Investigation of an outbreak of bloody diarrhea complicated with hemolytic uremic syndrome
Tel.: +995 32 224 46 60; fax: +995 32 22446 59.
Tel.: +404 639 2206.
Tel.: +995 32 398018.
Tel.: +231 17 54.
- DOI
- 10.1016/j.jegh.2014.03.004How to use a DOI?
- Keywords
- Bloody diarrhea; Hemolytic uremic syndrome; HUS; E. coli; O104:H4; STEC
- Abstract
In July–August 2009, eight patients with bloody diarrhea complicated by hemolytic uremic syndrome (HUS) were admitted to hospitals in Tbilisi, Georgia. We started active surveillance in two regions for bloody diarrhea and post-diarrheal HUS. Of 25 case-patients who developed HUS, including the initial 8 cases, half were ⩾15 years old, 67% were female and seven (28%) died. No common exposures were identified. Among 20 HUS case-patients tested, Shiga toxin was detected in the stools of 2 patients (one with elevated serum IgG titers to several Escherichia coli serogroups, including O111 and O104). Among 56 persons with only bloody diarrhea, we isolated Shiga toxin-producing E. coli (STEC) O104:H4 from 2 and Shigella from 10; 2 had serologic evidence of E. coli O26 infection. These cases may indicate a previously unrecognized burden of HUS in Georgia. We recommend national reporting of HUS and improving STEC detection capacity.
- Copyright
- © 2014 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Shiga toxin-producing Escherichia coli (STEC) can cause illness ranging from mild diarrhea, to bloody diarrhea, to the hemolytic uremic syndrome (HUS) – a life-threatening condition that manifests with a triad of: microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure [1]. HUS develops in approximately 6% of patients with STEC O157:H7 infection [2], but other non-O157 STEC strains have been implicated, particularly in the European region [3–7]. STEC are found in the intestinal tracts and excrement of a variety of animals, especially ruminants. Outbreaks of STEC infection are frequently associated with meat products, such as ground beef, as well as raw produce, dairy products, contaminated water, or contact with ruminant mammals or ill persons [8].
The national communicable disease surveillance system of Georgia monitors diarrheal diseases. National regulation mandates notification of diarrhea cases within 24 h after registration. More than three epidemiologically-linked cases trigger an investigation [9]. Lack of laboratory capacity, especially in rural areas, limits detection of enteric pathogens in patients with diarrheal illness. STEC infection complicated with HUS has never been officially reported in Georgia.
Between July and August 2009, eight patients with bloody diarrhea complicated by HUS were admitted to hospitals in Tbilisi, Georgia; two of those patients died. Stool samples of all patients were sent to the laboratory of the National Center for Disease Control and Public Health of Georgia (NCDC). After conducting bacterial culturing and serological testing, the laboratory of the NCDC reported seven possible E. coli O157 isolates from the eight hospitalized patients. An investigation was initiated to determine if these cases represented an outbreak, to identify the etiologic agent(s) and, if possible, to identify the source(s) of infections.
2. Materials and methods
2.1. Active surveillance
On 24 July 2009, active surveillance, case finding and data collection for bloody diarrhea and HUS was initiated at nine clinics in the two most affected regions: Tbilisi (5 clinics) and Shida Kartli (4 clinics). Most affected regions were identified based on analysis of line listing data and communication with the main regional clinics of Georgia. All nine clinics were located in hospitals with the capacity for dialysis or treatment of patients with diarrhea and that are major regional or central level clinics. Active surveillance efforts ceased on December 11, 2009.
Patients were identified based on clearly defined case definitions: a case of bloody diarrhea was defined as ⩾3 patients reported loose stools in 24 h containing visible blood. A case of HUS was defined as laboratory-confirmed anemia and kidney damage (elevated creatinine, hematuria, or proteinuria) occurring within 21 days after diarrheal illness [10].
Each of the nine selected surveillance clinics were provided with stool and serum collection kits and detailed instructions for specimen collection and storage. HUS and bloody diarrhea clinical description and patient management information were provided to physicians and supervisors of infectious disease and/or dialysis departments. At all nine clinics, a focal point was nominated for daily phone calls. These calls were for identification and reporting of any new cases of bloody diarrhea or HUS based on case definitions.
Stool samples were transported in sterile containers containing buffered glycerol saline solution for better preservation during transport. A portion of the stool sample was also inoculated into Buffered Peptone Broth (0.2% final concentration) for selective enrichment of E. coli and Salmonella spp. Samples (stool and stool inoculated in broth) were stored at 2–8 °Celsius (C) and picked up from collection sites in coolers containing cold packs. Samples were delivered to NCDC for testing on the same day from Tbilisi hospitals and within a maximum of 48 h from regional hospitals.
2.2. Epidemiologic and clinical data collection
Case-patients or their caregivers were interviewed and demographic, clinical, and exposure information was collected using structured questionnaires. Exposures assessed included food, water sources, animals, and ill persons that patients may have been exposed to within the 10 days before diarrhea onset. Most interviews were done in person at hospitals or during home visits. Additionally, field investigations were conducted in two villages: Dvani, located in the region of Kareli, and Mejvriskevi, located in the region of Gori. Both villages were located in the most affected area of the Shida Kartli region, in the mid-eastern part of the country, approximately 150 km from the capital, Tbilisi. During these field visits, case-patients were interviewed with the structured questionnaires and also non-structured, open-ended, hypothesis-generating conversations were initiated. To collect additional symptoms, laboratory, and treatment information on all patients, medical charts were made available by using an abstraction form developed for this investigation. Epi_Info v 3.5 was used for data entry, collection and analysis.
