STH risk factors.
2.11. Statistical Analysis
Data were entered using Microsoft Excel (version 2013; Microsoft Corp., Redmond, WA, USA). Descriptive statistics were used to compute the prevalence and Confidence Interval (CI) of the study population. Univariate analysis was used to determine the level of association with infection status, Odds Ratio (OR) and 95% CI were determined.
The Chi-square test was used to determine the relationship in the prevalence of STHs according to sex, age groups, and LGAs. All analyses were performed using SPSS software (version 22.0 for Windows; SPSS Inc., Chicago, IL, USA). Map visualization was carried out on DIVA-GIS version 7.5.0 (Developed by Robert J. Hijmans).
3. RESULTS
3.1. Demographics
A total of 1295 pupils submitted stool samples for examination; 683 (52.7%) of these children were males whereas 612 (47.3%) were females. The age distribution of the study population was as follows: 513 (39.6%), 769 (59.4%), and 13 (1.0%) for age groups 5–8 years, 9–12 years, and >12 years, respectively. All schools visited were located in the rural areas of the district.
3.2. Prevalence of STH Infections at School Level
The overall prevalence of STH infection was 17.1%, with prevalence rates ranging from 2.9% to 50.0% in schools. LGEA Primary School, Agbenema-Ife, Omala LGA (50.0%) had the highest rate of STH infections, followed by LGEA/Qua Ibo Church (QIC) Primary School, Ukwaja, Idah LGA (42.3%) and St. Martins de Pores Ejule, Ofu LGA (40.0%). STHs were found to be endemic in 42 of the 45 sampled schools in the nine LGAs of Kogi East Senatorial District. STH infection differed significantly among the schools (χ2 = 132.77, p < 0.05; Table 1).
| LGAs | Schools (n) | Number positive (prevalence in %) | |||
|---|---|---|---|---|---|
| STHs | Ascaris lumbricoides | Hookworms | Strongyloides stercoralis | ||
| Ankpa | All Saints Nur/Pri. Sch., Ikanekpo (21) | 8 (38.1) | 8 (38.1) | 0 (0) | 0 (0) |
| LGEA Pri. Sch., Opulega (25) | 5 (20.0) | 0 (0) | 5 (20.0) | 0 (0) | |
| Jephta Academy, Ogodo (37) | 6 (16.2) | 1 (2.7) | 4 (10.8) | 1 (2.7) | |
| LGEA Pri. Sch., Enokpoli (11) | 1 (9.1) | 0 (0) | 1 (9.1) | 0 (0) | |
| LGEA Central Pri. Sch., Enjema (18) | 3 (16.7) | 3 (16.7) | 0 (0) | 0 (0) | |
| Bassa | LGEA Pri. Sch., Akakana (29) | 3 (10.3) | 2 (6.9) | 1 (3.4) | 0 (0) |
| LGEA Anglican Pri. Sch., Oguma (31) | 6 (19.4) | 1 (3.2) | 5 (16.1) | 0 (0) | |
| Our Lady of Mercy, Sheria (36) | 3 (8.3) | 1 (2.8) | 2 (5.6) | 0 (0) | |
| LGEA/RCM Pri. Sch., Sheria (26) | 4 (15.4) | 4 (15.4) | 0 (0) | 0 (0) | |
| LGEA Pri. Sch., Londu (28) | 6 (21.4) | 5 (17.9) | 4 (14.3) | 0 (0) | |
| Dekina | LGEA Pri. Sch., Olubojo (27) | 7 (25.9) | 0 (0) | 7 (25.9) | 2 (7.4) |
| LGEA Pri. Sch., Ojofu (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| LGEA Pri. Sch., Ajiyolo-Akabe (30) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| LGEA Pri. Sch., Odu-Ogbaloto (35) | 5 (14.3) | 2 (5.7) | 5 (14.3) | 2 (5.7) | |
| LGEA Pri. Sch., Olofu (31) | 6 (19.4) | 0 (0) | 6 (19.4) | 0 (0) | |
| Ibaji | LGEA Pri. Sch., Itoduma (36) | 2 (5.6) | 0 (0) | 1 (2.8) | 1 (2.8) |
| LGEA Pri. Sch.., Onyedega (40) | 5 (12.5) | 2 (5.0) | 3 (7.5) | 0 (0) | |
