Tuberculous Meningitis in an Immunocompetent Host: A Diagnostic Challenge
 , Bader Shirah2, Nawal Abdelghaffar3, 4, Abdulrahman J. Alqahtani5, Mohammad Alshehri5
, Bader Shirah2, Nawal Abdelghaffar3, 4, Abdulrahman J. Alqahtani5, Mohammad Alshehri5- DOI
- 10.2991/dsahmj.k.200917.001How to use a DOI?
- Keywords
- Tuberculous meningitis; immunocompetent; brain biopsy; challenging diagnosis; Saudi Arabia
- Abstract
Tuberculosis is a major global public health problem, which poses significant diagnostic and treatment challenges. Tuberculous meningitis is the most severe form of extrapulmonary tuberculosis, which approximately affects 1% of all patients with active tuberculosis. It is considered a medical emergency as it causes neurological deficits in more than half of those affected and may lead to death despite advancement in antituberculous medications. In this article, we are reporting a challenging case of tuberculous meningitis with a delay in diagnosis and initiation of treatment. In addition, we summarize the challenges regarding the diagnosis and management of this disease. Although central nervous system tuberculosis is rare in immunocompetent hosts, such cases can be seen in neurology practice and represent a major clinical and diagnostic challenge. Although this case is not an uncommon scenario and no alternative diagnosis is suspected, the patient was not started on antituberculous medications because of an unjustified request for pathological diagnosis by the admitting team. Although Saudi Arabia is not one of the 22 countries with the highest number of tuberculosis cases worldwide, tuberculosis continues to represent a major health problem. Treating clinicians should try to use methods to increase the mycobacterial yield of cerebrospinal fluid smear examination. Multidisciplinary care, not a one-man show, should be practiced with cooperation and exchange of opinions between neurologists, infectious diseases specialists, neurosurgeons, and intensivists. Brain or meningeal biopsy is not a routine investigation tool and should never delay the initiation of antituberculous medications. Despite advancements in the field of tuberculosis science, it continues to represent a clinical and diagnostic challenge with a substantial percentage of morbidity and mortality.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Tuberculosis is a major global public health problem, which poses significant diagnostic and treatment challenges. Worldwide, it ranks as one of the top 10 causes of death. Approximately 10 million people are affected annually, among which 1.5 million die from the disease [1]. Tuberculous meningitis is the most severe form of extrapulmonary tuberculosis, which approximately affects 1% of all patients with active tuberculosis. It is considered a medical emergency as it causes neurological deficits in more than half of those affected and may lead to death despite advancement in antituberculous medications [2]. In this article, we are reporting a challenging case of tuberculous meningitis with a delay in diagnosis and initiation of treatment. In addition, we summarize the challenges regarding the diagnosis and management of this disease.
2. CASE REPORT
