Ground water as the source of an outbreak of Salmonella Enteritidis
- DOI
- 10.1016/j.jegh.2017.05.001How to use a DOI?
- Keywords
- Genotyping; Ground water; Pulsed-field gel electrophoresis; Salmonella Enteritidis; Water contamination
- Abstract
In September 2014, an outbreak of gastroenteritis was reported to the Public Health Institute of Šibenik and Knin County in Croatia. The outbreak occurred in the County center of Šibenik, a town with 50,000 inhabitants, and it lasted for 12 days. An epidemiological investigation suggested a nearby water spring as the source of the outbreak. Due to the temporary closure of the public water supply system, the inhabitants started to use untreated water from a nearby spring. Microbiological analysis revealed that the outbreak was caused by Salmonella enterica subsp. enterica serovar Enteritidis that was isolated from stool samples of the patients and ground water. The isolates were further analysed with pulsed-field gel electrophoresis using XbaI, which revealed an identical macrorestriction profile. Although 68 cases were reported, it was estimated that the actual number of affected persons was more than several hundred. In order to prevent further spread of disease, public advice was released immediately after the first epidemiological indication and a warning sign was placed at the incriminated water source, after microbiological confirmation. It is necessary to regularly monitor microbiological quality of ground water especially in urban areas and provide adequate education and awareness to the inhabitants regarding the risk of using untreated ground water.
- Copyright
- © 2017 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Salmonella is one of the most important causes of gastroenteritis worldwide. The symptoms of infection include fever, abdominal pain, diarrhoea, nausea, and sometimes vomiting. They usually appear 12–72 h after infection and last for 4–7 days, without any consequences for most patients [1]. The outbreaks usually occur due to the consumption of contaminated food and water [2–4]. Various sources of Salmonella spp. are found in the environment and they include livestock, wildlife, and poultry, but they also abound in natural waters [2,5]. More than 2500 Salmonella serovars are found in environmental samples where they can persist for long periods [2,3,5]. It has also been found that genotypically related strains can be isolated from water and wildlife samples, which suggests that they could be reservoirs for the dissemination of Salmonella in the environment [5]. Moreover, natural waters play an important role as vehicles of transmission of these microorganisms. Water contamination and outbreaks with nontyphoidal serovars are rarely reported, despite the fact that they are often present in surface waters [3,6–8]. Almost all outbreaks in developed countries are related to the use of untreated or inadequately treated water [3]. It has been estimated that ∼1.1 billion people globally drink unsafe water and as much as 88% of diarrhoeal disease in the world is attributable to unsafe water, sanitation, and hygiene [9]. Surveys of waterborne outbreaks of Salmonella are mainly conducted in developed countries where small numbers of such cases are reported. In the USA, there were 15 cases of nontyphoidal Salmonella outbreaks caused by drinking water from 1971 to 2000 and none were registered in the period from 2000 to 2012 [10–12]. However, Salmonella was not associated with waterborne outbreaks in England and Wales from 1992 to 2003 [12]. Contaminated waters are often used for irrigation or the washing of food products, so even though the true incidence of waterborne outbreaks caused by this microorganism is probably greater, it is not recognised as a waterborne disease [3]. The goal of our study was to find out the cause of the outbreak and to determine its source.
On September 12, 2014 a water supply system of the Croatian town of Šibenik (Fig. 1), which supplies ∼80,000 inhabitants, was temporarily closed due to extreme heavy rainfall and fear of possible pollution. That same day, the residents were warned that water from the pipes was not fit for human consumption and they were supplied with freshwater tanks. The first patients suffering from gastroenteritis contacted their family physicians on September 18, 2014. Five days later, an epidemiologist from the regional Public Health Institute was informed, by the microbiological laboratory, of a higher than usual number of Salmonella enterica subsp. enterica serovar Enteritidis (S. Enteritidis) in stool samples. By the end of the following week >68 cases were registered. The first epidemiological investigation revealed that two of the hospitalised patients consumed water from the ground water source, situated ∼15 km from the town of Šibenik (Fig. 2). The ground water from the source was sampled aseptically and sent to the microbiological laboratory for analysis, together with the water sample from the canister of one of the patients. The patient kept water from the source in the canister and consumed it repeatedly.
