A longitudinal cohort study of the relationship between Thimerosal-containing hepatitis B vaccination and specific delays in development in the United States: Assessment of attributable risk and lifetime care costs
- DOI
- 10.1016/j.jegh.2015.06.002How to use a DOI?
- Keywords
- Ethylmercury; Merthiolate; Thimerosal; Thiomersal; Vaccine
- Abstract
Epidemiological evidence suggests a link between mercury (Hg) exposure from Thimerosal-containing vaccines and specific delays in development. A hypothesis-testing longitudinal cohort study (n = 49,835) using medical records in the Vaccine Safety Datalink (VSD) was undertaken to evaluate the relationship between exposure to Hg from Thimerosal-containing hepatitis B vaccines (T-HBVs) administered at specific intervals in the first 6 months of life and specific delays in development [International Classification of Disease, 9th revision (ICD-9): 315.xx] among children born between 1991 and 1994 and continuously enrolled from birth for at least 5.81 years. Infants receiving increased Hg doses from T-HBVs administered within the first month, the first 2 months, and the first 6 months of life were significantly more likely to be diagnosed with specific delays in development than infants receiving no Hg doses from T-HBVs. During the decade in which T-HBVs were routinely recommended and administered to US infants (1991–2001), an estimated 0.5–1 million additional US children were diagnosed with specific delays in development as a consequence of 25 μg or 37.5 μg organic Hg from T-HBVs administered within the first 6 months of life. The resulting lifetime costs to the United States may exceed $1 trillion.
- Copyright
- © 2015 The Authors. Published by Elsevier Ltd. on behalf of Ministry of Health, Saudi Arabia.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Thimerosal is a mercury (Hg)-containing compound (49.55% Hg weight) used by pharmaceutical companies, and was developed in 1927. It has been added to a range of pharmaceutical products as an antimicrobial [1]. Following administration of Thimerosal-containing vaccines, Thimerosal rapidly dissociates into ethyl-Hg [2] which rapidly binds onto blood constituents [3] and is transported to many tissues in the body, including the brain [4]. Of particular concern, ethyl-Hg is actively transported across neuronal membranes [5], such as by the L-type neutral amino acid carrier transport system [6].
During the 1990s, US infants were exposed to significant amounts of organic Hg from Thimerosal-containing hepatitis B vaccine (T-HBV), diphtheria–tetanus–pertussis (DTP), and Haemophilus influenzae type B (Hib) vaccines administered at periodic intervals within the first 6 months of life. Typically, nominal concentrations of Thimerosal present in infant vaccines ranged from 0.005% to 0.01% (12.5 μg Hg/0.5 mL vaccine dose or 25 μg Hg/0.5 mL vaccine dose) [4]. Infants may have been exposed to bolus doses of organic Hg nominally ranging from 12.5 μg Hg to 62.5 μg organic Hg, collectively totaling up to nominally 200 μg organic Hg from Thimerosal-containing childhood vaccines during the first 6 months of life, representing >50% of all Hg exposure when considering environmental sources of Hg [4]. This dosing pattern continues unabated in many developing nations to the present day, and many US children continue to receive significant doses of organic Hg from the routinely recommended administration of Thimerosal-containing influenza vaccines (where >50% of all doses of influenza vaccine continue to contain 0.01% Thimerosal) given to pregnant women, infants, and young children [4].
In 2003, some of the co-authors of this article proposed that the hypothesis that exposure to Thimerosal-containing vaccines was associated with specific delays in development rested on indirect and incomplete information, primarily from analogies with methyl-Hg and levels of maximum Hg exposure from vaccines given to children [7]. It was suggested that the hypothesis was biologically plausible, but that the possible relationship between Thimerosal-containing vaccines and specific delays in development was unproven at that time. As of 2003, no peer-reviewed epidemiological studies in the scientific literature had evaluated the potential association between Thimerosal-containing vaccines and specific delays in development. Our study was the first epidemiological study to report a significant association between the administration of tens of millions of doses of Thimerosal-containing diphtheria–tetanus–acellular-pertussis (DTaP) vaccines to US children and specific delays in development based upon assessment of the Vaccine Adverse Event Reporting System (VAERS) database.
Subsequent studies in the United States have revealed significant associations between specific delays in development and administration of Thimerosal-containing vaccines to infants in the VAERS [7,8], the Vaccine Safety Datalink (VSD) [9,10], and the National Health and Nutrition Examination Survey (NHANES) [11] databases.
The purpose of the present study was to extend previous research by conducting a longitudinal cohort study of prospectively collected automated medical records in the VSD database to further evaluate the relationship between organic Hg exposure from T-HBVs administered in the first 6 months of life, and the risk of a child being diagnosed with specific delays in development (or learning disability).