| LGEA/Pilot Pri. Sch., Unale (40) | 3 (7.5) | 1 (2.5) | 1 (2.5) | 1 (2.5) | |
| LGEA Pri. Sch., Ejule-Ojebe (40) | 2 (5.0) | 2 (5.0) | 0 (0) | 0 (0) | |
| LGEA Pri. Sch., Odogwu (41) | 2 (4.9) | 0 (0) | 2 (4.9) | 0 (0) | |
| Idah | LGEA/QIC Pri. Sch., Ukwaja (26) | 11 (42.3) | 0 (0) | 11 (42.3) | 1 (3.8) |
| LGEA/Omepa Pri. Sch., Igalogba (24) | 3 (12.5) | 0 (0) | 3 (12.5) | 0 (0) | |
| St. Boniface Pri. Sch., Sabon Gari (21) | 4 (19.0) | 0 (0) | 4 (19.0) | 0 (0) | |
| Pilot Pri. Sch., Ede (29) | 2 (6.9) | 0 (0) | 3 (10.3) | 1 (3.4) | |
| LGEA/Ayegba Pri. Sch., Ubomu (24) | 2 (8.3) | 0 (0) | 2 (8.3) | 0 (0) | |
| Igalamela/Odolu | LGEA Pri. Sch., Ogbogbo 1 (29) | 2 (6.9) | 0 (0) | 1 (3.4) | 0 (0) |
| LGEA Pri. Sch., Ogbogbo 2 (22) | 6 (27.3) | 0 (0) | 5 (22.7) | 1 (4.5) | |
| LGEA Pri. Sch., Etutu (36) | 5 (13.9) | 1 (2.8) | 4 (11.1) | 0 (0) | |
| LGEA Pri. Sch., Ofuloko (20) | 7 (3.2) | 1 (5.0) | 6 (30.0) | 0 (0) | |
| LGEA Pri. Sch., Ujagba (9) | 2 (22.2) | 0 (0) | 0 (0) | 2 (22.2) | |
| Ofu | St. Martins Pri. Sch., Ejule (25) | 4 (16.0) | 1 (4.0) | 3 (12.0) | 1 (4.0) |
| LGEA Pri. Sch., Alome (22) | 6 (27.3) | 0 (0) | 6 (27.3) | 0 (0) | |
| St. Martins de Pores Pri. Sch., Ejule (40) | 16 (40.0) | 14 (35.0) | 3 (7.5) | 0 (0) | |
| RCM Pri Sch., Ikpokejo-Umomi (20) | 2 (10.0) | 0 (0) | 2 (10.0) | 0 (0) | |
| LGEA/UEC Pri. Sch., Ofakaga (30) | 4 (13.3) | 2 (6.7) | 2 (6.7) | 0 (0) | |
| Olamaboro | LGEA/RCM Pri. Sch., Ogugu (35) | 1 (2.9) | 1 (2.9) | 0 (0) | 0 (0) |
| LGEA/QIC Pri. Sch., Ogugu (36) | 3 (8.3) | 1 (2.8) | 2 (5.6) | 0 (0) | |
| St. Anthony Pri. Sch., Okpo (39) | 8 (20.5) | 2 (5.1) | 4 (10.3) | 1 (2.6) | |
| LGEA Pri. Sch., Ugbamaka-Igah (24) | 5 (20.8) | 0 (0) | 5 (20.8) | 0 (0) | |
| LGEA Pri. Sch., Igah-Ikeje (20) | 4 (20.0) | 0 (0) | 4 (20.0) | 0 (0) | |
| Omala | LGEA Central Pri. Sch., Abejukolo (40) | 9 (22.5) | 0 (0) | 9 (22.5) | 0 (0) |
| LGEA Pri. Sch., Opada (19) | 7 (36.8) | 0 (0) | 7 (36.8) | 0 (0) | |
| LGEA Pri. Sch., Agbenema-Ife (40) | 20 (50.0) | 0 (0) | 20 (50.0) | 0 (0) | |
| LGEA Islamiya Pri. Sch., Abejukolo (40) | 12 (30.0) | 1 (2.5) | 11 (27.5) | 0 (0) | |
| LGEA Pri. Sch., Ajiyolo-Ife (23) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Overall (1295) | 222 (17.1) | 56 (4.3) | 164 (12.7) | 14 (1.1) | |
| χ2 | 132.77 | 206.257 | 167.926 | 76.62 | |
| df | 44 | 44 | 44 | 44 | |
| p | 0.000* | 0.000* | 0.000* | 0.002* | |
Significant at p ≤ 0.05.
LGEA, Local Government Education Authority; ns, not significant at p > 0.05; QIC, Qua Ibo Church; Pri. Sch., primary school; RCM, Roman Catholic Mission; STH, soil-transmitted helminths; UEC, United Evangelical Church.
Prevalence of STHs in rural primary schools of Kogi East, Nigeria
The overall prevalence of A. lumbricoides infection was 4.3%, with prevalence ranging from 2.5% to 38.1% in schools. All Saint Nursery and Primary School, Ikanekpo, Ankpa LGA had the highest infection rate, followed by St. Martins de Pores Ejule, Ofu LGA with 35.0%. A. lumbricoides was endemic in 21 schools in eight LGAs. A. lumbricoides infection differed significantly among the schools (χ2 = 206.257, p < 0.05; Figure 2).
