Treatment Options during Cytokine Storm
 , Kentaro Oh-hashi3, Mahmoud Kandeel1, 4, *
, Kentaro Oh-hashi3, Mahmoud Kandeel1, 4, *- DOI
- 10.2991/dsahmj.k.210422.001How to use a DOI?
- Keywords
- Cytokine storm; monoclonal antibodies; inflammation; COVID-19
- Abstract
Cytokine storm is the major cause of death in patients with acute respiratory distress caused by COVID-19. Because cytokine storm is a life-threatening condition, it is important to treat patients with the most appropriate drugs depending on their infectious pathogen and medical status. In this short review, we highlight the pharmacological and nonpharmacological options for handling the cytokine storm. The potential drug targets comprise the use of small molecule or large macromolecules. These molecules include antibodies to bind with and inhibit the actions of interleukins, cytokine signal transducers such as Janus Kinases (JAKs), and cytokine receptors such as the cytokine receptor colony-stimulating factor 1 receptor (CSF1R). So far, dexamethasone appeared to be the pharmacological treatment of choice in managing or preventing cytokine release syndrome because of its safety and cost-effectiveness. Other pharmacological options either require more studies to prove their efficacy (e.g., anakinra and solnatide) or have shown poor efficacy in recent studies (e.g., tocilizumab). Moreover, specific immunoadsorption techniques can be safe and effective when considering nonpharmacological treatment.
- Copyright
- © 2021 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. CYTOKINE STORM
Cytokine storm is a syndrome characterized by multiorgan inflammation and severe hypotension caused by overdrawn excitation of immune system cells that lead to overproduction of different types of cytokines in blood plasma [1]. The elevated level of plasma cytokines is usually caused by various microorganisms’ infections. It could also be caused by using certain monoclonal antibody drugs, such as rituximab and alemtuzumab [2].
The main cytokines that lead to cytokine release syndrome are Tumor Necrosis Factor-alpha (TNF-α), interferon-gamma, Interleukin-1 beta (IL-1β), IL-4, IL-6, IL-13, IL-17, IL-18, IL-23, and the intracellular cytokine signal transducers Janus Kinase-1 (JAK-1) and JAK-3 [3]. Some of these cytokines are important in eradicating a specific pathogen. For instance, herpes zoster is cleared from the body with the aid of JAK-1 and JAK-3 activation. Thus, inhibiting these two cytokines could lead to the prolongation of herpes zoster infection period [4].
Furthermore, cytokine storm is one of the main causes of secondary capillary leak syndrome, which is described as leakage of intravascular fluid and blood because of the changed permeability of capillaries [5]. Hyperimmune stimulation could have a role in changing the permeability of capillaries to protein by inducing apoptosis and endothelial damage of microvascular cells [6]. There are possible cardiovascular and respiratory risks of secondary capillary leak syndrome including hypotension, arrhythmia, hypoxia, pneumonitis, and pulmonary edema [7].
Because cytokine storm and secondary capillary leak syndrome could lead to life-threatening conditions, it is important to treat patients with the most appropriate drugs depending on their infectious pathogen and medical status to overcome poor prognosis and decrease mortality rates. Targeting the cytokine storm-causing microorganism is the best way to minimize the inflammatory status in vital organs. However, specific drugs could be used nowadays or in the future to inhibit multiorgan inflammation-causing cytokines. The choice of a drug to treat the cytokine storm depends on the type of microorganism infection [8].
In general, bacterial infections are eradicated with the aid of TNF-α and IL-6 cytokines and JAK cytokine signal transducers; thus, inhibiting one of these cytokines could lead to severe prognosis, which may lead to death. However, during viral infections, JAKs are responsible for eliminating viruses including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19 from human cells. Thus, only JAK inhibitors are relatively contraindicated in this case [9].
2. PHARMACOLOGICAL OPTIONS DURING CYTOKINE STORM
2.1. Inhibition of IL-1β, IL-6, TNF-α, and JAKs
Anakinra is a protein molecule that acts as a reversible inhibitor of IL-1 type I receptors; therefore, anakinra can block the activity of two cytokines, IL-1α and IL-1β [10]. As anakinra has the ability to restrain the effect of IL-1β, cytokine storms caused by the macrophage activation syndrome could be prevented or treated by using anakinra [11]. The advantage of anakinra is its ability to reduce pain associated with the hyperinflammatory state of severely ill patients and is considered safe to use during pregnancy [12,13]. Moreover, inhibition of IL-1β by anakinra probably will not deteriorate or increase the risk of infection or course of infection for patients diagnosed with severe bacterial, viral, or fungal infection [14]. Some case reports have shown that anakinra could improve the condition of patients diagnosed with hypercytokinemia with a hyperinflammatory state caused by viral infection (e.g., COVID-19) [15,16].