2. Methods
The study protocol was approved by the US Centers for Disease Control and Prevention (CDC), the Institutional Review Board of Kaiser Permanente North-West (KPNW; ID: NW-05MGeie-01), and the Institutional Review Board of Kaiser Permanente Northern California (KPNC; ID: CN-03MGeie-01-H). Data were analyzed at the secure Research Data Center of the National Center for Health Statistics in Hyattsville, MD, USA. The views expressed in this study do not necessarily reflect those of the CDC or those of Kaiser Permanente.
The VSD project was created in 1991 by the National Immunization Program of the CDC, and VSD data collection and study methods have been previously described [12–16]. The project links medical event information, specific vaccine history, and selected demographic information from the computerized databases of several health maintenance organizations (HMOs).
2.1. Study participants
An overall cohort of over 1.9 million children enrolled in the VSD project (updated through the end of 2000) from KPNW, Kaiser Permanente Colorado, and KPNC was examined using SAS software (SAS Institute Inc., 100 SAS Campus Drive, Cary, NC 27513-2414, USA). Among the overall cohort of children, a subset of children with nonmissing date of birth and nonmissing sex (who were HMO-enrolled from their date of birth) was further examined.
The outcome files (inpatient and outpatient diagnoses) from this subset of children were then reviewed to find the first instance of International Classification of Disease, 9th revision (ICD-9)-diagnosed specific delays in development, including: reading disorder, unspecified (315.00), alexia (315.01), developmental dyslexia (315.02), specific spelling difficulty (315.09), dyscalculia (315.1), disorder of written expression (315.2), expressive language disorder (315.31), mixed receptive–expressive language disorder (315.32), speech and language developmental delay due to hearing loss (315.34), developmental articulation disorder (315.39), developmental coordination disorder (315.4), mixed development disorder (315.5), other specified delays in development (315.8), and unspecified delay in development (315.9). If there were multiple instances of the same diagnosis in a child, only the first instance was counted.
The records of the children diagnosed with specific delays in development were then reviewed to determine the mean age of initial diagnosis (2.63 years of age) and the standard deviation age of mean initial diagnosis (1.59 years of age). Then, using this information, we required that every member of the cohort assembled for our study had to be continuously enrolled from birth for at least 5.81 years (mean age of initial diagnosis + 2 × standard deviation of initial diagnosis). As a result, a cohort of 49,835 children (males = 25,624 and females = 24,210; male to female ratio = 1.06), born between 1991 and 1994, was examined in the present study.
2.2. Hepatitis B vaccine exposure
The vaccine file for the cohort was then reviewed to determine the exact dates of hepatitis B vaccine administration. Those members of the cohort receiving no doses of hepatitis B vaccine were also included in the present study. Overall among members of the cohort, Hg exposure was assigned as 12.5 μg organic Hg/dose for those receiving a pediatric hepatitis B vaccine or 0 μg organic Hg/dose for those receiving either combined Haemophilus influenzae type B (Hib)-hepatitis B vaccine or neither of the aforementioned vaccines (0 μg organic Hg/dose from T-HBV). In addition, to allow for a potential association between exposure and outcome, individuals diagnosed with specific delays in development before administration of the vaccines examined in the present study were excluded.
2.3. Statistical analyses
In all statistical analyses, the StatsDirect statistical software (Version 2.8.0, StatsDirect Ltd., 9 Bonville Chase, Altrincham, Cheshire WA 14 4QA, UK) was utilized, and a two-sided p value < 0.05 was considered statistically significant.
In the first set of experiments, the Fisher’s exact test statistic was utilized to examine the relationship between increasing amounts of organic Hg exposure from T-HBV administration and the frequency of diagnosed specific delays in development. In the first experimental group (Experiment I), the data were examined to determine the frequency of diagnosed specific delays in development among the cohort of children exposed to 12.5 μg organic Hg from T-HBV administered in the first month of life, in comparison with the frequency of diagnosed specific delays in development among the cohort of children exposed to 0 μg organic Hg from T-HBV administered in the first month of life. In the second experimental group (Experiment II), the data were examined to determine the frequency of diagnosed specific delays in development among the cohort of children exposed to 25 μg organic Hg from T-HBV administered in the first 2 months of life, in comparison with the frequency of diagnosed specific delays in development among the cohort of children exposed to 0 μg organic Hg from T-HBV administered in the first 2 months of life. In the third experimental group (Experiment III), the data were examined to determine the frequency of diagnosed specific delays in development among the cohort of children exposed to 37.5 μg organic Hg from T-HBV administered in the first 6 months of life, in comparison with the frequency of diagnosed specific delays in development among the cohort of children exposed to 0 μg organic Hg from T-HBV administered in the first 6 months of life. The aforementioned experiments were repeated by splitting the data so that male cohorts (Experiments IV–VI) and female cohorts (Experiments VII–IX) were analyzed separately. The overall null hypotheses for each of these experimental groups were that there would be no difference in the frequency of diagnosed specific delays in development regardless of exposure to organic Hg doses from T-HBVs.