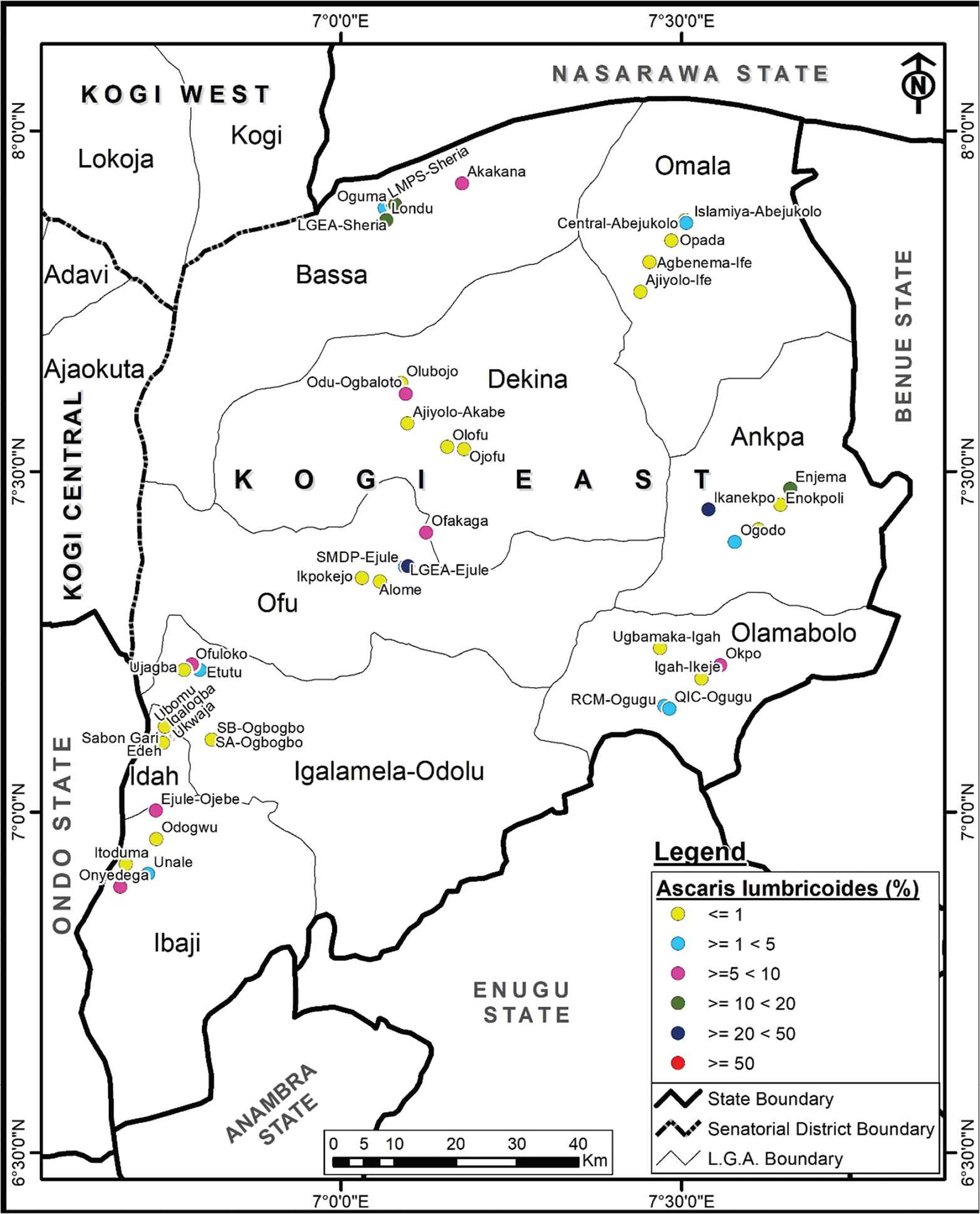
Spatial distribution of Ascaris lumbricoides in Kogi East, Nigeria.
Hookworms were found to be endemic in 36 schools in the nine LGAs, with an overall prevalence of 12.7% (ranging from 2.5% to 50.0% in schools). LGEA Primary School, Agbenema-Ife, Omala LGA (50.0%) had the highest prevalence of hookworm infection, followed by LGEA/QIC Ukwaja, Idah LGA with 42.3%. Hookworm infection differed significantly among the schools (χ2 = 167.926, p < 0.05; Figure 3).