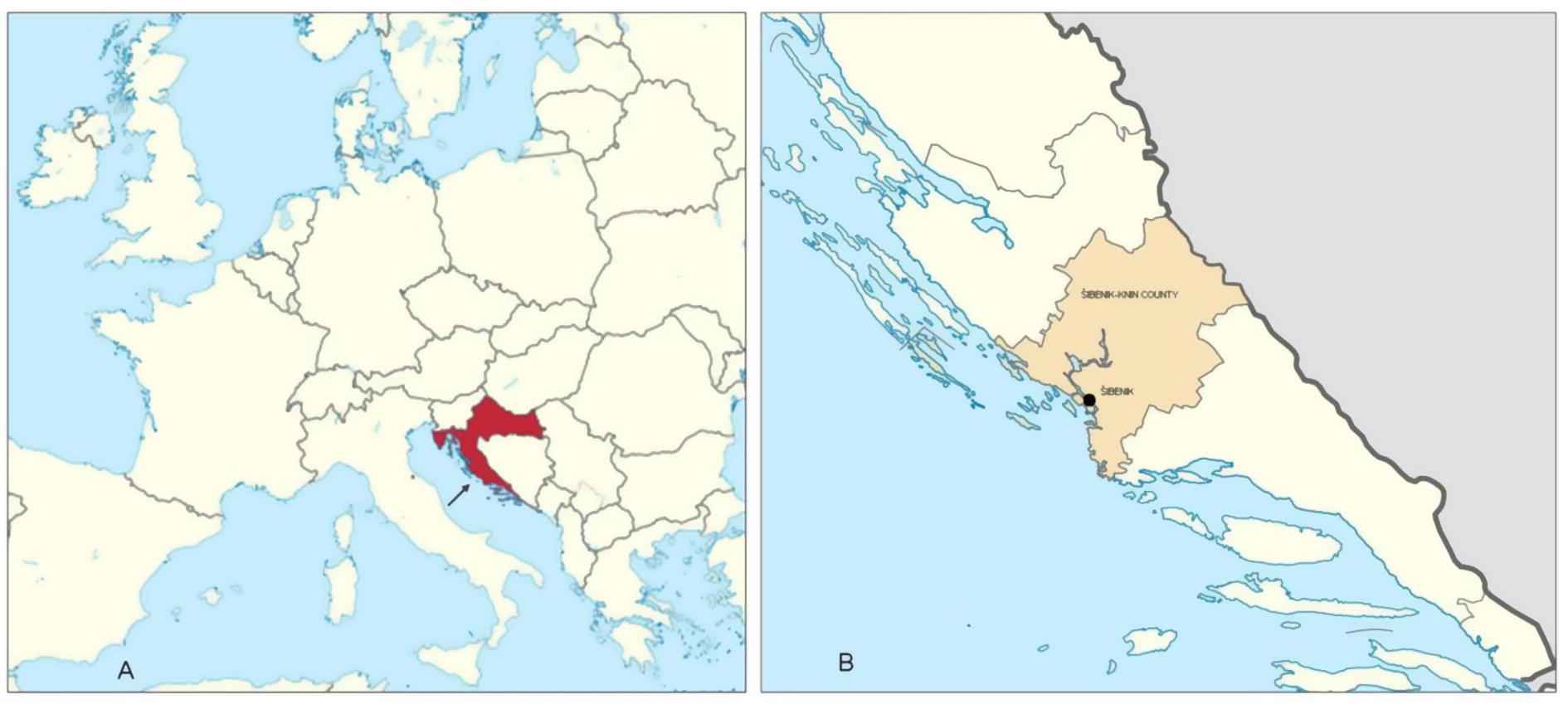
(A) Position of Croatia in Europe. (B) Šibenik-Knin County and location of the study.

Unchlorinated ground water source of the outbreak.
2. Materials and methods
2.1. Epidemiological data
Hypothesis-generating interviews conducted with 36 cases suggested that consumption of ground water from the source was the most important risk factor associated with disease. A case was defined as a person suffering from acute gastroenteritis with onset between September 12, 2014 and October 16, 2014 and with a stool sample positive for S. Enteritidis. All cases were interviewed by an epidemiologist in order to obtain standardised epidemiological information. The interview included personal information, data regarding illness, travel history, and all exposure-related variables (dietary habits, recreational activities, and pets). Detailed information was also collected on any water consumption including whether it was consumed from ground water springs or water bottles that were filled at the spring. The surrounding area was explored by epidemiologists and local hunters, searching for any animal traces or biological waste.