The second set of experiments examined the potential dose–response relationship between increasing amounts of organic Hg exposure from T-HBV administration and the frequency of diagnosed specific delays in development utilizing the linear regression and Fisher’s exact statistical tests. The linear regression test was employed to compare the frequency of diagnosed specific delays in development/μg organic Hg exposure from T-HBVs administered within the first 6 months of life. Additionally, the Fisher’s exact statistical test was used to determine the discrete relative risks for the frequency of diagnosed specific delays in development in the cohorts receiving exposure to 12.5 μg organic Hg, 25 μg organic Hg, or 37.5 μg organic Hg from T-HBV doses, in comparison with the cohort receiving 0 μg organic Hg from Thimerosal-free hepatitis B vaccine doses and/or no hepatitis B vaccinations within the first 6 months of life. The overall null hypotheses for each of these comparisons were that there would be no difference in the frequency of diagnosed specific delays in development regardless of exposure to organic Hg from Thimerosal-containing hepatitis vaccines.
3. Results
Table 1 displays the frequency of diagnosed specific delays in development in relation to exposure to organic Hg from T-HBVs administered at specific intervals within the first 6 months of life. It was observed in Experiment I that infants receiving 12.5 μg organic Hg from T-HBVs (4.25% diagnosed with specific delays in development) in comparison with infants receiving 0 μg organic Hg from T-HBVs (3.49% diagnosed with specific delays in development) within the first month of life were significantly more likely to have received a specific delays in development diagnosis (relative risk = 1.220, p < 0.0001). In Experiment II, it was found that infants receiving 25 μg organic Hg from T-HBVs (4.25% diagnosed with specific delays in development) in comparison with infants receiving 0 μg organic Hg from T-HBVs (3.50% diagnosed with specific delays in development) within the first 2 months of life were significantly more likely to have received a specific delays in development diagnosis (relative risk = 1.214, p < 0.0001). Finally, in Experiment III, it was revealed that infants receiving 37.5 μg organic Hg from T-HBVs (4.46% diagnosed with specific delays in development), in comparison with 0 μg organic Hg from T-HBVs (1.67% diagnosed with specific delays in development) were significantly more likely to have received a specific delays in development diagnosis (relative risk = 2.667, p < 0.0001). Table 2 (Experiments IV–VI) and Table 3 (Experiments VII–IX) show similar patterns of increased relative risk when the data were separated by the sex of the vaccine recipients. The attributable risk percent was significantly increased among boys receiving 37.5 μg organic Hg from T-HBVs in comparison with 0 μg organic Hg from T-HBVs within the first 6 months of life (attributable risk percent = 4.091%, 95% confidence interval = 3.065–5.117) versus girls receiving 37.5 μg organic Hg from T-HBVs in comparison with 0 μg organic Hg from T-HBVs within the first 6 months of life (attributable risk percent = 1.406%, 95% confidence interval = 0.541–2.271).
| Group examined | Number diagnosed with specific delays in development (%) | Number of individuals in the cohort | Relative risk (95% CI) | Attributable risk % (95% CI) | Population attributable risk % (95% CI) |
|---|---|---|---|---|---|
| Experiment I | |||||
| 12.5 μg Organic mercury within the first mo | 828 (4.25) | 18,637 | 1.220* (1.117–1.332) | 0.767 (0.420–1.14) | 7.64 (4.2–11.08) |
| 0 μg Organic mercury within the first mo | 1127 (3.49) | 31,198 | |||
| Experiment II | |||||
| 25 μg Organic mercury within the first 2 mo | 829 (4.25) | 18,660 | 1.214* (1.112–1.327) | 0.751 (0.403–1.10) | 7.57 (4.072–11.07) |
| 0 μg Organic mercury within first 2 mo | 1105 (3.50) | 30,444 | |||
| Experiment III | |||||
| 37.5 μg Organic mercury within the first 6 mo | 641 (4.46) | 13,725 | 2.667* (1.866–3.812) | 2.89 (2.11–3.46) | 59.62 (45.87–73.38) |
| 0 μg Organic mercury within the first 6 mo | 31 (1.67) | 1822 | |||
CI = confidence interval.
p < 0.001.