2.3. Microbiological, molecular and serological testing
After the first registered cases of HUS in Georgia, the necessary bacteriological, serological, and molecular diagnostic reagents and appropriate training were kindly provided by colleagues from the United States Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, to the laboratories at the NCDC. The following methods were used to identify enteric pathogens in stool samples: (1) isolation of enteric pathogens (including Salmonella, Shigella, E. coli) on selective and differential media (Soy broth, Sorbitol broth, Endo agar, Hektoen enteric agar, Kligler agar, SS agar, Sorbitol MacConkey agar, CHROMagar O157); (2) identification of suspect isolates by biochemical assays using the API-20E Enteric Identification System (BioMerieux, Inc., Hazelwood, MO), and serotyping by agglutination assays, including those for E. coli sero-groups O157, O26, O45, and O111; 3) detection of fecal Shiga toxin using a commercial EIA (Premier EHEC, Meridian Bioscience Europe, Italy); and (4) determination of the presence of Shiga toxin genes (stx1 and stx2), as well as the intimin (eae), and enterohemolysin (ehxA-also referred to in the literature as hlyA and E-hly) genes in E. coli bacterial growths/cultures and stool samples in enrichment broths using conventional multiplex polymerase chain reaction (PCR) conditions as described [11]. Aliquots of serum, stool specimens, and stool broth enrichments from 20 patients were sent to the CDC-Atlanta for additional testing, including primary isolation, O:H serotyping in a microtiter format essentially as described by Ewing [12], pulsed-field gel electrophoresis (PFGE) [13], antimicrobial susceptibility testing [14], and PCR-based detection of STEC [15,16] and enteroaggregative E. coli (EAEC) [17,18] virulence factors. For the EAEC PCR, the primers for aggR and aatA (aggR gene is a virulence regulator in EAEC, and aatA is a plasmid encoded gene [pCVD432] and an EAEC virulence factor) were combined, and the PCR was performed as described by Schmidt et al. [17]. Unpaired serum specimens collected from patients with HUS were tested for antibodies to several E. coli lipopolysaccharide antigens (O157, O111, O26, O104) using in-house developed assays (CDC-Atlanta) [19].
2.4. Retrospective assessment of HUS cases and dialysis utilization
To better assess if the number of HUS cases observed represented a true increase over baseline, hospital medical records were reviewed and physicians from the department of kidney diseases at the “A. Tsulukidze-National Center of Urology” and from the department of kidney diseases in “M. Iashvili-Children Central Hospital” were interviewed to find other diagnosed HUS cases or similar conditions meeting this HUS case definition during the last three years. Cases were identified by physician recall and by a review of acute dialysis cases in the dialysis registry. Because of administrative issues at some clinics, access to review the medical charts was not allowed for all surveillance sites.
3. Results
87 total bloody diarrhea cases were identified with registration dates between June 1 and December 11, 2009; 62 patients had uncomplicated bloody diarrhea and another 25 also developed HUS. Samples were collected for 76 patients; 56 with bloody diarrhea only and 20 HUS cases. Most HUS and bloody diarrhea cases occurred in the warmer months.
3.1. Uncomplicated bloody diarrhea
In 62 patients with uncomplicated bloody diarrhea, the most common symptoms were abdominal pain (32%) and vomiting (32%). The most affected age groups were children <5 (7 persons) and the ⩾15-year olds (28 persons). However, age was unknown for 26 patients (Table 1). Registered patients resided in 10 out of the 12 regions of Georgia. No deaths were recorded among these 62 patients.
| Characteristics | Case-patients without HUS (N = 62) | Case-patients with HUS (N = 25) | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Age (years) | ||||
| <5 | 7 | 11 | 4 | 16 |
| 5–14 | 1 | 2 | 8 | 32 |
| 15+ | 28 | 45 | 13 | 52 |
| Unknown | 26 | 42 | ||
| Sex | ||||
| Male | 27 | 44 | 8 | 32 |
| Female | 31 | 50 | 17 | 68 |
| Unknown | 4 | 6 | ||
| Place of residence | ||||
| Rural Dvani | 1 | 8 | 3 | 13 |
| Rural Mejvriskevi | 0 | 0 | 1 | 4 |
| Other rural villages | 4 | 31 | 12 | 52 |
| Urban | 8 | 62 | 7 | 30 |
Demographic characteristics of patients with bloody diarrhea and HUS (N = 87).
3.2. Hemolytic uremic syndrome
Of the 25 patients who developed HUS, 68% were female and 52% were ⩾15 years of age (Table 1). All HUS case-patients required dialysis. Seven (28%) patients died, including 2 children <5 years of age and 5 adults ⩾15 years old. HUS case-patients resided in 8 regions of Georgia; however, incidence rates were highest in regions neighboring Tbilisi (Fig. 2). Among all 25 cases, the first HUS case was registered on July 10, 2009, and the last case on October 12, 2009. The distribution includes two waves of HUS cases with the second wave beginning around September 10 (Fig. 1). Sixty-nine percent of HUS case-patients resided in rural areas (Table 1). Three HUS case-patients resided within 100 meters of each other in one village. In this village, it was also found that three dogs with gastrointestinal symptoms had died around the same time that the patients with HUS had first become ill.