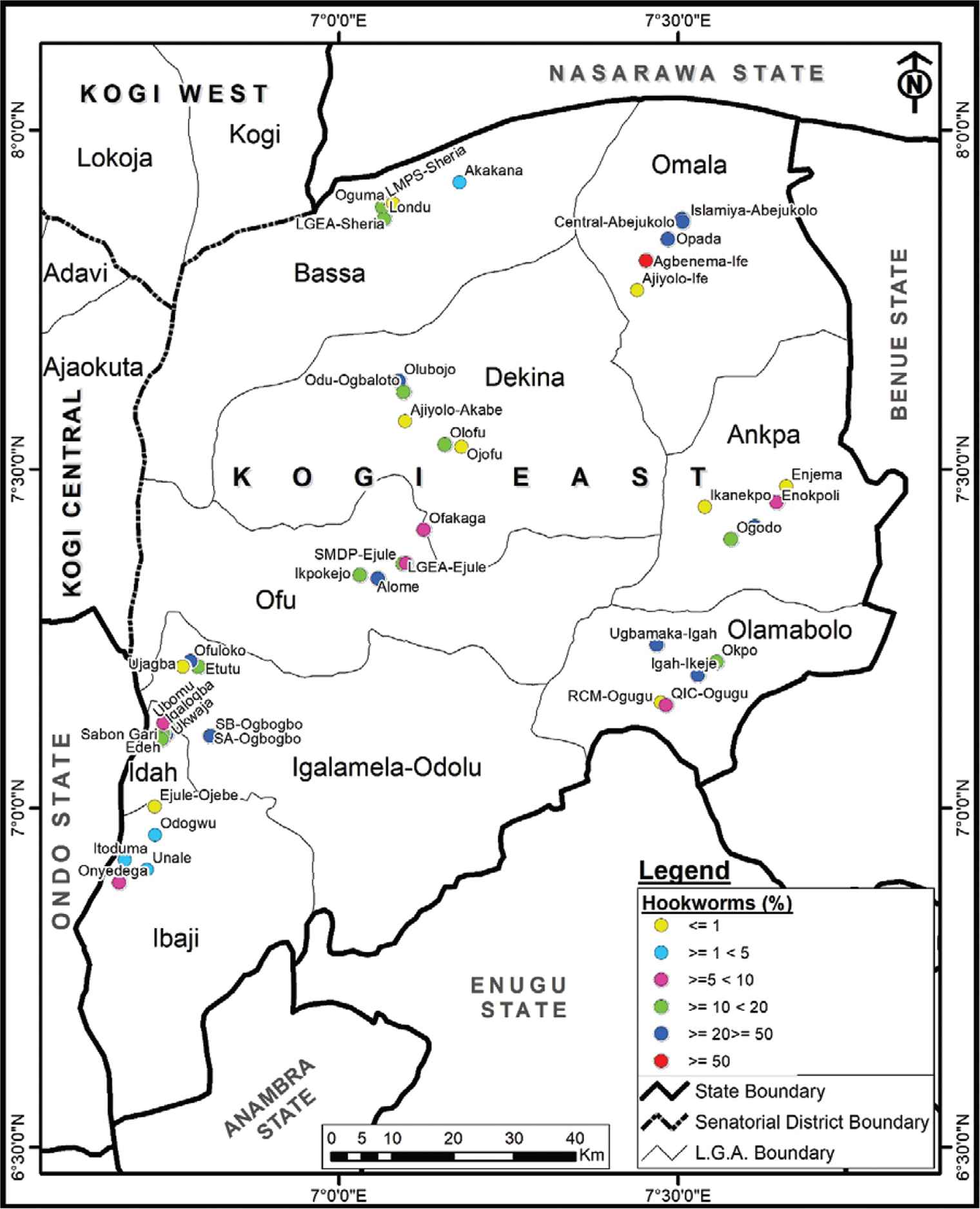
Spatial distribution of hookworms infection Kogi East, Nigeria.
Strongyloides stercoralis was endemic in 11 schools in seven LGAs with an overall prevalence of 1.1% (ranging from 2.5% to 22.2%). LGEA Primary School (Ujagba, Igalamela LGA) had the highest S. stercoralis infection. Prevalence differed significantly among the schools (χ2 = 76.62, p < 0.05; Figure 4).
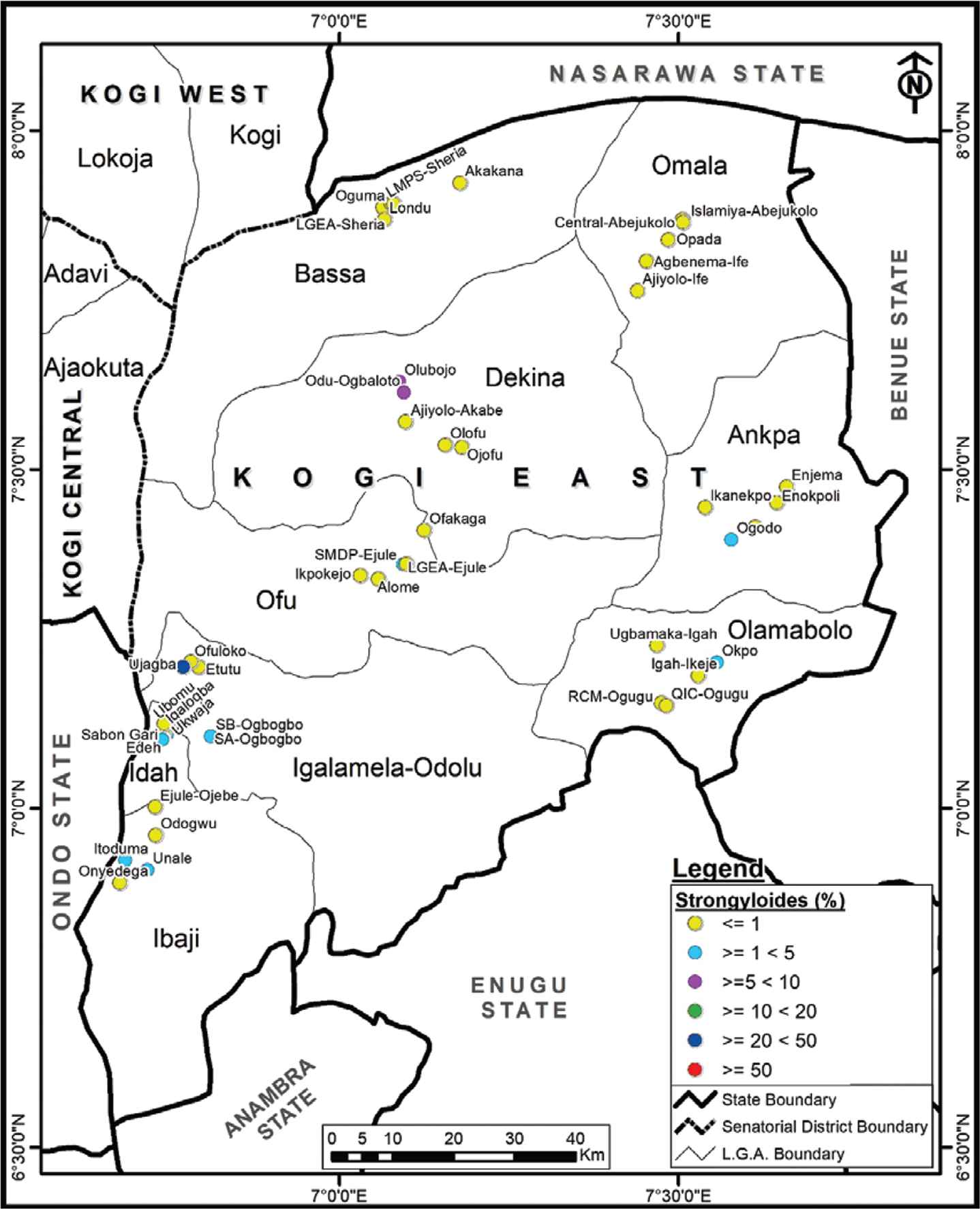
Spatial distribution of Strongyloides stercoralis in Kogi East, Nigeria.
3.3. Prevalence of STH Infections at LGA Level
The prevalence of STHs at the LGA level was determined by pooling together the prevalence of the five schools sampled within the LGA. The prevalence of STH infection was highest in Omala LGA (29.6%), followed by Ofu LGA (23.4%), and Ankpa LGA (20.5%); meanwhile, Ibaji LGA had the lowest prevalence (7.1%). Prevalence rates ranged from 7.1% to 29.6% at the LGA level. Prevalence differed significantly at the LGA level (χ2 = 40.758, p < 0.05; Table 2).
| Categories (n) | Number positive (prevalence in %) | |||
|---|---|---|---|---|
| STHs | Ascaris lumbricoides | Hookworms | S. stercoralis | |
| LGAs | ||||
| Ankpa (112) | 23 (20.5) | 12 (10.7) | 10 (8.9) | 1 (0.9) |
| Bassa (150) | 22 (14.7) | 13 (8.7) | 12 (8.0) | 0 (0) |
| Dekina (143) | 18 (12.6) | 2 (1.4) | 18 (12.8) | 4 (2.8) |
| Ibaji (197) | 14 (7.1) | 5 (2.5) | 7 (3.6) | 2 (1.0) |
| Idah (124) | 22 (17.7) | 0 (0) | 23 (18.5) | 2 (1.6) |
| Igalamela (116) | 22 (19.0) | 2 (1.7) | 16 (13.8) | 3 (2.6) |
| Ofu (137) | 32 (23.4) | 17 (12.4) | 16 (11.7) | 1 (0.7) |