A summary of the frequency of diagnosed specific delays in development in comparison with organic mercury exposure from Thimerosal-containing hepatitis B vaccine administration within the Vaccine Safety Datalink (VSD) database.
| Group examined | Number of males diagnosed with specific delays in development (%) | Number of males in the cohort | Relative risk (95% CI) | Attributable risk % (95% CI) | Population attributable risk % (95% CI) |
|---|---|---|---|---|---|
| Experiment IV | |||||
| 12.5 μg Organic mercury within the first mo | 567 (5.62) | 9514 | 1.231* (1.108–1.369) | 1.057 (0.508–1.61) | 7.965 (3.846–12.08) |
| 0 μg Organic mercury within the first mo | 771 (4.57) | 16,110 | |||
| Experiment V | |||||
| 25 μg Organic mercury within the first 2 mo | 568 (5.63) | 9525 | 1.223* (1.1–1.359) | 1.025 (0.4727–1.58) | 7.798 (3.614–11.98) |
| 0 μg Organic mercury within the first 2 mo | 758 (4.60) | 15,709 | |||
| Experiment VI | |||||
| 37.5 μg Organic mercury within the first 6 mo | 443 (6.00) | 6946 | 3.148* (1.974–5.018) | 4.091 (3.065–5.117) | 65.57 (50.13–81) |
| 0 μg Organic mercury within the first 6 mo | 18 (1.90) | 927 | |||
CI = confidence interval.
p < 0.001.
A summary among males of the frequency of diagnosed specific delays in development in comparison with organic mercury exposure from Thimerosal-containing hepatitis B vaccine administration within the Vaccine Safety Datalink (VSD) database.
| Group examined | Number of females diagnosed with specific delays in development (%) | Number of females in the cohort | Relative risk (95% CI) | Attributable risk % (95% CI) | Population attributable risk % (95% CI) |
|---|---|---|---|---|---|
| Experiment VII | |||||
| 12.5 μg Organic mercury within the first mo | 261 (2.78) | 9122 | 1.207* (1.031–1.413) | 0.4765 (0.0682–0.885) | 7.247 (1.061–13.43) |
| 0 μg Organic mercury within the first mo | 356 (2.31) | 15,088 | |||
| Experiment VIII | |||||
| 25 μg Organic mercury within the first 2 mo | 261 (2.78) | 9134 | 1.207* (1.03–1.415) | 0.4773 (0.0678–0.887) | 7.376 (1.073–13.68) |
| 0 μg Organic mercury within the first 2 mo | 347 (2.30) | 14,735 | |||
| Experiment IX | |||||
| 37.5 μg Organic mercury within the first 6 mo | 198 (2.84) | 6779 | 1.982* (1.136–3.459) | 1.406 (0.541–2.271) | 46.5 (18.54–74.46) |
| 0 μg Organic mercury within the first 6 mo | 13 (1.43) | 895 | |||
CI = confidence interval.
p < 0.05.
A summary among females of the frequency of diagnosed specific delays in development in comparison with organic mercury exposure from Thimerosal-containing hepatitis B vaccine administration within the Vaccine Safety Datalink (VSD) database.
Table 4 displays the relative risk estimates for increasing doses of organic Hg exposure from T-HBVs administered within the first 6 months of life. It was observed that the cohort of infants receiving 12.5 μg organic Hg from T-HBVs (2.873% diagnosed with specific delays in development), in comparison with 0 μg organic Hg from T-HBVs (1.701% diagnosed with specific delays in development) within the first 6 months of life were nonsignificantly more likely to be diagnosed with specific delays in development (relative risk = 1.669, p = 0.0958). It was found that the cohort of infants receiving 25 μg organic Hg from T-HBVs (3.761% diagnosed with specific delays in development), in comparison with 0 μg organic Hg from T-HBVs within the first 6 months of life, were significantly more likely to be diagnosed with specific delays in development (relative risk = 2.166, p < 0.0001). It was determined that the cohort of infants receiving 37.5 μg organic Hg from T-HBVs (4.670% diagnosed with specific delays in development) within the first 6 months of life, in comparison with 0 μg organic Hg from T-HBVs within the first 6 months of life, were significantly more likely to be diagnosed with specific delays in development (relative risk = 2.667, p < 0.0001).
| Exposure | Number diagnosed with specific delays in development (%) | Number of individuals in the cohort | Outcome measurement |
|---|---|---|---|
| 0 μg Organic Hg (reference dose) | 31 (1.701) | 1822 | |
| 12.5 μg Organic Hg | 21 (2.873) | 731 | |
| Relative risk (95% CI) | 1.669 (0.971–2.864) | ||
| p | 0.096 | ||
| Attributable risk (95% CI) | 0.0112 (−0.000606–0.0264) | ||
| Population attributable risk % (95% CI) | 16.191 (−2.355–34.737) | ||
| 25 μg Organic Hg | 1262 (3.761) | 33,557 | |
| Relative risk (95% CI) | 2.166 (1.526–3.0814) | ||
| p | <0.001 | ||
| Attributable risk (95% CI) | 0.0195 (0.0124–0.0249) | ||
| Population attributable risk % (95% CI) | 52.551 (36.192–68.910) | ||
| 37.5 μg Organic Hg | 641 (4.670) | 13,725 | |
| Relative risk (95% CI) | 2.667 (1.872–3.808) | ||
| p | <0.001 | ||
| Attributable risk (95% CI) | 0.0279 (0.0203–0.0340) | ||
| Population attributable risk % (95% CI) | 59.622 (45.865–73.379) |
CI = confidence interval.