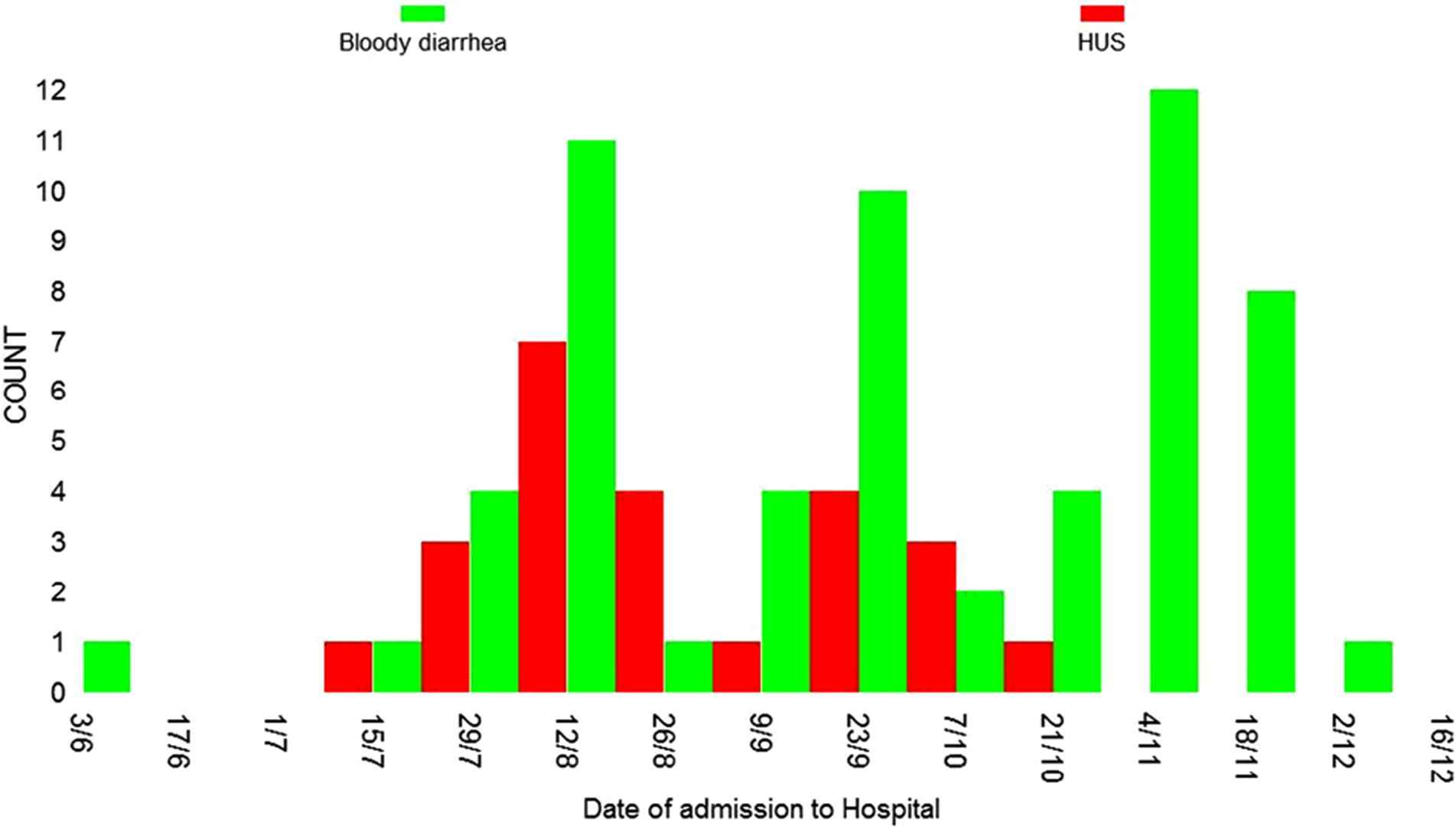
Distribution of HUS and bloody diarrhea cases by date of admission to the hospital, during outbreak of HUS in the republic of Georgia, 2009.
3.3. Exposures
Thirteen (21%) of 62 patients with uncomplicated bloody diarrhea and 19 (76%) of 25 patients with HUS were able to be interviewed about exposures to foods, water sources, animals, and ill persons. Food and other exposure-specific attack rates and attack rate ratios (RR) (Table 2) were calculated. For HUS case patients, it was found that the highest RR was associated with the consumption of ice cream (RR = 1.4), contact with domestic or farm animals during the 10 days before the onset (RR = 1.9), and eating food outside the home during the 10 days before the onset (RR = 1.5).
| Food products used or other exposition | Exposed | Unexposed | Attack rate ratio RR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HUS | Bloody diarrhea | N | Attack rate % | HUS | Bloody diarrhea | N | Attack rate % | ||
| Cheese | 5 | 8 | 13 | 38 | 14 | 5 | 19 | 74 | 0.5 |
| Fresh tomato | 6 | 6 | 12 | 50 | 13 | 7 | 20 | 65 | 0.8 |
| Sausage | 6 | 5 | 11 | 55 | 13 | 8 | 21 | 62 | 0.9 |
| Apple | 3 | 7 | 10 | 30 | 16 | 6 | 22 | 73 | 0.4 |
| Ice cream | 6 | 2 | 8 | 75 | 13 | 11 | 24 | 54 | 1.4* |
| Grapes | 3 | 3 | 6 | 50 | 16 | 10 | 26 | 62 | 0.8 |
| Dairy (excluding cheese and ice cream) | 1 | 5 | 6 | 17 | 18 | 8 | 26 | 69 | 0.2 |
| Beef | 1 | 4 | 5 | 20 | 18 | 9 | 27 | 67 | 0.3 |
| Chicken | 0 | 4 | 4 | 0 | 19 | 9 | 28 | 68 | 0.0 |
| Fresh greens | 1 | 3 | 4 | 25 | 18 | 10 | 28 | 64 | 0.4 |
| Contact with domestic or farm animals during 10 days before onset | 14 | 5 | 19 | 74 | 5 | 8 | 13 | 38 | 1.9* |
| Ate food outside home during10 days before onset | 13 | 6 | 19 | 68 | 6 | 7 | 13 | 46 | 1.5* |
| Contact with ill patient who had diarrhea | 0 | 5 | 5 | 0 | 19 | 8 | 27 | 70 | 0.0 |
Attack rate is more than 1.
Distribution of potential risk factors among interviewed (N = 32) patients with bloody diarrhea (N = 13) and those with HUS (N = 19).
Data on antibiotic treatment after admission to the hospital were available for 35 patients, among which 13 (37%) had uncomplicated bloody diarrhea and 22 (63%) had HUS. Ten patients (77%) with uncomplicated bloody diarrhea and 20 (90%) with HUS had been treated with antibiotics by a physician either before or after hospital admission.