| Olamaboro (154) | 21 (13.6) | 4 (2.6) | 15 (9.7) | 1 (0.6) |
| Omala (162) | 48 (29.6) | 1 (0.6) | 47 (29.0) | 0 (0) |
| Overall (1295) | 222 (17.1) | 56 (4.3) | 164 (12.7) | 14 (1.1) |
| χ2 | 40.758 | 58.001 | 63.621 | 10.605 |
| df | 8 | 8 | 8 | 8 |
| p | 0.000* | 0.000* | 0.000* | 0.225 ns |
| Sex | ||||
| Male (683) | 124 (18.2) | 33 (4.8) | 90 (13.2) | 7 (1.0) |
| Female (612) | 98 (16.0) | 23 (3.8) | 74 (12.1) | 7 (1.1) |
| 1295 | 222 (17.1) | 56 (4.3) | 164 (12.7) | 14 (1.1) |
| χ2 | 1.043 | 0.899 | 0.344 | 0.043 |
| df | 1 | 1 | 1 | 1 |
| p | 0.307 ns | 0.343 ns | 0.558 ns | 0.836 ns |
| Age group (years) | ||||
| 5–8 (513) | 85 (16.6) | 25 (4.9) | 59 (11.5) | 5 (1.0) |
| 9–12 (769) | 137 (17.8) | 31 (4.0) | 105 (13.7) | 9 (1.2) |
| >12 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1295 | 222 (17.1) | 56 (4.3) | 164 (12.7) | 14 (1.1) |
| χ2 | 3.053 | 1.121 | 3.194 | 0.254 |
| df | 2 | 2 | 2 | 2 |
| p | 0.217 ns | 0.571 ns | 0.203 ns | 0.881 ns |
| Class | ||||
| One (161) | 31 (19.3) | 11 (6.8) | 19 (11.8) | 2 (1.2) |
| Two (243) | 35 (14.4) | 9 (3.7) | 27 (11.1) | 2 (0.8) |
| Three (191) | 32 (16.8) | 7 (3.7) | 25 (13.1) | 2 (1.0) |
| Four (244) | 38 (15.6) | 9 (3.7) | 29 (11.9) | 1 (0.4) |
| Five (193) | 34 (17.6) | 8 (4.1) | 24 (12.4) | 3 (1.6) |
| Six (263) | 52 (19.8) | 12 (4.6) | 40 (15.2) | 4 (1.5) |
| Overall (1295) | 222 (17.1) | 56 (4.3) | 164 (12.7) | 14 (1.1) |
| χ2 | 3.543 | 3.164 | 2.353 | 2.101 |
| df | 5 | 5 | 5 | 5 |
| p | 0.617 ns | 0.675 ns | 0.799 ns | 0.835 ns |
Significant at p ≤ 0.05.