A summary of discrete risk ratio estimates for diagnosed specific delays in development with increasing exposure to organic mercury (Hg) from Thimerosal-containing hepatitis B vaccine administration within the first 6 months of life.
In addition, the linear regression test was used to compare the frequency of diagnosed specific delays in development/μg organic Hg exposure from T-HBVs administered within the first 6 months of life; it revealed, as shown in Fig. 1, a significantly positive dose-dependent correlation between increasing organic Hg exposure from T-HBVs and an increasing incidence of diagnosed specific delays in development. The following equation explains the distribution of the data using the linear regression test: percent of children diagnosed with specific delays in development = (0.0784 × μg organic Hg) + 1.782 (p = 0.0023, 95% confidence interval of slope value = 0.0623–0.0944, r = 0.998, 95% confidence interval of r = 0.892–0.999, r2 = 0.995).
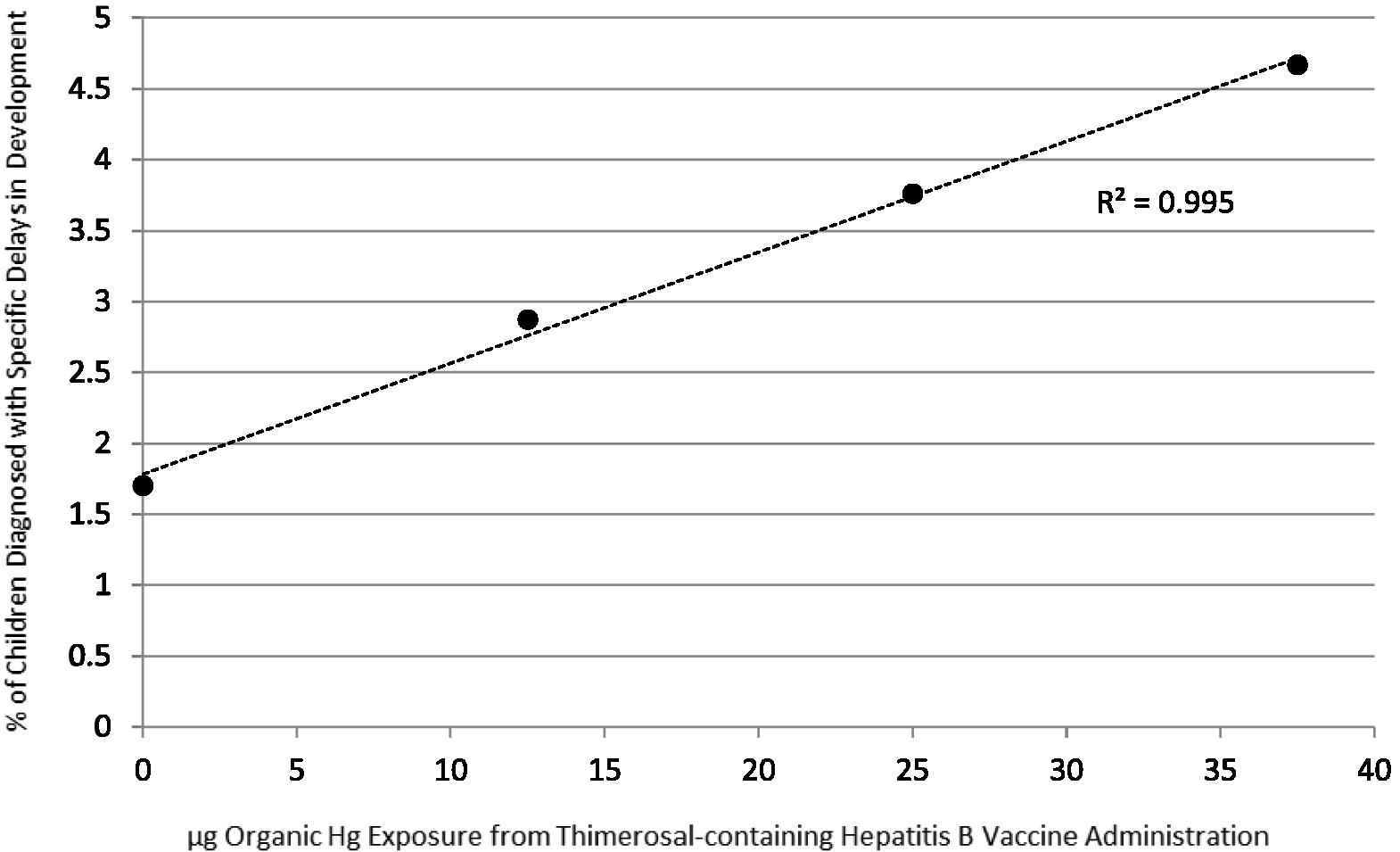
A summary of the percent of children diagnosed with specific delays in development in comparison with the dose of organic Hg received from Thimerosal-containing hepatitis B vaccines administered within the first 6 months of life. The following equation explains the distribution of the data using the linear regression test: percent of children diagnosed with specific delays in development = (0.0784 × μg organic Hg) + 1.782 (p = 0.0023, 95% confidence interval of slope value = 0.0623–0.0944, r = 0.998, 95% confidence interval of r = 0.892–0.999, r2 = 0.995).