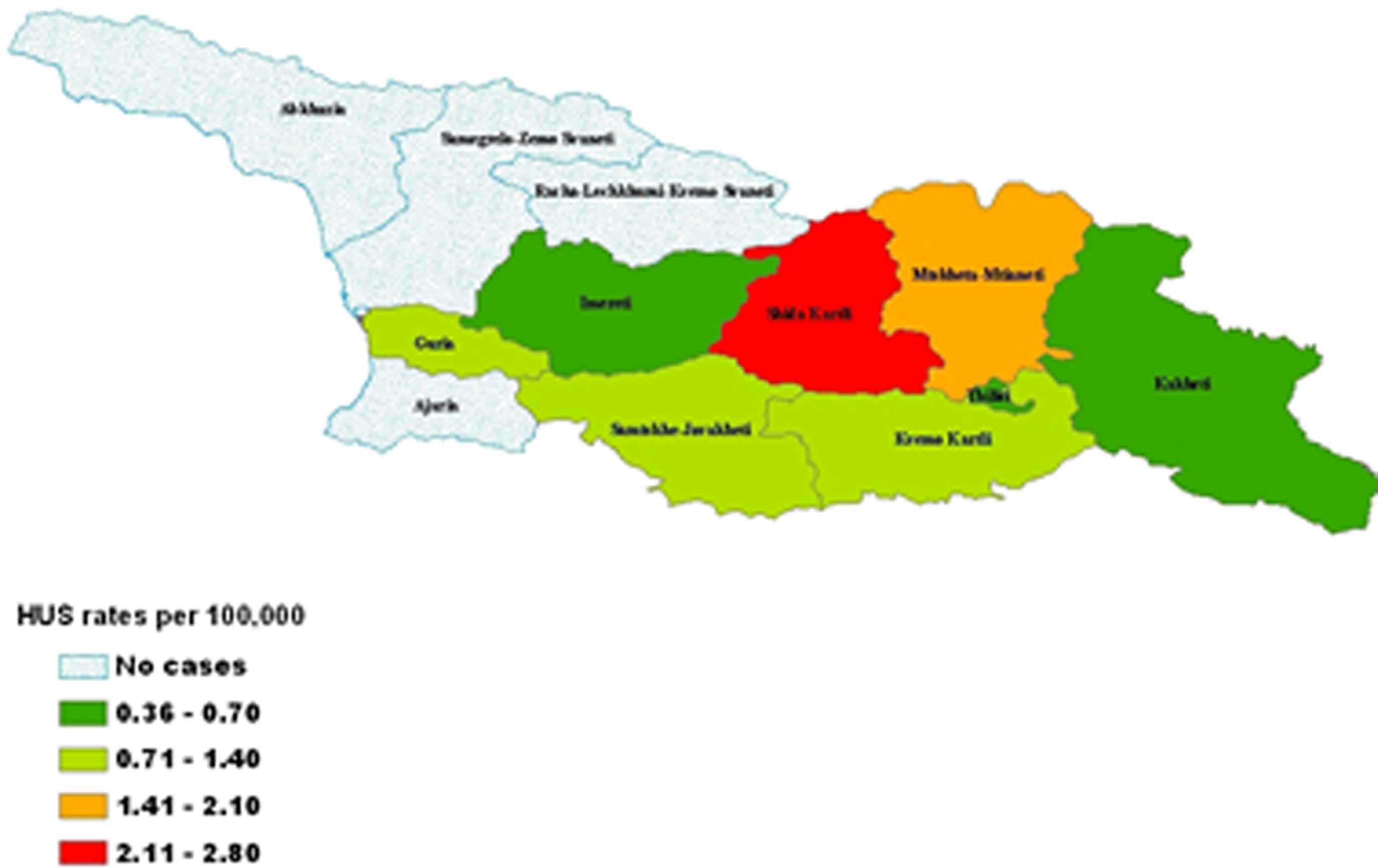
Geographic distribution of HUS rates in the republic of Georgia, 2009.
Thirty-six patients reported self-antibiotic treatment prior to admission to the hospital; 14 (39%) presented with uncomplicated bloody diarrhea and 22 (61%) with HUS. Six of the 7 (86%) patients who have died, were treated with antibiotics by physicians and 1 had also self-treated with antibiotics. It was unable to be established what types of antibiotics were taken, what the usage interval was or what dosage was taken by the patients prior to hospitalization.
3.4. Retrospective review of HUS cases
Review of the medical records at the “M. Iashvili-Children Central Hospital” revealed 2 HUS cases in 2008 and 9 in 2009. One case of HUS was registered at the “A. Tsulukidze-Urology National Center” in 2007, 2 in 2008, and another 2 in 2009.
3.5. Laboratory findings
The laboratory of NCDC tested a total of 76 fecal specimens from patients with bloody diarrhea cases; 20 of these specimens were from those with HUS. A total of 66 samples from 20 patients (12 with bloody diarrhea and 8 HUS patients) were also sent to the CDC (Atlanta, Georgia) for further testing. Combined laboratory results from tests done both at the NCDC and the CDC are summarized in Table 3.