LGEA, Local Government Education Authority; ns, not significant at p > 0.05; STH, soil transmitted helminths.
Prevalence of STHs according to categories in rural schools of Kogi East, Nigeria
The prevalence of A. lumbricoides infection ranged from 0.6% in Omala LGA to 12.4% in Ofu LGA. Prevalence of A. lumbricoides differed significantly at the LGA level (χ2 = 58.001, p < 0.05).
The prevalence of hookworm infection ranged from 3.5% in Ibaji LGA to 29.0% in Omala LGA. No significant difference was observed in the prevalence of hookworms at the LGA level (p > 0.05).
The prevalence of S. stercoralis infection at the LGA level was lower than 3.0%, with prevalence ranging from 0.6% in Olamaboro LGA to 2.8% in Dekina LGA. No significant difference (p > 0.05) in prevalence was observed.
3.4. Prevalence According to Sex, Age Group, and Class
The prevalence of STH infection was higher in males (18.2%) than in females (16.0%). This is also applicable to A. lumbricoides with 4.8% prevalence in males and 3.8% in females, and hookworms with prevalence of 13.2% in males and 12.1% in females (Table 2). Nevertheless, no significant difference (p > 0.05) was observed in the prevalence of all STHs between male and female pupils; however, the odds of male pupils being infected with A. lumbricoides (OR = 1.300; 95% CI, 0.755–2.240) and hookworms (OR = 1.103; 95% CI, 0.794–1.533) was higher compared with that of females (Table 3).
| Categories | Odds ratio of pupils infected (95% CI) | |||
|---|---|---|---|---|
| STHs | Ascaris lumbricoides | Hookworms | Strongyloides stercoralis | |
| Sex | ||||
| Male (683) | 1.163 (0.870–1.556) | 1.300 (0.755–2.240) | 1.103 (0.794–1.533) | 0.895 (0.312–2.566) |
| Female (612) | 1 | 1 | 1 | 1 |
| Age group (y) | ||||
| 5–8 (513) | 1 | 1 | 1 | 1 |
| 9–12 (769) | 1.092 (0.811–1.469) | 0.820 (0.478–1.406) | 1.217 (0.866–1.710) | 0.665 (0.192–2.309) |
| >(13) | – | – | – | – |
| Class | ||||
| One (161) | 1 | 1 | 1 | 1 |
| Two (243) | 0.706 (0.415–1.200) | 0.525 (0.212–1.296) | 0.934 (0.501–1.744) | 0.660 (0.092–4.732) |
| Three (191) | 0.844 (0.489–1.456) | 0.519 (0.196–1.371) | 1.126 (0.595–2.129) | 0.841 (0.117–6.040) |
| Four (244) | 0.774 (0.459–1.305) | 0.522 (0.211–1.290) | 1.008 (0.544–1.867) | 0.327 (0.029–3.638) |
| Five (193) | 0.897 (0.523–1.537) | 0.590 (0.231–1.503) | 1.061 (0.559–2.018) | 1.255 (0.207–7.606) |
| Six (263) | 1.034 (0.630–1.696) | 0.652 (0.281–1.514) | 1.341 (0.747–2.407) | 1.228 (0.222–6.781) |
CI, confidence interval; ns, not significant at p > 0.05.
Univariate analysis of STH risk factors in rural schools, Kogi East, Nigeria
The prevalence of STH infections do not differ significantly (p > 0.05) among age groups and according to the class of pupils (Table 2). The age group of 9–12 years (17.8%) had a higher prevalence than the age group of 5–8 years (16.6%), and pupils aged >12 years had no parasites. Pupils within the age group of 9–12 years had a higher odds of being infected with hookworms (OR = 1.217; 95% CI, 0.866–1.710) than those within the age group of 5–8 years. The odds of being infected increases for hookworms and S. stercoralis as the pupils progress from junior class to senior class (Table 3).