Nevertheless, tocilizumab (also called atilizumab) is a monoclonal antibody that blocks the IL-6 receptor, thereby inhibiting the IL-6 signal pathway. Tocilizumab was approved by the United States Food and Drug Administration (FDA) to treat rheumatoid arthritis, giant cell arteritis, and cytokine storm. However, tocilizumab should not be used or should only be used with strict precautions in patients with bacterial infections [17]. During the COVID-19 pandemic, tocilizumab was frequently used to deal with many cases of cytokine release syndrome, which raised and led to increased mortality rates globally. However, recent studies found that, compared with placebo, tocilizumab has no significant efficacy in improving the prognosis of COVID-19 patients [18–20].
Meanwhile, anti-TNF-α (e.g., adalimumab) and anti-JAK (e.g., baricitinib) drugs are suggested for prophylaxis and treatment of cytokine storm syndrome, although these drugs are not officially indicated in this case, are not favored, and must be used with strict precautions in primary or secondary bacterial infections [21]. A specific TNF-α inhibitor, XPro1595, is currently under phase 2 clinical trials (Table 1) [22].
| Study title | Trial phase | Molecular target | Conditions | Interventions |
|---|---|---|---|---|
| A phase 2 study to evaluate axatilimab for hospitalized patients with respiratory involvement secondary to COVID-19 | Phase 2 | The cytokine receptor colony stimulating factor 1 receptor (CSF-1R) | Respiratory distress caused by COVID-19 | Randomized, double-blind, placebo-controlled, 29-day study to assess the efficacy and safety of axatilimab in patients with COVID-19 |
| XPro1595 for the treatment of pulmonary complications from COVID-19 | Phase 2 | Inhibition of TNF-α | Respiratory distress caused by COVID-19 | Double-blind, randomized, placebo-controlled clinical trial of XPro1595 in participants with pulmonary complications caused by COVID-19 infection |
| A study to assess the efficacy and safety of gimsilumab in subjects with lung injury or acute respiratory distress syndrome secondary to COVID-19 | Phase 2 | Granulocyte-macrophage colony stimulating factor | Lung injury or acute respiratory distress syndrome caused by COVID-19 | Randomized, double-blind, placebo-controlled study to assess the efficacy and safety of gimsilumab in patients with lung injury or acute respiratory distress syndrome secondary to COVID-19 |
Current clinical trials to investigate the cytokines storm associated with COVID-19 and its treatment
2.2. Inhibition of IL-4, IL-13, IL-17, and IL-23
A drug approved by the US FDA in 2017 called dupilumab is a monoclonal antibody that binds to IL-4 and inhibits the signaling pathways of both IL-4 and IL-13. Dupilumab was shown to be effective in treating moderate to severe cases of atopic dermatitis and asthma. Because dupilumab targets IL-4 and IL-13, it could be suggested for treatment or prevention of multiorgan inflammation caused by infection stimulation of IL-4 and IL-13. By inhibiting IL-13, the biosynthesis of immunoglobulin-E and mucus will be reduced. This effect could be beneficial in patients with inflammation in the upper and lower respiratory tract, and it is noted that inhibiting IL-13 will not increase the risk of bacterial, viral, or fungal infections [23].
Brodalumab, ixekizumab, and secukinumab are described as monoclonal antibody inhibitors of IL-17 signaling pathways. Brodalumab acts by blocking the type A IL-17 receptors, thus inhibiting the binding of some types of IL-17 (IL-17A, IL-17F, and IL-17C), whereas ixekizumab and secukinumab bind to IL-17A and inhibit its binding to its receptor. These IL-17 inhibitors are approved for treatment of psoriasis and ankylosing spondylitis, but because of the ability to reduce IL-17, it is suggested to treat cytokine storm syndrome and reduce the mortality rate. Inhibiting IL-17 cytokine could worsen the fungal infections, but not bacterial and viral infections [24].
Risankizumab, a newer monoclonal antibody approved by the US FDA in 2019, targets IL-23 cytokine and inhibits its inflammatory consequences. It was officially approved to treat moderate and severe cases of plaque psoriasis, but owing to the inhibition of IL-23, it is suggested to be used for prevention or treatment of cytokine storm. The advantage of blocking IL-23 is the reduction of multiorgan damage without any risk of infection prolongation during bacterial, fungal, or viral infections (Figure 1) [25].