| ID | Status | Testing of fresh stool or stool enrichment broths | Pathogenic stool isolates | Serum ELISA titers | |
|---|---|---|---|---|---|
| Shiga toxin ELISA |
Virulence genes | ||||
| 1 | HUS | + | E. coli O111 | O104-IgG 1280 O111, O26, O45-IgG 640 |
|
| 2 | HUS | – | |||
| 3 | HUS | − | − | – | – |
| 4 | HUS | − | − | – | |
| 5 | HUS | + | – | ||
| 6 | HUS | − | – | – | |
| 7 | HUS | – | – | ||
| 8 | BD | E. coli O111 | |||
| 9 | BD | − | − | E. coli O111 | |
| 10 | BD | E. coli O111 | |||
| 11 | BD | − | − | E. coli O26 | |
| 12 | BD | − | − | E. coli O26 | |
| 13 | BD | − | − | E. coli O26, S. dysenteriae type 3 | |
| 14 | BD | − | − | E. coli O45 | |
| 15 | BD | − | – | – | |
| 16 | BD | − | − | – | |
| 17 | BD | − | − | – | |
| 18 | BD | − | − | – | |
| 19 | BD | − | − | – | – |
| 20 | BD | − | − | – | – |
| 21 | BD | − | – | – | |
| 22 | BD | stx2a, aatA | E. coli O104:H4# | ||
| 23 | BD | eae, stx2 | E. coli+,^ | ||
| 24 | BD | − | – | ||
| 25 | BD | − | – | ||
| 26 | BD | − | stx2a, aatA, eae^ | E. coli O104:H4# | – |
| 27 | BD | – | O26-IgG 1280 | ||
| 28 | BD | − | – | ||
| 29 | BD | − | S. boydii | ||
| 30 | BD | − | S. boydii | ||
| 31 | BD | − | S. boydii | ||
| 32 | BD | − | S. flexneri | O26-IgM, IgG 640 | |
| 33 | BD | S. sonnei | |||
| 34 | BD | S. sonnei | |||
| 35 | BD | S. sonnei | |||
| 36 | BD | S. sonnei | |||
| 37 | BD | S. sonnei | |||
Isolate was not serogrouped.
stx2a-positive, eae-negative, ehxA-negative, aatA-positive (CDC Atlanta).
eae, stx2 positive (NCDC).
Summary of laboratory results for 37 patients with bloody diarrhea (BD) or HUS.
Examination of clinical samples from patients with HUS (Table 3) revealed the following results:
- •
Preliminary results presumptive for E. coli O157 obtained, at the beginning of the study, at the NCDC laboratory based on bacterial culture and serotyping with available diagnostic sera were not corroborated by CDC-Atlanta.
- •
Shiga toxin was demonstrated by EIA from the stool of a patient who died with HUS. In addition, the patient had elevated antibody titers to E. coli O104, O26, and O111.
- •
One patient with negative bacteriology results had Shiga toxin detected by EIA from stool.
- •
Shigella species were not isolated from persons with HUS.
Among those with uncomplicated bloody diarrhea, the following results were obtained:
- •
10 persons had Shigella species isolated from their stools; S. boydii (3 patients), S. sonnei (5 patients), S. dysenteriae type 3 (1 patient), and S. flexneri (1 patient). The serum specimen from the patient with S. flexneri was also positive for E. coli O26 antibodies (Table 3).
- •
18 persons had various E. coli stool isolates; 3 were sero-grouped as O111, 3 as O26 (1 had S. dysenteriae type 3 in his stool), 2 as O104:H4, and 1 as O45 (Table 3).
- •
One person did not have any E. coli isolates, but was positive for antibodies to E. coli sero-group O26.
- •
Samples from 2 patients were positive for the eae (intimin) gene (Fig. 3 gel lanes 7 and 21) by conventional multiplex PCR initially performed at NCDC on stool enrichment broths. A second specimen from one of these patients (Table 3 patient #23) was also positive for stx2 (gel lane 8). Virulence genes were not initially demonstrated at NCDC for patient 26, but were positive at CDC-Atlanta (Table 3).
- •
Only 2 E. coli O104:H4 isolates (patients 22 and 26) were eventually confirmed to carry Shiga toxin (stx2a) genes (CDC-Atlanta). The virulence gene and antimicrobial resistance profiles of these two O104:H4 isolates, as well as other E. coli strains are summarized in Table 4. Like the strains that caused the severe outbreak of bloody diarrhea in Germany in 2011, both strains from Georgia were stx2a-positive, eae-negative, ehxA-negative, aggR-positive and aatA-positive. Both O104 isolates were resistant to ampicillin, streptomycin, sulfisoxazole, and trimethoprim/sulfamethoxazole. One was also resistant to tetracycline. In contrast to the strains from Germany, both strains from Georgia were susceptible to third-generation cephalosporins [14].
- •
PFGE analysis of the strains listed in Table 4 showed that the two STEC O104:H4 isolates from Georgia clustered with the two isolates from the 2011 outbreak in Germany and were clearly separable from an EAEC O104:H4 control strain from the Central African Republic and the STEC O104:H21 strain from Montana, USA.