4. Discussion
The results of the present study confirm and extend previous epidemiological studies finding a significant relationship between exposure to Thimerosal-containing childhood vaccines and an increased risk of specific delays in development. It was observed, in the overall cohort and the cohorts separated by sex, that infants who received increased organic Hg from T-HBVs, in comparison with infants who received no organic Hg from T-HBV within the first month of life, the first 2 months of life, and the first 6 months of life, were significantly more likely to subsequently be diagnosed with specific delays in development. Further, it was observed that there was a significant dose-dependent relationship between increasing doses of organic Hg from T-HBVs administered within the first 6 months of life and the eventual risk of a child being diagnosed with specific delays in development.
In further considering the implications of the present study regarding US children, it is important to note that in 1991, the Advisory Committee on Immunization Practices recommended that infants should receive their hepatitis B vaccine doses as follows: first dose between birth and 2 months of age, second dose between 1 month of age and 4 months of age, and third dose between 6 months of age and 18 months of age [17]. Subsequently, from 1991 through 2001, most doses of hepatitis B vaccine routinely administered to US infants contained 12.5 μg organic Hg/dose [18,19].
The results of the present study reveal that a cohort of children receiving 25 μg organic Hg from T-HBVs or 37.5 μg organic Hg from T-HBVs within the first 6 months of life was significantly more likely to be diagnosed with specific delays in development than a cohort of children receiving 0 μg organic Hg from T-HBVs administered within the first 6 months of life (relative risk = 2.31, 95% relative risk confidence interval = 1.632–3.285, p < 0.0001, attributable risk = 0.02196, 95% attributable risk confidence interval = 0.0149–0.0272). Then, utilizing the yearly live birth estimates of the CDC [20] to estimate the size of the US birth cohort between 1991 and 2001, along with the aforementioned calculation of the risk posed by receipt of 25 μg organic Hg from T-HBVs or 37.5 μg organic Hg from T-HBVs within the first 6 months of life, in comparison with 0 μg organic Hg from T-HBVs administered within the first 6 months of life, it was possible to estimate the attributable number of children diagnosed with specific delays as a result or organic Hg exposure from Thimerosal-containing vaccines. Table 5 estimates the number of children impacted based upon different exposure estimates in the US infant population to T-HBVs. It was estimated, utilizing the calculated exposure percentage derived from our analysis of the VSD (95% estimated exposure) database, that 913,518 children born between 1991 and 2001, were diagnosed with specific delays in development as a consequence of receipt of 25 μg organic Hg from T-HBVs or 37.5 μg organic Hg from T-HBVs administered within the first 6 months of life. It was estimated, utilizing a much more conservative estimated exposure percentage (50% estimated exposure), that 480,799 children born between 1991 and 2001, were diagnosed with specific delays in development as a consequence of receipt of 25 μg organic Hg from T-HBVs or 37.5 μg organic Hg from T-HBVs administered within the first 6 months of life. It is important to note from the data presented in Fig. 1 of our study that while organic Hg exposure from T-HBVs administered within the first 6 months of life significantly contributed to an increased number of children being diagnosed with specific delays in development, such exposure was not the sole cause of this diagnosis (i.e., 1.701% of children were diagnosed with specific delays in development in the absence of any organic Hg exposure from T-HBVs administered within the first 6 months of life).
| Estimated exposure percentage (%) | Estimated number of children exposed | Estimated number of children diagnosed with specific delays in development |
|---|---|---|
| 95 | 41,597,291 | 913,518 |
| 85 | 37,218,629 | 817,358 |
| 75 | 32,839,966 | 721,198 |
| 50 | 21,893,311 | 480,799 |
The bolded-italicized text is the estimated percentage of children receiving 25 μg organic Hg or 37.5 μg organic Hg from Thimerosal-containing hepatitis B vaccines administered within the first 6 months of life based upon the present analysis of the Vaccine Safety Datalink (VSD) database [(33,557 + 13,725)/(1822 + 731 + 33,557 + 13,725) × 100 = 95%].
A summary of the estimated number of US children born between 1991 and 2001 (n = 43,597,291) and diagnosed with specific delays in development attributable to receipt of 25 μg organic mercury (Hg) or 37.5 μg organic Hg from Thimerosal-containing hepatitis B vaccines administered within the first 6 months of life.