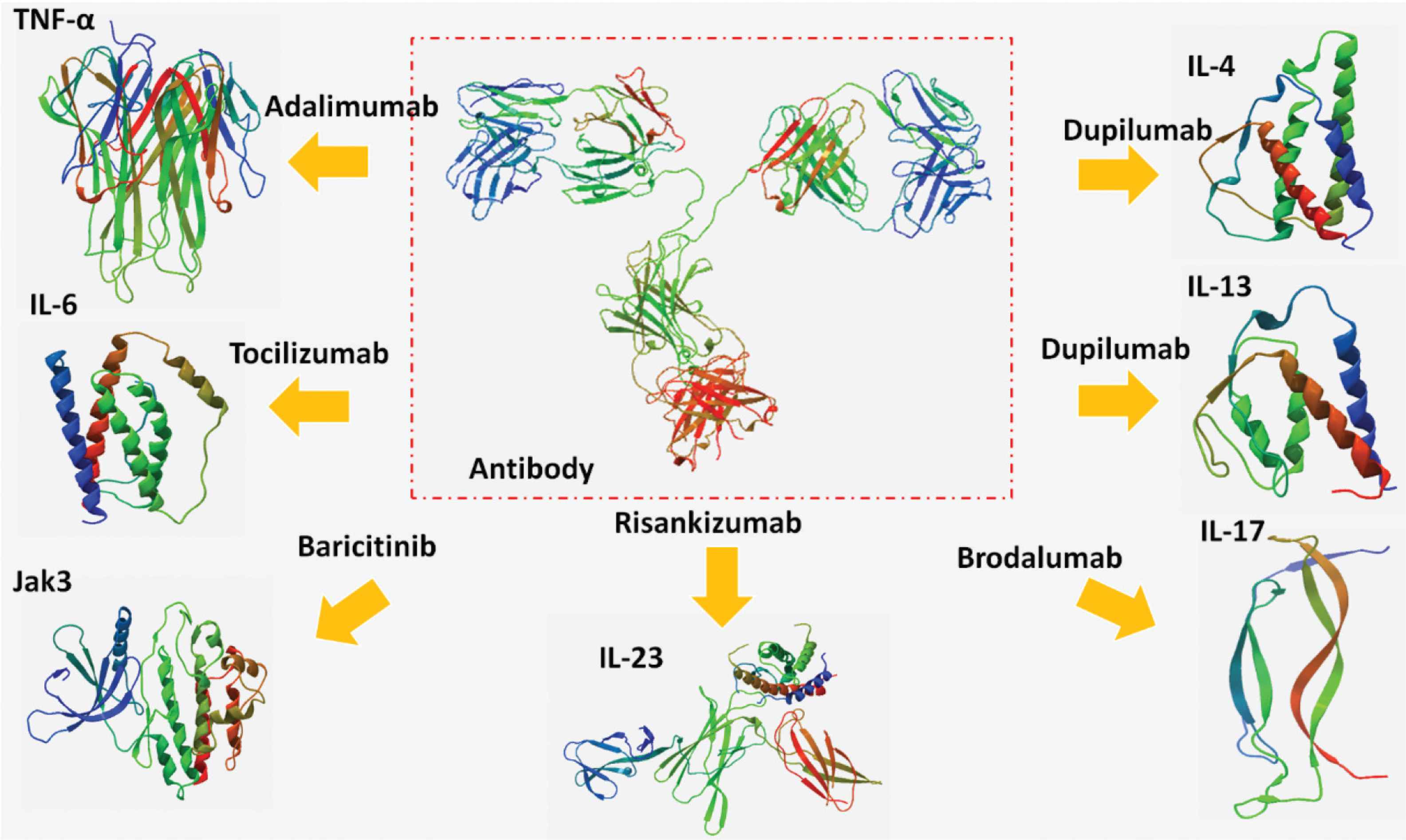
The antibodies targeting tumor necrosis factor-alpha (TNF-α), interleukin (IL)-4, IL-6, IL-13, IL-17, IL-23, and Janus Kinase-3 (JAK-3).
2.3. Antibodies against Receptors Involved in Cytokine Storm
There are currently two clinical trials investigating the effect of antibodies against three important receptors for inflammation signaling (Table 1). The first study aims to prevent macrophage production, differentiation, and function by targeting the cytokine Colony-Stimulating Factor 1 Receptor (CSF1R) using axatilimab. The second trial targets the granulocyte-macrophage CSF using gimsilumab [26,27].
2.4. Corticosteroids
Glucocorticoids (not mineralocorticoids) are potent suppressors of many cytokines’ biosynthesis that increase the inflammatory status in vital organs. This glucocorticoid mechanism comprises inhibition of a transcription factor called nuclear factor kappa light chain enhancer of activated B cells (NF-κB), which enhances the transcription of cytokine genes to increase their production [28]. The use of glucocorticoids (e.g., dexamethasone) for patients with cytokine storm depends on the cause of increased cytokine biosynthesis and the benefit/risk ratio, because glucocorticoids may increase the duration of bacterial and viral infections and their pathogenesis effect, and glucocorticoids could also increase the risk of insulin resistance [29].