A 60-year-old female presented to the emergency department with headache, neck pain, and low back pain that started 6 months ago. Two weeks prior to presentation, she had difficulty walking with no sphincter control disturbance or sensory symptoms. She had no history of diabetes mellitus, infectious disease, or dyslipidemia. She is a housewife who is married and living with her children. Although no family history of tuberculosis was documented, she gave a strong history of contact with a patient who has open pulmonary tuberculosis that was not on treatment. Her systemic examination was normal including vital signs, lung auscultation, cardiac examination, and skin inspection. As part of her investigations, she had normal complete blood count, renal and liver function tests, and electrolytes including sodium, magnesium, and phosphate. Her erythrocyte sedimentation rate was high at 64. The result of her human immunodeficiency virus test was negative, and the chest X-ray was normal. Magnetic Resonance Imaging (MRI) of the whole spine revealed intramedullary dorsal (thoracic) ring-enhancing lesion at the level of T10–T11 (Figure 1). MRI of the brain showed diffuse meningeal enhancement with no intraparenchymal cerebral or cerebellar lesions (Figure 2). Cerebrospinal Fluid (CSF) analysis revealed a lymphocytic pleocytosis with a leukocyte count of 90 cells (reference range, 0–5), lymphocyte predominance (87%), raised protein of 236 mg/dL (reference range, 15–60 mg/100 mL), low CSF glucose 18 mg/dL (reference range, 50–80 mg/100 mL). CSF Gram stain, culture, and sensitivity were negative for all bacteria including mycobacterium tuberculosis. In addition, the Polymerase Chain Reaction (PCR) for viral, bacterial, and mycobacterial DNA was negative. Unfortunately, and despite tuberculous meningitis being the most likely diagnosis based on clinical and paraclinical data, the patient was not started on antituberculous medications. The treating infectious diseases consultant requested a brain and meningeal biopsy, which was declined by the patient’s family. The consultant refused to start the patient on antituberculous medications if no tissue is obtained for pathological confirmation of diagnosis. The patient’s condition deteriorated, and she was admitted to the intensive care unit with intubation and mechanical ventilation. The patient developed status epilepticus and was started on levetiracetam 1000 mg twice daily. She developed focal neurological deficits and multiple cranial nerve involvement. A repeated MRI of the brain showed an acute ischemic infarction with restricted left capsular, medial temporal, and hippocampal lesions in the apparent diffusion coefficient map (Figure 3). It also revealed persistence and even worsening of meningeal enhancement. Repeated CSF analysis revealed low glucose at 8, high protein at 255, and white blood cells count of 190 with lymphocytes (85%) and neutrophils (15%). Owing to the family request (based on neurology team recommendation) as well as clinical and radiological deterioration, the patient was started on antituberculous medications (rifampicin 600 mg, isoniazid 300 mg, pyrazinamide 1500 mg, and ethambutol 1200 mg) and oral corticosteroids through a nasogastric tube. This was several weeks after admission to the intensive care unit. Despite the advanced stage of the disease, the development of endarteritis obliterans with multiple ischemic strokes, and poor clinical condition, the patient’s condition improved, and her seizures stopped. Her neurological status improved, and she became more alert with better power in all limbs, and after 3 weeks, she was weaned off ventilation and was extubated. The patient was transferred to a rehabilitation center for an aggressive physiotherapy regimen, and the family was instructed to continue the antituberculous medications for a full 12-month period. Unfortunately, the patient died from neurological complications related to tuberculous meningitis (multiple infarcts with superadded massive pulmonary embolism).
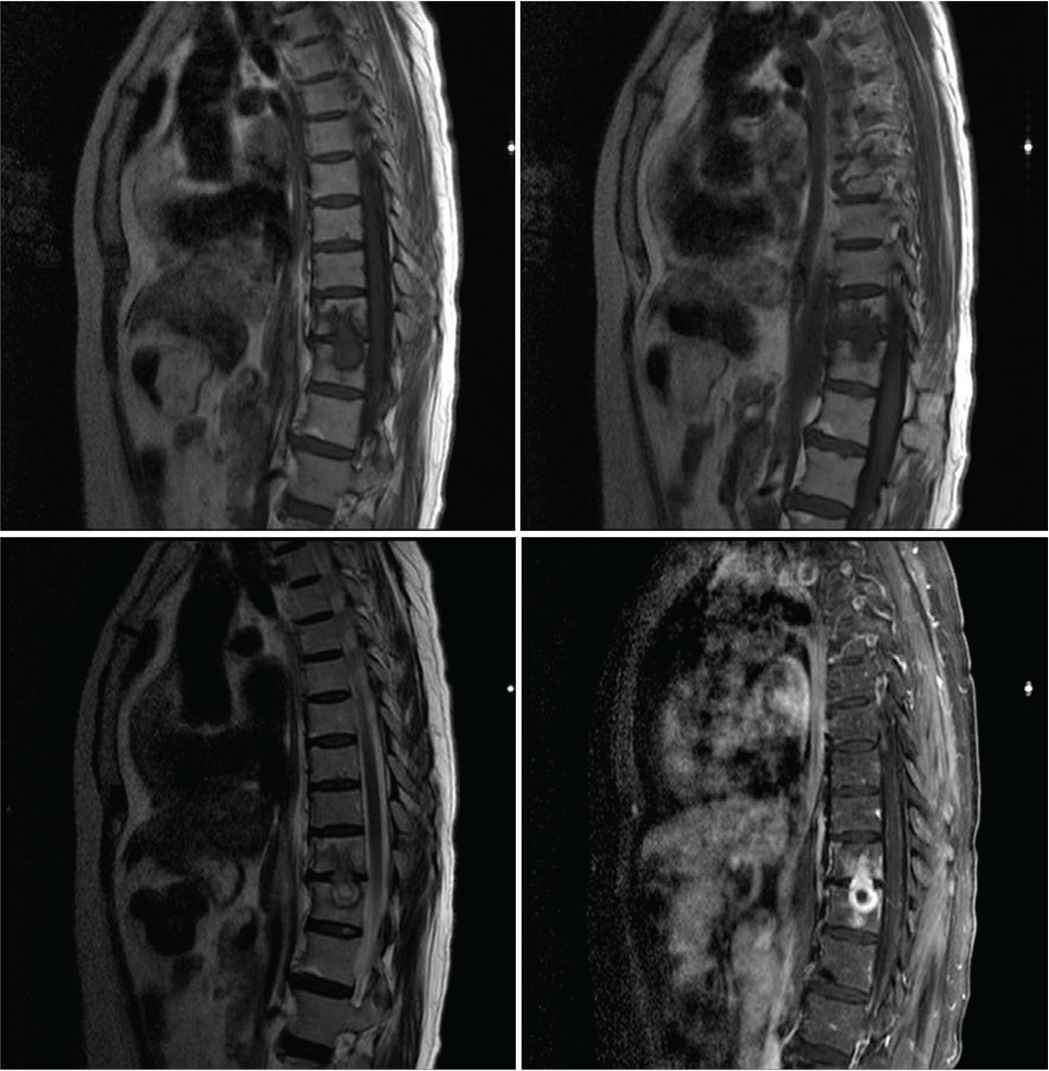
Magnetic Resonance Imaging (MRI) scan of the whole spine showing intramedullary dorsal (thoracic) ring-enhancing lesion at the level of T10–T11.