The long-term consequences of such a large number of Americans being diagnosed with specific delays in development from the administration of T-HBVs are very serious. For example, investigators reported that individuals diagnosed with learning disabilities are more prone than the rest of the population to chronic health problems, including epilepsy, dementia, hepatitis, peptic ulcer, dysphagia, and problems related to sensory impairment, as well as more likely to suffer more from age-related diseases such as stroke, cardiovascular disease, and malignancy [21]. A recent study estimated that the simple incremental cost of a learning disability from birth to retirement is $1.982 million/person with a learning disability [22]. It is important to consider that many individuals diagnosed with learning disabilities have a wide range of severities, from mild to profound, but the lifetime incremental cost of a learning disability estimate utilized in the present study is compatible with those reported for other developmental disabilities, with a spectrum of severity such as autism spectrum disorder [23] or attention-deficit/hyperactivity disorder [24]. Therefore, utilizing the range of estimated attributable number of children diagnosed with specific delays in development from receipt of 25 μg organic Hg from T-HBVs or 37.5 μg organic Hg from T-HBVs administered within the first 6 months of life, the lifetime cost estimates to the United States would range from $952,943,618,000 (50% estimated exposure) to $1,810,592,676,000 (95% estimated exposure). It is also important to note that when considering our cost estimates, the present study did not consider other potential confounding factors that may have impacted our calculations.
Many recent studies support the biologically plausible role of organic Hg exposure from Thimerosal-containing vaccines in the pathogenesis of specific delays in development [25]. For example, administration of Thimerosal mimicking the US early childhood vaccination schedule of the 1990s to infant monkeys, resulted in significant and persistent levels of Hg in the brain [26]. Ethyl-Hg is able to enter into the brain and remain as ethyl-Hg, but it can also become and persist as methyl-Hg or inorganic Hg [27,28]. Once in the brain, Hg has many adverse effects [29]. In addition, studies have revealed that administration of Thimerosal mimicking the US early childhood vaccination schedule of the 1990s yielded significant pathology or clinical symptoms in mice [30], rats [31–33], hamsters [34], and monkeys [35] that are consistent with those observed in children diagnosed with specific delays in development.
In addition, one of the main effects of Hg in the brain is axonal degeneration, particularly of large caliber axons which tend to be long-range axons interconnecting distant parts of the brain [29]. This loss of long-range axons can result in a reduction of long-range connectivity. Long-range connectivity is particularly important in many processes in the brain, such as reading, hearing, coordination, and speech/language [36–41]. Research shows that abnormalities in the long-range neural tracts associated with these processes can result in specific delays in development. For example, investigators showed that the growth pattern of long-range connections in the brain predicts how a child’s reading skills will develop [40]. Those investigators stated that literacy requires the integration of activity in brain areas involved in vision, hearing, and language and, because these areas are distributed throughout the brain, it requires more speed-efficient long-range neural networks to bring about efficient communication overall between these regions. Investigators have also found lack of axonal integrity in the long-range axons of the arcuate fasciculus in children with delayed speech development [41]. In addition to Hg causing axonal degeneration, particularly of these critically important long-range axons that would require axon guidance, studies have shown that Hg also inhibits axon guidance [42]. Finally, studies revealed Hg (and specifically Thimerosal) disrupts neuronal cell maturation [42,43], which is another issue noted in children with reading and speech/language delay [41].
As discussed previously, the results observed in the present study are consistent with and extend previous studies conducted in the VAERS [7,8], VSD [9,10], and NHANES [11] databases, showing a significant association between organic Hg exposure from Thimerosal-containing childhood vaccines and specific delays in development.
In contrast, the results of the present study differ from several other studies that failed to find a consistent significant relationship between specific delays in development and organic Hg exposure from Thimerosal-containing childhood vaccines [44,45]. A recent review has critically examined many of these studies, and found that their results are uninterpretable [46].
For example, some of these studies examined cohorts with significantly different childhood vaccination schedules and with different diagnostic criteria for outcomes than those used in the United States. As another example, these other studies employed different epidemiological methods, especially with respect to the issue of a sufficient follow-up period for individuals in the cohorts examined. The method used to measure how a follow-up period is determined for individuals is a critical issue in all studies examining the relationship between exposures and the subsequent risk of the diagnosis of specific delays in development. This is the case because the risk of an individual being diagnosed with specific delays in development is not uniform throughout his/her lifetime. Therefore, any follow-up method that fails to appropriately consider the lag-time between birth and the individual’s age of an initial diagnosis for specific delays in development, will likely not be able to observe the true relationship between exposure to Hg through vaccination and the subsequent risk of a specific delays in development diagnosis.