2.2. Microbiological analysis of stool samples
Stool samples were inoculated in Selenite Broth and xylose lysine deoxycholate (XLD) agar (Becton Dickinson, Franklin Lakes, NJ, USA) and incubated at 37 °C for 24–48 h in air atmospheric. Suspicious nonlactose fermenting and H2S-positive colonies (red colonies with black pigmentation and pink halo) were biochemically tested and identified with an automated VITEK 2 Compact System (bioMerieux, l’Etoile, France). Such colonies were also serotyped by group- and type-specific antibodies that react with O and H determinants (BioRad, Paris, France). Susceptibility of the strains to ampicillin/amoxicillin, amoxicillin–clavulanic acid, ceftazidime, ceftriaxone, pefloxacin (screen for ciprofloxacin resistance), chloramphenicol, and trimethoprim + sulfamethoxazole was determined using a disc diffusion test (Mast Diagnostics, Bootle, Merseyside, UK). Our laboratory participates in the United Kingdom National External Quality Assessment Schemes for general bacteriology and antimicrobial susceptibility. The results of disc diffusion tests were interpreted according to the European Committee on Antimicrobial Susceptibility Testing criteria (version 4.0, 2014) and Committee for Antibiotic Resistance Surveillance in Croatia [12–14].
2.3. Microbiological analysis of water
Two samples of water, one from the spring and the other from the canister of the patient, were tested for Salmonella spp. according to International Organisation for Standardisation 19250:2013 [15]. Samples of water from the spring were taken in a 1-L sterile bottle and the patients brought their canisters with water that were filled at the same place. After inoculation in nonselective and selective broths, samples were transferred onto XLD agar and Brilliance Salmonella Agar (Oxoid, Basingstoke, UK). All suspicious (nonlactose fermenting and H2S positive) colonies that appeared as red with black centers on XLD or purple on Brilliance Salmonella Agar were biochemically tested using API 20E (bioMerieux). The isolates were serotyped and further tested for susceptibility as described above for colonies from stool samples.
2.4. Pulsed-field gel electrophoresis analysis
In order to determine their clonal relationship, 13 isolates of S. Enteritidis from stool samples and one from water were analysed by pulsed-field gel electrophoresis (PFGE). These 13 stool isolates were chosen for PFGE analysis as representative samples, according to epidemiological interviews. The plugs were prepared according to the protocol described by Ribot et al. [16] and afterwards restricted with XbaI for 3 h at 37 °C. Salmonella Braenderup H9812 strain was used as a size standard. Electrophoresis was performed using 1.4% certified PFGE agarose gel (Bio-Rad, Germany) in CHEF DRII/III (Bio-Rad, Germany), according to the conditions described in the PulseNet PFGE protocol for Salmonella issued by the US Centers for Disease Control and Prevention. Obtained fingerprinting patterns were analysed by Molecular Analyst Fingerprinting software (Bio-Rad, Germany). Dice similarity coefficients were calculated using pairwise comparison, with optimization and a position tolerance of 1.5%. The dendrogram was created using the unweighted pair group method with arithmetic averages.
3. Results
From September 12, 2014 to September 30, 2014, when the water supply system in Šibenik County was closed, following extreme heavy rainfall and fear of possible pollution, 68 inhabitants reported gastrointestinal symptoms. Seven of them were admitted to the hospital due to the severity of their symptoms. Microbiological analysis of stool samples confirmed S. Enteritidis in all 68 patients and in the water samples. Their antimicrobial profile was identical. The isolates were sensitive to all antibiotics used: ampicillin/amoxicillin, amoxicillin–clavulanic acid, ceftazidime, ceftriaxone, pefloxacin, chloramphenicol, and trimethoprim + sulfamethoxazole.
During the outbreak, which lasted for 12 days, 34 of 36 interviewed patients confirmed their consumption of ground water from the spring. Two of the patients could not remember consuming the incriminated water. In this outbreak, there was no mortality recorded. Thirty-seven patients were male and 31 were women with an average age of 28.2 years and 23.5 years, respectively.
All human and water isolates analysed by PFGE exhibited an identical macrorestriction profile and were indistinguishable from one another (Fig. 3).