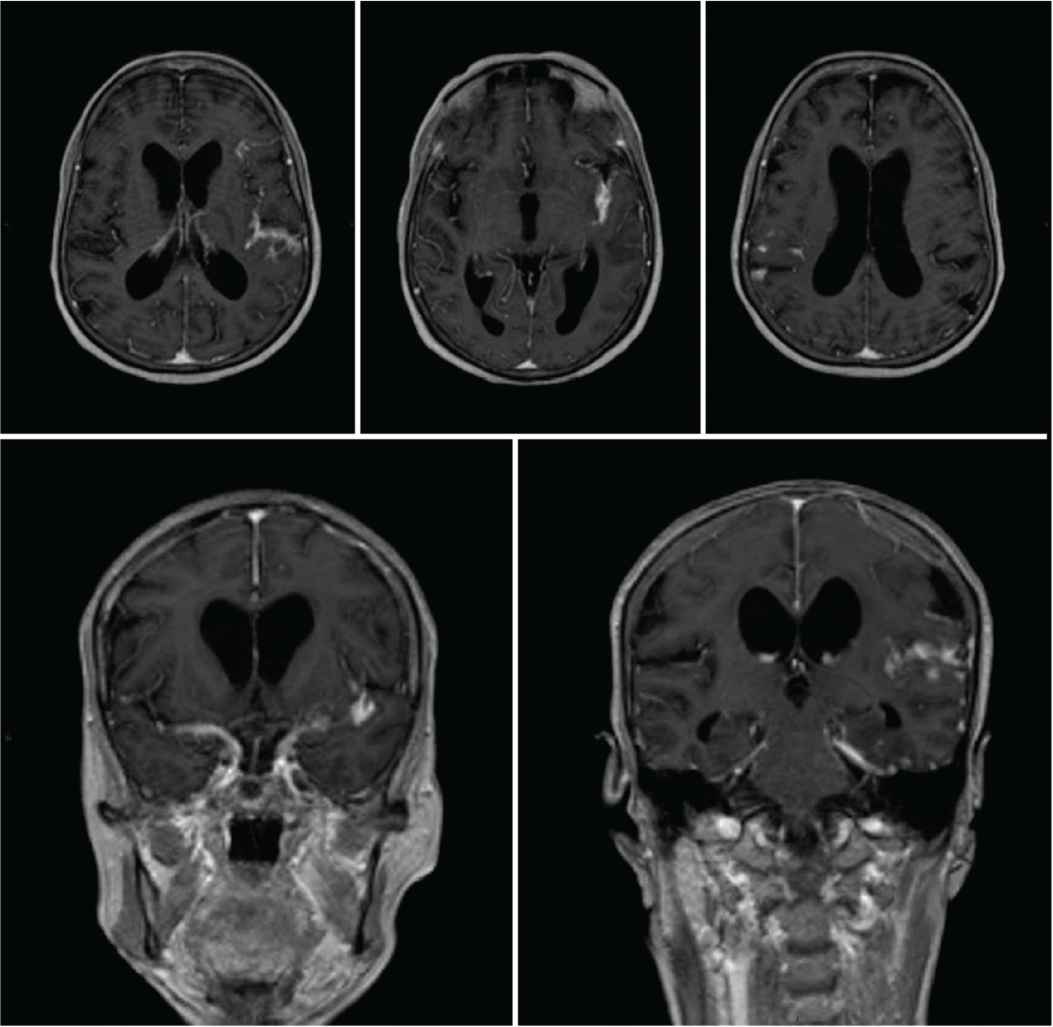
Magnetic Resonance Imaging (MRI) scan of the brain showing diffuse meningeal enhancement with no intraparenchymal cerebral or cerebellar lesions.