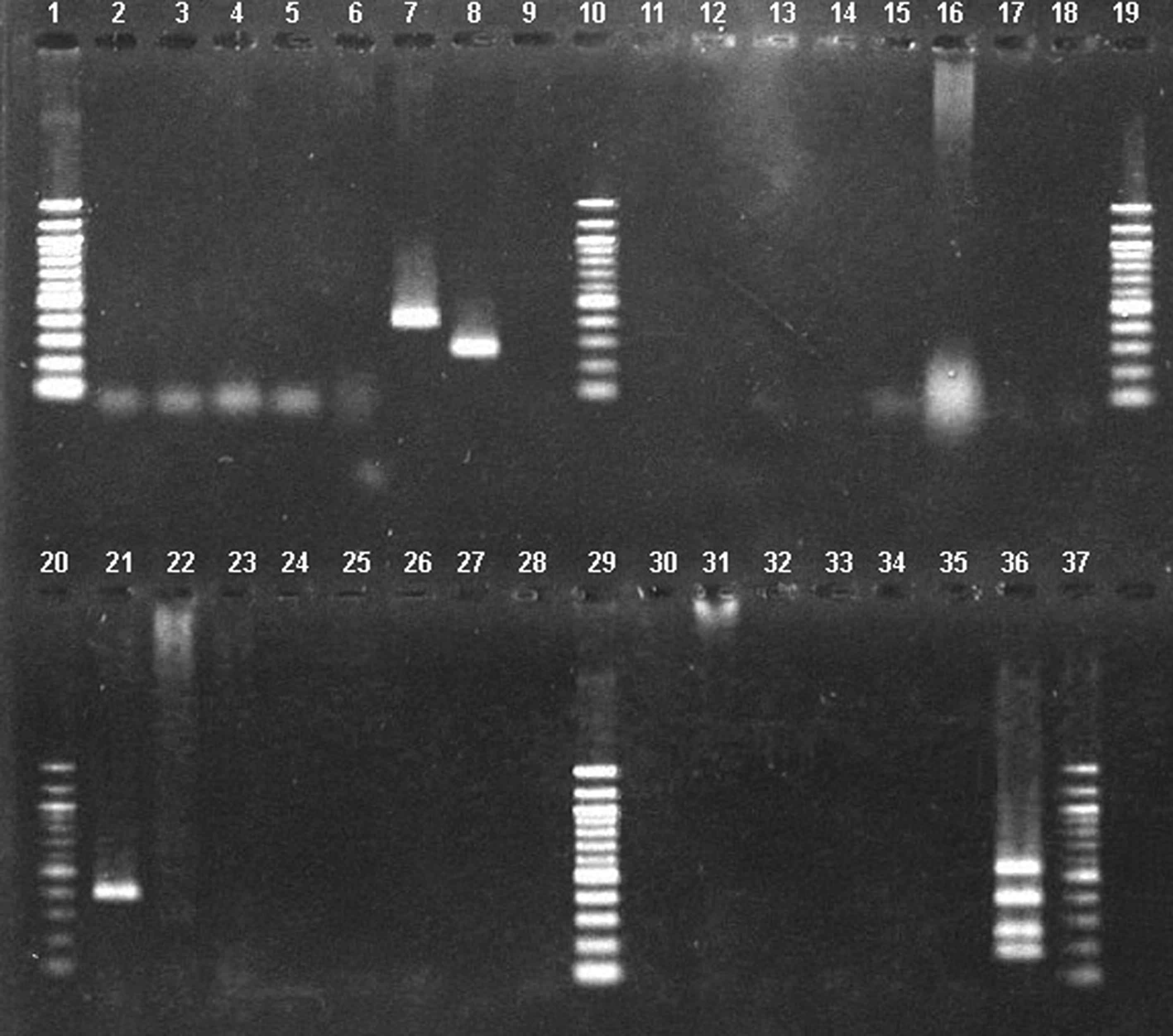
Conventional multiplex PCR of E. coli isolates and stool enrichment broth from patients with bloody diarrhea. This is located under Fig. 3 as legend. 100 bp Molecular marker ladders are located on the outer and central lanes (1, 10, 19, 20, 29, and 37) of each gel. Lane 35 contains a negative control; lane 36 contains E. coli O157 positive control and indicates location of the ehxA (534 bp), eae (384 bp), stx2 (255 bp), and stx1 (180 bp) genes. Lanes 7 (eae positive) and 8 (stx2 positive) are from stool isolates; lane 21 (eae positive) is from a stool enrichment broth.
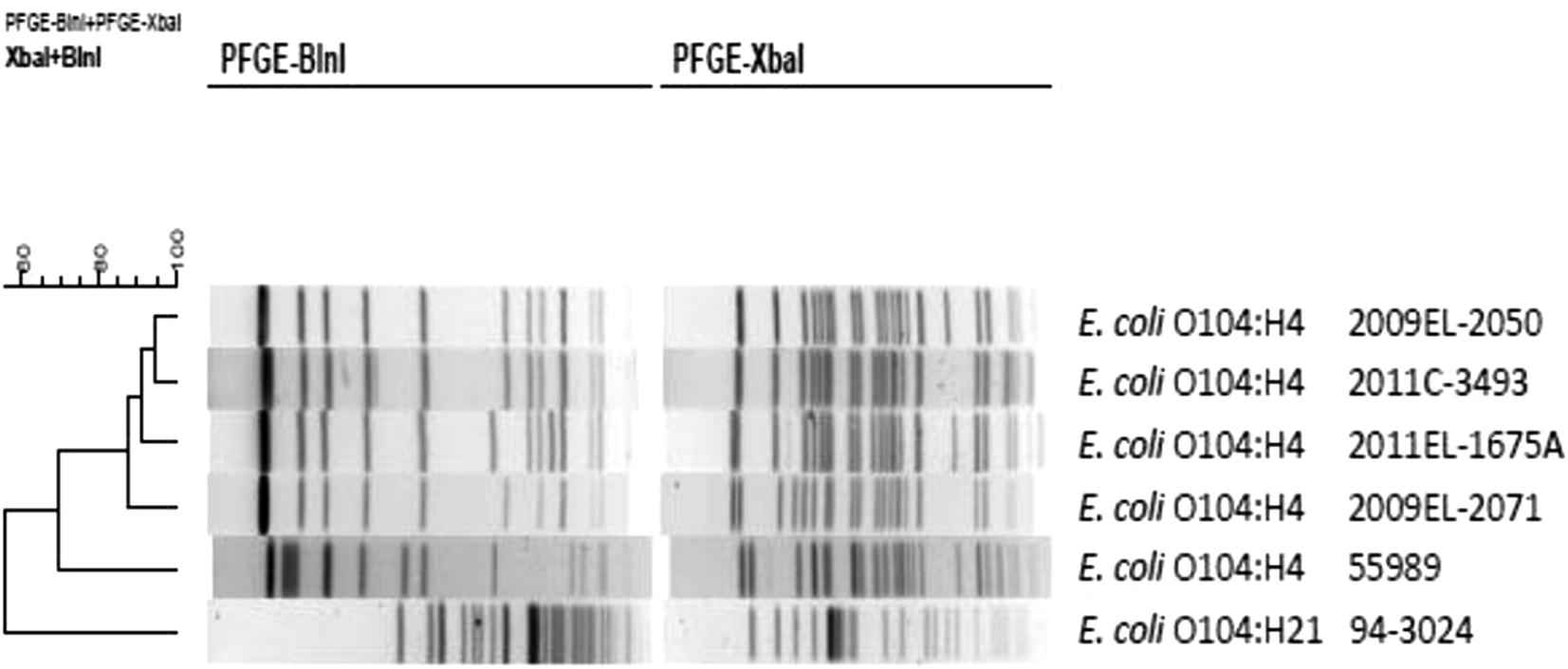
Macrorestriction analysis (BlnI and XbaI) by pulsed-field gel electrophoresis of Escherichia coli O104 isolates described in Table 4. This is located under Fig. 4 as legend. Dendrogram is based on combined PFGE patterns and was generated by BioNumerics 5.2 (Applied Maths, Inc., Austin, Texas, USA). Similarity analysis was performed using the Dice coefficient and clustering was performed using the unweighted pair-group method with arithmetic averages (UPGMA).