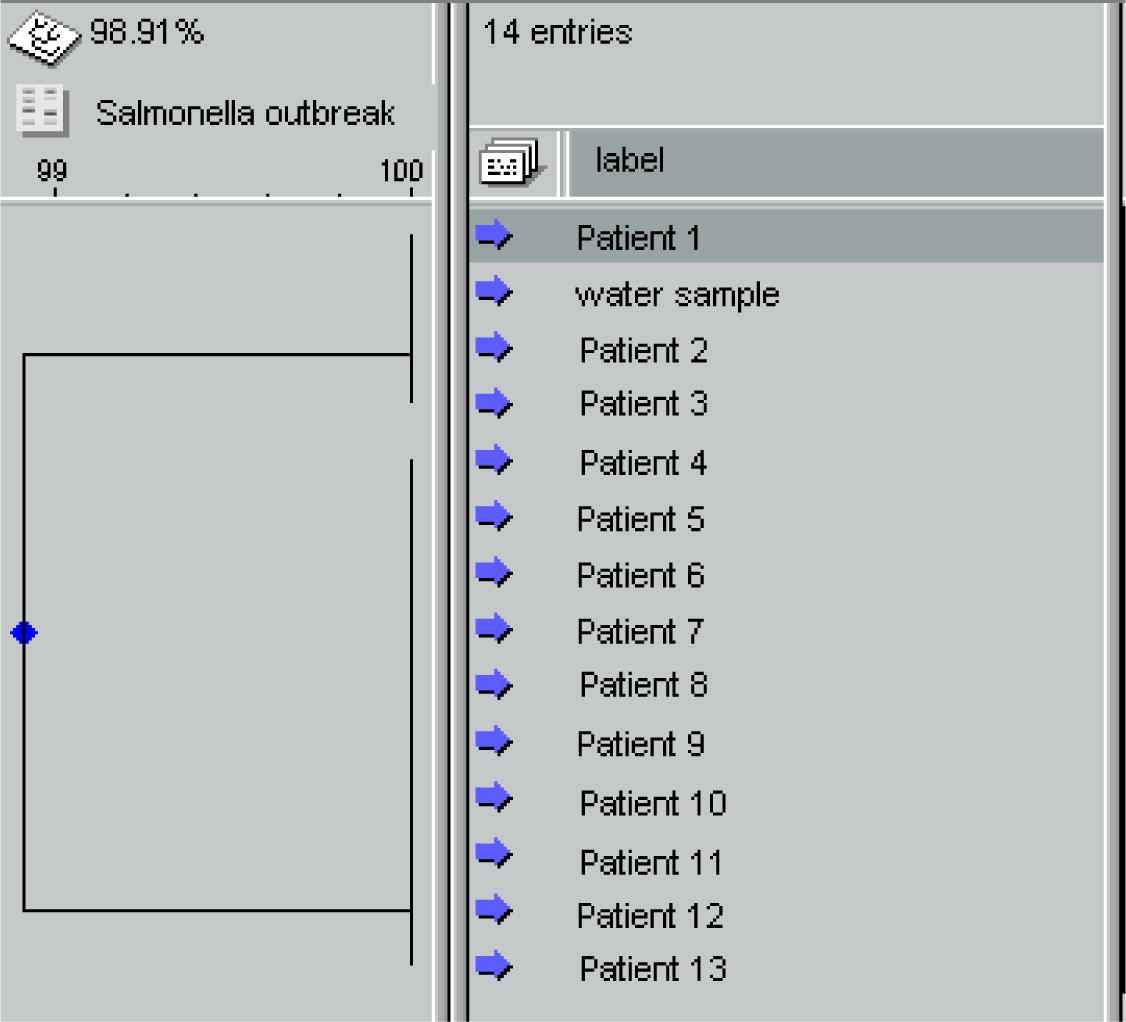
Dendogram based on XbaI-digested DNA from stool and water isolates of S. Enteritidis.