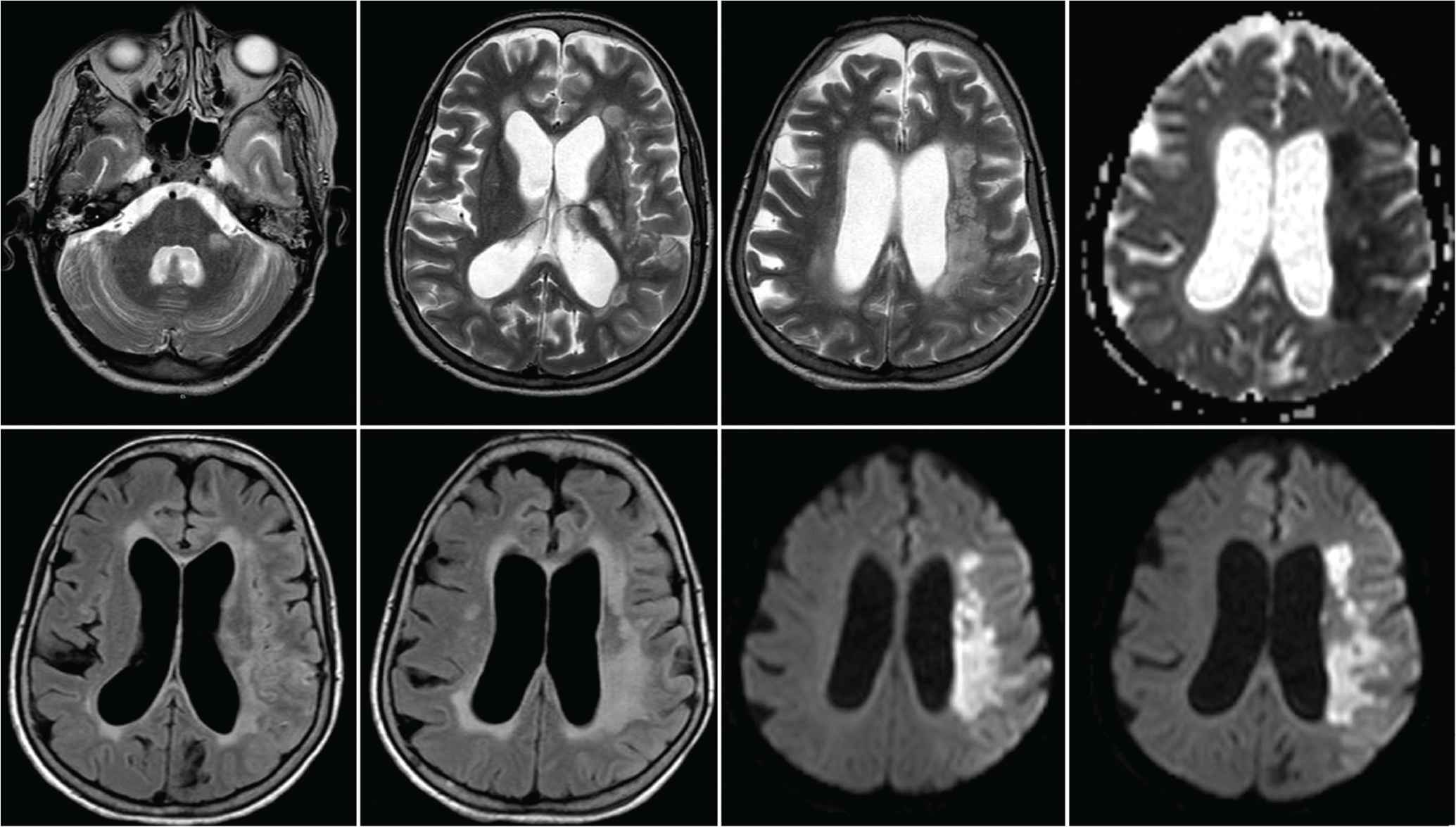
Magnetic Resonance Imaging (MRI) scan of the brain showing an acute ischemic infarction with restricted left capsular, medial temporal, and hippocampal lesions in the apparent diffusion coefficient map.