3.5. Risk Factors Associated with STH Infections in Kogi East District
The risk factors associated with STH in Kogi East are presented in Table 3. All of the human risk factors examined did not significantly (p > 0.05) influence the transmission of STHs (Table 4).
| Risk factor/response (n) | Status | χ2 | df | p (ns) | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||||
| Source of water for drinking | |||||||
| Pipe water (79) | 16 (20.3) | 63 (79.7) | 0.129 | 1 | 0.719 | 1.165 | 0.650–2.087 |
| Borehole (676) | 121 (17.9) | 555 (82.1) | 1 | ||||
| River/stream (540) | 85 (15.7) | 455 (84.3) | 0.847 | 1 | 0.358 | 0.857 | 0.632–1.161 |
| Method of fecal disposal | |||||||
| Water closet system (231) | 31 (13.4) | 200 (86.6) | 1 | ||||
| Pit latrine (442) | 75 (17.0) | 367 (83.0) | 1.185 | 1 | 0.276 | 1.318 | 0.839–2.073 |
| Open defecation (622) | 116 (18.6) | 506 (81.4) | 2.874 | 1 | 0.090 | 1.479 | 0.963–2.271 |
| Wash hands after using toilet | |||||||
| Yes (188) | 25 (13.3) | 163 (86.7) | 1 | ||||
| No (1107) | 197 (17.8) | 910 (82.2) | 1.983 | 1 | 0.159 | 1.412 | 0.902–2.210 |
| Wash hands prior to eating | |||||||
| Yes (1237) | 210 (17.0) | 1027 (83.0) | 1 | ||||
| No (58) | 12 (20.7) | 46 (79.3) | 0.308 | 1 | 0.579 | 1.276 | 0.664–2.450 |
| Walk bare feet | |||||||
| Yes (739) | 135 (18.3) | 604 (81.7) | 1.355 | 1 | 0.244 | 1.205 | 0.897–1.619 |
| No (556) | 87 (15.6) | 469 (84.4) | 1 | ||||
| When last were you dewormed? | |||||||
| 3 mo (149) | 20 (13.4) | 129 (86.6) | 1 | ||||
| 6 mo (338) | 54 (16.0) | 284 (84.0) | 0.344 | 1 | 0.558 | 1.226 | 0.705–2.133 |
| No (808) | 148 (18.3) | 660 (81.7) | 1.758 | 1 | 0.185 | 1.446 | 0.874–2.393 |
| Finger nail status | |||||||
| Trimmed (442) | 75 (17.0) | 367 (83.0) | 1 | ||||
| Untrimmed (853) | 147 (17.2) | 706 (82.8) | 0.002 | 1 | 0.966 | 1.019 | 0.751–1.383 |
CI, confidence interval; ns, not significant at p > 0.05.
Risk factors associated with STHs in Kogi East, Nigeria
4. DISCUSSION
This study attempted to compare the current status and risk factors associated with STHs in pupils of rural schools of Kogi East. This study revealed that STH infections remain a major public health problem in Kogi State and are prevalent among schoolchildren in the rural communities of Kogi East [22]. The overall prevalence of STHs observed in this study was higher than that observed in Chencha town, Ethiopia (2.2%) [23]; Were-abaye, Ethiopia (12.22%) [24]; Babile town, Ethiopia (13.8%) [25]; Zana wereda, Ethiopia (12.7%) [4]; and Cameroon (6.3%) [26]; but lower than that observed in Adwa town, Ethiopia (69.0%) [27]; Lumame town, Ethiopia (54%) [28]; Northern Gondar, Ethiopia (66.7%) [29]; Zegie Peninsula, Ethiopia (69.1%) [30]; Jimma, Ethiopia (45.6%) [25]; Ogun State, Nigeria (34.6%) [31]; and Jos, Nigeria (42.6%) [32]. They attributed the differences in prevalence to the topography of the study areas, living standards, as well as personal and environmental hygiene of the communities studied. They also reported that poor sanitary facilities of the schools contributed to the higher prevalence. Water, sanitation, and hygiene infrastructures were lacking in most of the schools visited. Blum and Hotez [33] stated that differences in the fundamental biology and thermo-tolerance of STHs could play a large role in determining their prevalence in this decade.
The overall prevalence of A. lumbricoides in this study was lower than what was observed in school-age pupils of rural areas of Southern China (18.5%) [34]; southern rural Lao People’s Democratic Republic (PDR) (27.4%) [35]; Jimma, Ethiopia (23.6%) [25]; Zana wereda, Ethiopia (11.5%) [4]; Ogun State, Nigeria (29.3%) [31]; and Jos, Nigeria (25.7%) [32]. The possible reason for the higher prevalence in those studies compared with this study could be that the environment of where those studies were carried out may be more favorable for completion of the life cycle of the parasite such as fertile soil, and humid and wet environment. Kounnavong et al. [35] observed that improper disposal of human waste, contaminated drinking water, and poor sanitation might be responsible for infections with A. lumbricoides in resource-poor settings.
The overall prevalence of hookworms in this study was higher than those reported in studies conducted in Chencha town, Ethiopia (2.2%) [23] and Babile town, Ethiopia (6.7%), [36] and almost similar to that of Jimma town, Ethiopia (12.9%) [37] but lower than that observed in Zana wereda, Ethiopia (32.7%) [4]. Hookworms were the most prevalent and the most widespread STH parasite in Kogi East. A similar observation was reported in Ogun State, Nigeria [31,38]. The authors attributed this to the location of Nigeria in the equatorial zone with favorable environment for the development of the larvae of hookworms. Several studies have reported that hookworm larvae in African soils remain viable up to land surface temperature of 40°C or higher [39–41], unlike A. lumbricoides and T. trichiura eggs that cease to develop at 38°C [39,42]. Furthermore, the motility of infective hookworm larvae [39–41] and accelerated development (3–100 days) in comparison with 10–30 days and 28–84 days for T. trichiura and A. lumbricoides eggs, respectively, could confer advantages, as do adult hookworms’ longer life spans [42], and hence the high prevalence.