4. Discussion
This report describes a water outbreak caused by non-typhoid Salmonella in Croatia. In 1986, an outbreak of gastroeneterocollitis caused by four different types of Salmonella (S. Typhi, S. Enteritidis, S. Manhattan and S. Anatum), Shigella flexneri, and hepatitis A virus was recorded in one Croatian city. Epidemiological investigation revealed that the probable cause of this outbreak was leakage of sewage water in the water supply system. Nevertheless, none of the pathogens was isolated from water samples [17]. The outbreak described in this paper was caused by S. Enteritidis from an unchlorinated ground water source that could have been due to the popular belief that water from all ground water sources is safe for consumption. However, it is the first water outbreak in which we proved that Salmonella isolates from water and patients were identical. The town of Šibenik together with its surroundings is connected to the public water supply system. After its closure, due to the fear of contamination, the residents started to consume water from a nearby spring source, despite the fact that they were supplied with fresh water tanks. Epidemiological investigation together with microbiological and molecular analysis proved that the outbreak occurred as a result of consumption of the ground water from the spring contaminated with S. Enteritidis. The ground water might have been contaminated by animal faeces or human waste leaking from the septic tanks of the surrounding houses that are not connected to the sewage infrastructure. One possible source of Salmonella in ground water is improperly composted manure, whereas it can be transferred to fresh water through the flow paths of the soil or in the surface run-off water [18]. The area around this water spring is famous for Mediterranean agriculture. The inhabitants are mostly cultivating olive trees, fig trees, and grapevines, and use animal waste as natural fertiliser. Since early autumn is the usual time for intensive fertilisation, the underground water could have been contaminated with high levels of faecal microorganisms. Moreover, the extreme quantity of rainfall that affected the area in mid-September (>250 L/m2) may have caused the unusual surface runoff, especially since this spring is situated in a karst terrain. Karst aquifers generally act as poor filtering agents because of their thin soils and lack of granular texture, so they are far more susceptible to microbial contamination than other geologic media. It has been found that they are also characterised by sudden variations in microbiological water composition as a reaction to hydrologic events such as rainfall [19,20].
Microorganisms show significantly higher survival in sediment compared to overlying water [20,21]. Salmonella spp. can survive in soil for prolonged periods and can be transported to freshwater sources in high numbers [18]. It has also been proved that runoff can be an important source of Salmonella load in surface waters. A correlation between rainfall and outbreaks of waterborne disease has been found. Furthermore, 51% of all outbreaks in the USA from 1948 to 1994 were preceded by an extreme rainfall event [3,22].
Although it has been proved that Salmonella can persist in the environment for several years (5), it cannot multiply in water due to lack of nutrients; in groundwater most microorganisms are in a viable but noncultivable state [20]. In this outbreak, a sufficient infectious dose was probably reached by the continuous use of the contaminated water for drinking and preparation of food.
Although the outbreak lasted for 12 days, a more serious outcome was prevented due to the excellent cooperation between the epidemiologists and government institutions and their prompt response. The announcement regarding the general use of all ground water was released the same day after assumption that the cases were connected with consumption of spring water. A warning sign was also placed at the water source immediately after microbiological confirmation.
A definitive identification of the microbial agent that caused this outbreak was obtained by PFGE. It revealed identical genotypes of S. Enteritidis isolated from water and human samples. Two of the interviewed patients denied consumption of water from the spring. This finding is in accordance with the fact that contaminated water often remains unrecognised as the source of an outbreak since it is used for cooking or washing food products [3]. PFGE is a valuable typing method that is used to facilitate an epidemiological investigation and to investigate the source of the microorganisms in the surface water environment. This method has in recent times become a powerful tool and is used almost routinely in public health laboratories [2,23].
This study has several potential limitations. The results of water analysis from the patients’ canisters could be questionable. They filled the canisters themselves and kept them in their houses, so they might have been contaminated subsequently. The epidemiologists were informed late about the possible outbreak due to the fact that children and adults were admitted to different hospital wards (paediatrics and infectious diseases). Two interviewed patients denied drinking the ground water, so it can be assumed that they forgot about it or that they were infected by food contaminated with the incriminated water. There was also a lack of a control group of healthy individuals exposed to the incriminated water because most of them refused to co-operate.
Such studies can help to raise awareness regarding potential risk of pathogens from ground water [24]. Similarly, the results of our study indicate the need to regularly monitor the microbiological quality of groundwater and provide adequate education to the inhabitants regarding the risk of using untreated ground water.
Conflicts of interest
None declared.
Acknowledgements
We are grateful to Professor Marija Tonkić for providing us with the necessary equipment and supporting our work. We also acknowledge Dr Katarina Šiško-Kraljević for her co-operation with the PFGE method.
References
Cite this article
TY - JOUR AU - Ana Kovačić AU - Željko Huljev AU - Edita Sušić PY - 2017 DA - 2017/05/22 TI - Ground water as the source of an outbreak of Salmonella Enteritidis JO - Journal of Epidemiology and Global Health SP - 181 EP - 184 VL - 7 IS - 3 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2017.05.001 DO - 10.1016/j.jegh.2017.05.001 ID - Kovačić2017 ER -