3. DISCUSSION
Central Nervous System (CNS) tuberculosis is a devastating medical condition caused by Mycobacterium tuberculosis manifesting as meningoencephalitis, abscesses, or tuberculomas. It was universally fatal in the era prior to the discovery of antituberculous medications, and it continues to represent a major health problem with a mortality rate of 20–50% of patients, and many of the survivors have significant neurological deficits. It accounts for approximately 1% of all cases of tuberculosis and remains a formidable diagnostic challenge. Risk factors to acquire this disease include children, immunocompromised patients, malnutrition, malignancies, and alcoholism [3].
The clinical presentation of tuberculous meningitis is highly variable with a prodromal period of 2–4 weeks. Symptoms during this stage may include fatigue, fever, myalgia, malaise, and anorexia. In 75% of cases, a meningitic state ensues with headache, nausea, vomiting, photophobia, stiffness of the neck, and continuation of fever. In 25–50% of patients, cranial nerve palsies may occur. The most common cranial nerves involved are the abducens (CN VI) and the oculomotor (CN III). Fever, neck rigidity, and other signs of bacterial meningitis may be absent, which makes the clinical suspicion of tuberculous meningitis difficult [4]. Other clinical presentations may include hydrocephalus caused by disturbance of CSF circulation and seizures caused by parenchymal damage. Infarction due to vasculitis may cause hemiparesis, altered consciousness, aphasia, or visual field defects. Hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion or renal salt-wasting syndrome may occur in up to 50% of cases. Other less common clinical presentations include personality changes and slowly progressive dementia [5]. If tuberculous meningitis remains untreated, it progresses over time with subsequent elevation of intracranial pressure, coma, more focal neurological deficit, and death. Survivors of tuberculous meningitis including mental retardation in children, sensorineural hearing loss, permanent cranial nerve palsies, epilepsies, and hydrocephalus with or without ventriculoperitoneal shunting [6].
Intracranial tuberculomas occur in 1% of patients with CNS tuberculosis. It is thought to arise when tubercles multiply in the brain parenchyma forming microscopic granulomatous foci with gradual enlargement and no rupture into the subarachnoid space. It is believed that the most common route of spread is hematogenous with seeding of the organism into subependymal or subpial regions (rich foci) [7]. In 15–33% of cases, tuberculomas are multiple involving supratentorial and infratentorial regions of the brain. They usually present with symptoms and signs of space-occupying lesions as focal neurological deficits with no evidence of systemic disease. The neuroimaging features are nonspecific and may present a diagnostic challenge with broad differential diagnoses including neoplasms, sarcoidosis, fungal infections, and pyogenic abscesses [8].
Cerebrospinal fluid analysis is of critical importance to diagnose tuberculous meningitis as early as possible. It typically shows lymphocytic pleocytosis, elevated protein, and lowered glucose concentrations. In tuberculous meningitis, pleocytosis in the CSF is usually less than 1000/µL and peripheral blood leukocytosis of less than 15,000 × 103/mL. Culture of the CSF sample is positive in 5–58% of patients with tuberculous meningitis. If positive, it allows for drug sensitivity testing and strain subanalysis. Although CSF examination is the mainstay of diagnosis for tuberculous meningitis, it remains a clinical and diagnostic challenge. Among different challenges facing the neurologist is the atypical CSF findings such as normal glucose, neutrophil predominance pleocytosis, and negative PCR for acid-fast bacilli (up to 20%). The low sensitivity for CSF tuberculosis PCR can be explained by the low bacillary load in the CSF, PCR inhibitors in the CSF, and small sample volume [9].
Cerebrospinal fluid examination using Ziehl–Neelsen stain to demonstrate acid-fast bacilli should be sent routinely for any patient with suspected meningitis, especially in highly endemic areas. Although the specificity is 100%, the sensitivity is as low as 5–25%. Culture of CSF with the identification of mycobacterium tuberculosis is considered the gold standard technique for the diagnosis of this disorder. Several culture techniques have been evaluated for their performance including culture on solid medium, the use of automated systems, and microscopically observed drug susceptibility assay. Results of different types of cultures are obtained only after 2–8 weeks of incubation, which may delay clinical decision making. The sensitivity of these different culture techniques ranges from 40% to 60%, which is another barrier for accurate and fast diagnosis [10].