| Strain | Serotype | Country | Year of isolation | stx1 | stx2 | eae | ehxA | aatA | aggR | AMP* | STR | SIX | TMP-SXT | TET | CTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009EL-2050 | O104:H4 | Georgia | 2009 | − | + (stx2a) | − | − | + | + | R (>32) | R (>64) | R (>256) | R (>4) | R (32) | S (⩽0.25) |
| 2009EL-2071 | O104:H4 | Georgia | 2009 | − | + (stx2a) | − | − | + | + | R (>32) | R (>64) | R (>256) | R (>4) | S (⩽4) | S (⩽0.25) |
| 2011C-3493 | O104:H4 | USA-travel to Germany | 2011 | − | + (stx2a) | − | − | + | + | R (>32) | R (>64) | R (>256) | R (>4) | R (32) | R (>64) |
| 2011EL-1675A | O104:H4 | USA-travel to Germany | 2011 | − | + (stx2a) | − | − | + | + | R (>32) | R (>64) | R (>256) | R (>4) | R (32) | R (>64) |
| 55989 | O104:H4 | Central African Republic | 1996–1999 | − | − | − | − | + | + | NT^ | NT | NT | NT | NT | NT |
| 94–3024 | O104:H21 | USA-Montana | 1994 | − | + (stx2a, stx2d) | − | + | − | − | S (2) | S (<32) | S (⩽16) | S (⩽0.12) | S (⩽4) | S (⩽0.25) |
Interpretive criteria to categorize minimum inhibitory concentration results as susceptible (S), intermediate (I) or resistant (R) are based on current guidelines provided by the Clinical and Laboratory Standards Institute. Values in parentheses are minimum inhibitory concentration of antibiotic in micrograms per milliliter. Antibiotic abbreviations: AMP = ampicillin; STR = streptomycin; SIX = sulfisoxazole; TET = tetracycline; TMP-SMX = trimethoprim–sulfamethoxazole; CTX = ceftriaxone. The full panel of antibiotics tested by the National Antimicrobial Resistance Monitoring System (NARMS) is reported in reference 24.
NT = not tested.
Origin, serotype, virulence gene and antibiotic resistance profiles of E. coli O104 strains.
4. Discussion
Georgia experienced many HUS and bloody diarrhea cases during the period July–December 2009 in most of its regions. Because of the wide geographical distribution of patients, no reported exposures to widely distributed foods or other products, and a considerable diversity in the types of pathogens detected, it was considered that most of the cases represented a previously undetected burden of sporadic HUS and bloody diarrhea or several small unrelated outbreaks, or both. However, based on this assessment of past dialysis usage, more HUS cases appear to have occurred in 2009 relative to preceding years. Although this increase could be an artifact of enhanced surveillance, it does suggest that, for unknown reasons, the incidence of HUS did increase in 2009. In the years since, a higher incidence of toxigenic E. coli cases has also been reported by the NCDC (unpublished data).
This study demonstrated that laboratory specimens from a few cases were positive for pathogenic organisms, including enteroaggregative Shiga toxin-positive E. coli O104:H4 and the Shigella species. Additional microbiological and serological evidence show that E. coli with the same or serologically related O antigens as STEC O26, STEC O45 and STEC O111, were circulating among those with bloody diarrhea at or before the time of this investigation. The isolation of Shigella species in 10 (34%) of the 29 patients with bloody diarrhea from whom the specimens were collected shows the high incidence of these infections in those patients at the time. No Shigella dysenteriae type 1 – a pathogen that also produces Shiga toxin and causes HUS – was identified. This organism, however, is fragile and may not survive long in clinical specimens [31].
Even though the purpose of this surveillance was to identify infections capable of causing HUS, a relatively broad case definition was chosen which included any bloody diarrhea illness. As expected, the selection of this sensitive case definition captured a large group of illnesses, including STEC infections, other infections such as shigellosis, and many illnesses with no etiology determined. Although this limited the specificity of these surveillance efforts to target HUS-causing organisms, this choice of case definition was necessary given the very limited number of diarrheal illnesses in Georgia in which an etiology is determined. HUS affected a significantly larger proportion of females than males; this phenomenon is similar to the epidemiological picture of the enteroaggregative STEC O104:H4 outbreak in Germany in 2011 and also has been observed for STEC O157 in the United States [2,20]. The patient profile in the Georgian cases may be related to close contact, eating, and living behaviors between adult women and their children <15 years old.
The case fatality rate of 28% among HUS patients is high compared with reports in the literature [1,2,20]. Several possible explanations for the observed elevated mortality include: (1) virulence of the causative agent(s) or host-pathogen interactions [21]; (2) disease transmission factors that relate to the dose of pathogens ingested; (3) lack of financial means and awareness on the part of patients about the importance of early referral to hospital at the time of bloody diarrhea; (4) limited familiarity of physicians in Georgia with HUS clinical management or disease epidemiology; and (5) delays in accessing appropriate levels of care, especially in rural areas. Georgia has dialysis capacities only in the urban centers, which means that patients must be referred to and transported to these units even though they may be located far from their locations of residence.