Dexamethasone, a selective glucocorticoid receptors agonist, has been commonly used in COVID-19 patients to treat viral pneumonia and prevent cytokine storm, as it showed better outcomes than placebo [30]. Although dexamethasone’s efficacy in reducing mortality rates is controversial, its use among patients showed no harmful effects in general and is probably considered a cost-effective treatment option when compared with placebo or tocilizumab in treating cytokine release syndrome [31–33].
2.5. Specific Pharmacological Treatment Options for Secondary Capillary Leak Syndrome
Apart from cytokine suppression agents, some nominated drugs could have a substantial role in treating life-threatening hypoxia and hypotension caused by capillary leak syndrome.
Solnatide, a drug described as a peptide molecule consisting of 17 amino acids, has the ability to activate Epithelial Sodium Channels (ENaCs), which are found in the lung, colon, distal and collecting tubes of nephrons, sweat glands of the skin, and reproductive tracts in both human sexes [34,35]. The activation of ENaCs in the lung by solnatide could enhance the function of capillaries as tight junction barriers to decrease fluid leakage from blood vessels, thus improving lung function and overcoming hypoxia and pulmonary edema [36,37].
By contrast, the drug FX06 (also called Bβ15–42) is a fibrin-derived peptide that induces a tight junction of endothelial lining in the capillaries by maintaining the function of Vascular Endothelial cadherin (VE-cadherin), which is required to ensure the availability of a tied endothelial barrier between cells. The exact mechanism of action of FX06 is through the inhibition of the activity of one of the src family kinases called Fyn, which is responsible for the disorganization of VE-cadherin-dependent cell–cell junction; therefore, FX06 could preserve the normal fluid permeability and enhance respiratory tract work [38,39].
3. NONPHARMACOLOGICAL TREATMENT OPTIONS DURING CYTOKINE STORM
3.1. Immunoadsorption Therapy
Immunoadsorption is one of the methods used to purify blood from pathogenic cytokines, chemokines, and antibodies [40,41]. There are various methods for immunoadsorption treatment involving the use of different selective, semiselective, and nonselective binders in the immunoadsorption column to adsorb specific or several harmful immunoglobulins [42]. Previous studies revealed that immunoadsorption could enhance organs’ functions in patients diagnosed with septic shock [43,44].
Specific extracorporeal devices were invented to act like immunoadsorption tools to remove pathogenic endotoxins and cytokines. These devices can adsorb IL-6, which is known as one of the potent inflammatory mediators in COVID-19 patients; therefore, extracorporeal immunoadsorption may have a major role as a life-saving treatment option for severe COVID-19 cases with cytokine storm [42,44].
3.2. Plasmapheresis
Plasmapheresis, also called plasma exchange, is the process of removing all protein molecules from a patient’s plasma including pathogenic immunoglobulins and cytokines. The removed plasma is replaced by a solution containing human albumin or fresh frozen plasma [45]. Unlike immunoadsorption, plasmapheresis removes harmful and beneficial plasma proteins, which in turn, may lead to a number of adverse effects such as immunosuppression, hypotension, and transfusion reactions [46,47].
Plasma exchange was used in some studies during the COVID-19 pandemic to treat patients infected with SARS-CoV-2 and diagnosed with cytokine release syndrome. These studies suggested considering plasmapheresis as a treatment option to treat cytokine storm; however, its complications and inaccessibility make it difficult to apply in every patient [48,49].
4. CONCLUSION AND RECOMMENDATION
Various approved and nonapproved medications could prevent or treat cytokine release syndrome and may have the ability to reduce the mortality rate with significant evidence. More clinical trials are necessary to confirm the safety and efficacy of most nominated drugs to avoid capillary leak syndrome.
So far, dexamethasone appears to be the pharmacological treatment of choice in managing or preventing cytokine release syndrome because of its safety and cost-effectiveness. Other pharmacological options either require further studies to prove their efficacy (e.g., anakinra and solnatide) or have shown poor efficacy in recent studies (e.g., tocilizumab). Furthermore, specific immunoadsorption techniques can be safe and effective when considering nonpharmacological treatment.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
Funding
No financial support was provided.
REFERENCES
Cite this article
TY - JOUR AU - Abdullah Alkattan AU - Kentaro Oh-hashi AU - Mahmoud Kandeel PY - 2021 DA - 2021/04/29 TI - Treatment Options during Cytokine Storm JO - Dr. Sulaiman Al Habib Medical Journal SP - 48 EP - 52 VL - 3 IS - 2 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.210422.001 DO - 10.2991/dsahmj.k.210422.001 ID - Alkattan2021 ER -