Radiological investigations including Computed Tomography (CT) scan and MRI of the brain can be helpful in early diagnosis, especially when clinical symptoms and signs raise the suspicion for CNS tuberculosis. The whole neuroaxis should be imaged, and a careful look for coexisting extraneural manifestations of tuberculosis is rewarded. MRI is superior to CT scan in sensitivity and specificity, and considered the modality of choice for radiological assessment of CNS tuberculosis [11]. The characteristic imaging findings of tuberculous meningitis include basal cisternal and leptomeningeal enhancement, hydrocephalus, periventricular infarcts, and tuberculomas. These manifestations may occur alone or in combination, and may not be detected until the late stage of the disease [12]. Chest radiographs are positive in up to 50% of cases with tuberculous meningitis [1].
Brain biopsy is an important invasive modality for the investigation of several neurological and neurosurgical disorders. In CNS tuberculosis, brain biopsy, whether open or stereotactic, is only rarely indicated in certain cases of tuberculomas. Indications for biopsy include lack of definitive diagnosis and persistence or paradoxical growth of presumed tuberculoma despite medical therapy. Meningeal biopsy is extremely rarely done for the diagnosis of tuberculous meningitis [13]. In our case, the treating infectious diseases consultant insisted on performing brain biopsy prior to starting antituberculous medications. Antituberculous medications were only started when the neurology team took over the management of this case, which was late. Unfortunately, this patient died from neurological complications related to tuberculous meningitis (multiple infarcts with superadded massive pulmonary embolism). Whether this catastrophic result was preventable is a subject of investigation and further research.
The World Health Organization guideline for the treatment of CNS tuberculosis was based on the recommendation for the treatment of pulmonary tuberculosis with no controlled trials to determine the optimal drug regimen and treatment duration. The current guidelines established by the World Health Organization and the British Infection Society are more than a decade old, and the issue of pathological diagnosis is not required prior to starting antituberculous medications. The recommended first-line treatment agents for CNS tuberculosis are pyrazinamide, ethambutol, isoniazid, and rifampicin. The first two agents are used for 2 months, and the last two agents are used for a total of 10–12 months. This is achieved through two phases with the initial phase using all four medications together and the second phase using isoniazid and rifampicin only. As early treatment can dramatically improve morbidity and mortality, CNS tuberculosis should be on top of the list for the differential diagnosis of space-occupying lesions in highly endemic countries for tuberculosis [14]. Antituberculous medications should never be withheld in suspected cases waiting for pathological diagnosis. Adjunctive corticosteroid therapy should be given to all patients with tuberculous meningitis regardless of disease severity at presentation. Dexamethasone is given at a dose of 0.4 mg/kg/day with a tapering course over 6–8 weeks [15].
4. CONCLUSION
Although CNS tuberculosis is rare in immunocompetent hosts, such cases can be seen in neurology practice and represent a major clinical and diagnostic challenge. Although this case is not an uncommon scenario and no alternative diagnosis is suspected, she was not started on antituberculous medications because of an unjustified request for pathological diagnosis by the admitting team. Despite the fact that Saudi Arabia is not one of the 22 countries with the highest number of tuberculosis cases worldwide, tuberculosis continues to represent a major health problem. Treating clinicians should try to use methods to increase the mycobacterial yield of CSF smear examination. Multidisciplinary care, not a one-man show, should be practiced with cooperation and exchange of opinions between neurologists, infectious diseases specialists, neurosurgeons, and intensivists. Brain or meningeal biopsy is not a routine investigation tool and should never delay the initiation of antituberculous medications. Despite advancements in the field of tuberculosis science, it continues to represent a clinical and diagnostic challenge with a substantial percentage of morbidity and mortality.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
HA and BS contributed in study conceptualization and writing (original draft) the manuscript. NA, AJA, and MA contributed in data curation, formal analysis, and wrote (review and editing) the manuscript. All authors approved the final version for publication.
REFERENCES
Cite this article
TY - JOUR AU - Hussein Algahtani AU - Bader Shirah AU - Nawal Abdelghaffar AU - Abdulrahman J. Alqahtani AU - Mohammad Alshehri PY - 2020 DA - 2020/09/26 TI - Tuberculous Meningitis in an Immunocompetent Host: A Diagnostic Challenge JO - Dr. Sulaiman Al Habib Medical Journal SP - 16 EP - 20 VL - 3 IS - 1 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200917.001 DO - 10.2991/dsahmj.k.200917.001 ID - Algahtani2020 ER -