Because HUS is not a reportable condition and was rarely registered previously in Georgia, physicians are not aware of adequate diagnostic procedures and clinical case management. The fact that many patients were initially treated with antibiotics by physicians even though they presented with classical HUS manifestations is of concern and attests to the lack of general knowledge that treatment of bloody diarrhea with antibiotics may precipitate HUS if STEC has not been ruled out as a cause [22,23]. An informal interview of physicians suggested a lack of knowledge about HUS diagnosis and clinical management. Nonetheless, some physicians working at the A. Tsulukidze-National Center of Urology and Infectious Diseases, AIDS and Clinical Immunology Research Center recalled cases of HUS during the past several years and were aware of the clinical condition. At present, no national clinical guidelines for antibiotics use in the treatment of bloody diarrhea exists even though antibiotics have wide availability and usage in Georgia. Early administration of intravenous fluids may reduce complications and death of patients with STEC infections capable of causing HUS [24].
Although a search into the possible sources of infections is warranted in the context of a possible outbreak of STEC infections, this study was unable to identify a single unifying exposure among case-patients. The following individual risk factors for developing HUS were identified: eating food outside the home during the 10 days before the onset of symptoms, contact with domestic animals, and eating ice cream. Nevertheless, this study did demonstrate that robust surveillance and timely laboratory diagnostics could enable the early detection of localized clusters of illness and possibly allow interventions to limit the extent and severity of the clinical illness. Through this surveillance, rapid identification of a cluster of 3 HUS cases in a small rural village in the Shida Kartli region was possible. There is much uncertainty as to what role, if any, the ill dogs played in disease transmission in this village. Dogs infected with STEC can develop HUS [25]; however, no laboratory isolation attempt was made from animal specimens.
Until the very large outbreak of STEC O104:H4 in Germany in 2011 and a smaller outbreak among travelers returning from Turkey, this STEC serotype was rarely described. The virulence gene profiles of both STEC O104:H4 isolates matched those of the German outbreak strain (stx2a-positive, eae-negative hly-negative, aaT-positive, and aggR-positive) [14,21]. The Georgian isolates, however, show 1–2 band difference from the German outbreak strains by xbaI and blnI restriction enzyme analysis using the PulseNet non-O157 protocol [13,26]. In addition, both the Georgian and German strains had similar antimicrobial susceptibility patterns except the German strain showed resistance to ceftriaxone [21,27].
No etiology was determined for most illnesses. This could be due in part to the high frequency of patients treated with antibiotics either as prescribed by a physician or through self-treatment and due to the general difficulty in isolating STEC organisms. Due to a variety of reasons, including costs of seeking medical help and perhaps the distances between rural communities and medical centers, many Georgians regularly self-treat with available antibiotics.
This investigation had several limitations that likely further limited the ability to determine the etiology of illnesses. First, the investigation was initiated at the end of July, whereas cases of bloody diarrhea began in June. Access to laboratory supplies and training of staff were not appropriate at the initial stages of the investigation, and the sample collection network only became operational in the middle of August. Secondly, there was a general lack of experience in laboratory detection of STEC [28] and understanding the importance of collecting stool samples early after the onset of diarrhea. In many instances, stool samples were collected more than several days after the onset of diarrhea. Also, it was not possible to determine the types, dosage and the duration of use of antibiotics that may have been taken by patients prior to hospitalization. HUS typically develops in approximately 5–7 days after the onset of diarrhea and by that time, the amount of STEC in the stool is often very limited and diarrhea has often ceased [29]. Thirdly, at the time of the HUS reports in 2009, neither specialty hospitals nor NCDC laboratories had sufficient capacity and funding to fully detect, isolate, identify, and subtype STEC. Fourthly, suboptimal specimen storage and transportation may have further limited identification of the pathogens. Review of the literature showed that there is a lack of information on the epidemiology of bloody diarrhea and HUS caused by STEC in the region, including Georgia [30] and the Newly Independent States (NIS).
Several recommendations can be made based on these findings to better understand the epidemiology of these infections in this region of the world. First, in order to ensure early detection of outbreaks and to better describe the epidemiology of diarrheal diseases and HUS in Georgia, it should be mandatory to report HUS and bloody diarrhea cases nationally. Secondly, training of physicians is needed to increase awareness of the clinical presentations, diagnosis and treatment of HUS, including the importance of reporting every HUS case. Thirdly, the fact that a strain of STEC O104:H4 nearly identical to that responsible for the deadly outbreak in Europe in 2011 was isolated highlights the need to collaborate across borders to improve investigation and control efforts.
In the course of this investigation, proper collection, storage and transportation of samples were identified as a weakness that needs to be addressed in Georgia. These elements are part of the basic prerequisite for surveillance and outbreak investigation. Furthermore, there must be adequate capacity in terms of reference laboratory infrastructure and expertise for testing collected stool samples during these outbreaks. Finally, it is recommended that the feasibility of laboratories, particularly those in referral hospitals and in the rural regions, or in regional public health centers, to implement testing for the timely identification of STEC or Shiga toxin in clinical specimens be evaluated.
Conflict of interest
None.
Acknowledgements
We gratefully acknowledge the technical assistance of Maurice Curtis, Jessica Halpin and Sung Im for performing the PFGE analysis.
References
Cite this article
TY - JOUR AU - Otar Chokoshvili AU - Khatuna Lomashvili AU - Naile Malakmadze AU - Marika Geleishvil AU - Jonas Brant AU - Paata Imnadze AU - Nazibrola Chitadze AU - Lia Tevzadze AU - Gvantsa Chanturia AU - Tea Tevdoradze AU - Tengiz Tsertsvadze AU - Deborah Talkington AU - Rajal K Mody AU - Nancy Strockbine AU - Russell A Gerber AU - Edmond Maes AU - Thomas Rush PY - 2014 DA - 2014/04/30 TI - Investigation of an outbreak of bloody diarrhea complicated with hemolytic uremic syndrome JO - Journal of Epidemiology and Global Health SP - 249 EP - 259 VL - 4 IS - 4 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2014.03.004 DO - 10.1016/j.jegh.2014.03.004 ID - Chokoshvili2014 ER -