Trichuris trichiura was not endemic in Kogi East Senatorial District, Nigeria. A systematic meta-analysis on the prevalence of STH infection in Nigeria [22] also revealed that T. trichiura was not endemic in Kogi State.
The findings of this study revealed that age, sex, and class of pupils had no significant effect on STH infection in Kogi East.
The univariate analysis revealed the risk factors that significantly played an important role in the prevalence of STH infections in Kogi East. It was observed in this study that as the age of the children increased, so did the odds of having hookworm infection, whereas the odds of having A. lumbricoides infection decreased. Increased age is a protective factor for both A. lumbricoides and T. trichiura infections [43–46] and a risk factor for hookworm infections [47–50] as observed in these studies. A similar observation was reported in Honduras by Gabrie et al. [51], who stated that the odds of being infected with A. lumbricoides reduced by 20% as the age increased by 1 year and increased for hookworms by 20% as the age increased by 1 year. They explained that no plausible validity of why a particular age group should have high odds of infection than others and advised that further studies to evaluate deworming treatment and its differential effect on age groups will be useful.
It was observed in this study that male pupils had higher odds of STH infections compared with females. A similar observation was reported in children of Lao PDR [52], schoolchildren of periurban Guinea-Bissau [50], West African children [49], schoolchildren in Sri Lanka [53], and schoolchildren in Cote d’Ivoire [54]. No tangible reasons have been offered to explain the differential exposure of males and females. However, several studies have indicated that the social roles and behaviors of male pupils (in comparison with female pupils) could have led to higher exposure to infections. Further studies to evaluate the impact of social behaviors between sexes of pupils will help provide a clearer understanding.
The low prevalence of STH infections in this study was attributed to the deworming activities carried out by KSMoH in collaboration with WHO. However, the school-based deworming programme did not cut across most of the schools in rural areas. Some schools reported that no deworming drugs have been administered in the past 3 years especially in Omala LGA.
During the study, it was observed that river water delivered by tankers to villagers is the major source of drinking water in most of the villages. This drinking water is often contaminated with infectious human feces. A similar observation was reported in Southwestern China [55]. Therefore, it is recommended that safe drinking water sources be provided to rural schools of Kogi East. In addition, the unavailability of school hygiene and sanitation facilities contributed immensely to the infections observed. A study carried out in Kenya highlighted the need for improved school hygiene and sanitation to reduce STH infections after school-based deworming [56]. Ziegelbauer et al. [57] carried out a meta-analysis of the effect of sanitation on STHs and reported a reduction in infection rates between 40% and 50% where adequate sanitation is available. It is also recommended that functional toilet facilities should be made available in rural schools of Kogi State.
This study did not measure the actual usage of functional sanitary facilities available in the schools and homes of the children sampled. Therefore, further studies should be carried out to ascertain whether children were able or willing to use sanitary facilities at home or in schools were they are available.
5. CONCLUSION
This study revealed that STH is endemic among rural pupils of Kogi East. The overall prevalence of STH infections in Kogi East is 17.1%, with A. lumbricoides, hookworms, and S. stercoralis having prevalence rates of 4.3%, 12.7%, and 1.1%, respectively. Hookworms was the most prevalent STH in Kogi East, whereas T. trichiura was not endemic in Kogi East, Nigeria. Intensifying the distribution of antihelminthic drugs to rural schools will help further reduce the prevalence of infections. The findings of this study revealed the need for the use of integrated management strategies to control STH infections such as the inclusion of health education programme alongside school-based deworming.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
CAY, EK and SAL contributed in study conceptualization and writing (review and editing) of the manuscript. CAY contributed in data curation, formal analysis, and writing (original draft). EK, SAL and JK contributed in project administration and supervision. EK, SAL and JK contributed in review and editing of the manuscript. All authors contributed in validation of the manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the Chairman, Kogi State Universal Basic Education Board, Lokoja, Kogi State, Nigeria; the Education Secretaries of Ankpa, Bassa, Dekina, Ibaji, Idah, Igalamela/Odolu, Ofu, Olamaboro and Omala LGEAs Kogi State; the Commissioner of Health and Director NTDs Unit, Kogi State Ministry of Health, Lokoja for the permission to carry out the study in schools. We also thank the Department of Animal and Environmental Biology, Kogi State University, Anyigba, for providing the laboratory space for parasitological examinations.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Clement Ameh Yaro AU - Ezekiel Kogi AU - Sodangi Abdulkarim Luka AU - Junaidu Kabir PY - 2020 DA - 2020/02/21 TI - School-based Cross-sectional Survey on Soil-transmitted Helminths in Rural Schools of Kogi East, Nigeria JO - Dr. Sulaiman Al Habib Medical Journal SP - 10 EP - 19 VL - 2 IS - 1 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200211.002 DO - 10.2991/dsahmj.k.200211.002 ID - Yaro2020 ER -