5. Strengths/limitations
A strength of the present study was its examination of a cohort of children from the VSD database. The VSD database observations were made based upon retrospective assessment of prospectively collected medical records of patients enrolled in various HMOs. All members of the cohort examined had to be enrolled from birth and were required to be continuously enrolled for a sufficient time to ensure that there was a very small chance that, during additional follow up, any additional members of the cohort would be medically diagnosed with specific delays in development. As a result, any factors associated with enrollment (i.e., adjustment for potential independent variables were not necessary because enrollment was from birth) or healthcare-seeking behavior (i.e., adjustment for potential access/availability of healthcare was continuous among all members of the cohort) were minimized. In addition, members of the cohort diagnosed with specific delays in development were specifically evaluated to ensure that only those cases diagnosed with specific delays in development following vaccine administration were considered in the present analyses.
Another strength of the present study was that it mathematically ensured that all the cohorts examined in the VSD had adequate lengths of follow-up time. Specifically, the outcome files (inpatient and outpatient diagnoses) were examined to determine the age when children continuously enrolled in the VSD from birth were first diagnosed with specific delays in development. The observed mean age of initial diagnosis was 2.63 years of age, with a standard deviation of initial diagnosis age of 1.59 years. Then, using this information, every member of the cohort assembled for our study was required to have been continuously enrolled from birth for at least 5.81 years (mean age of initial diagnosis + 2 × standard deviation of initial diagnosis). Given the available review period (1991 through 2000) in the VSD, all children in the cohorts examined were born between 1991 and 1994. Based on the data for age of initial diagnosis for the specific delays in development studied, this was a sufficient period to ensure that, with further follow-up, very few children would receive a specific delays in development diagnosis presuming a normal distribution of data, and there is mathematically <2.5% chance of these individuals being diagnosed with specific delays in development with additional follow-up time beyond 5.81 years.
Another strength of the present study was that the VSD data were collected independently of the study design. The VSD data records analyzed were collected as part of the routine healthcare individuals received through their participation with their respective Kaiser health plans, and as such, the healthcare providers were not able to anticipate a potential association between vaccine exposures and health outcomes.
However, the results of the present study may have a number of potential limitations. It is possible that the results observed may have occurred from unknown biases or confounders present in the datasets examined. This seems unlikely because other control outcomes (i.e., outcomes that are not biologically plausibly linked to postnatal organic Hg exposure from Thimerosal-containing vaccines) were previously extensively examined in the VSD database, and no similar patterns of significant associations were observed for those outcomes.
Another potential limitation of the present study is that the results observed for specific delays in development may be the result of statistical chance. However, such a possibility would be unlikely given the limited number of statistical tests performed, the highly significant results observed, and the consistency in the direction and magnitude of the results observed.
Still, other potential limitations of the present study include the possibilities that some of the individuals in the cohorts examined in the VSD database may have had more subtle neurological dysfunction that was not brought to the attention of their healthcare providers; healthcare providers may have misdiagnosed some individuals; or some vaccine exposures may not have been appropriately classified. While these limitations, possibly present in the data examined in the current study, should not have significantly impacted the results observed, it is unclear how differential application would have occurred to affect the study cohorts examined, based upon the Thimerosal doses that the individuals received. Moreover, misclassification occurring in the data examined would tend to bias any results observed toward the null hypothesis, since such effects would result in individuals being placed in the wrong exposure and/or outcome categories examined, and this would result in decreased statistical power to determine true potential exposure–outcome relationships.
In addition, another potential limitation of the present study is that exposure to other sources of Hg were not evaluated. The individuals examined in the present study very likely incurred other organic Hg exposure from other Thimerosal-containing childhood vaccines, breastfeeding, formula feeding, and, to a lesser extent, dental amalgams, fish, or other environmental sources. While these other sources of Hg may play a significant involvement in the pathogenesis specific delays in development, these Hg exposures, not accounted for in this study, would actually tend to bias the results observed toward the null hypothesis because they potentially would confound the specific exposure classifications of Hg examined. For example, individuals classified as having lower organic Hg exposure from Thimerosal-containing vaccines may have actually received high doses of Hg from other sources, and individuals having higher organic Hg exposure from Thimerosal-containing vaccines may have actually received low doses of Hg from other sources, with the net result tending to minimize the magnitude of the associations observed.
Finally, the current study suffers from the potential limitation that analyses were not conducted to further explore the precise timing and cumulative doses of organic-Hg from all Thimerosal-containing childhood vaccines associated with maximum adverse consequences. In future studies, it would be worthwhile to explore these precise timing and cumulative-dose phenomena. In addition, evaluating other neurodevelopmental outcomes, as well as other covariates such as race, birth weight, gestational age, socioeconomic status, environmental conditions, as well as any complications during pregnancy, etc., that may affect the magnitude of the adverse effects found, would be valuable.
6. Conclusion
The present study provides compelling new evidence to confirm and extend previous epidemiological studies finding a significant relationship between organic Hg exposure from Thimerosal-containing childhood vaccines and the subsequent increased risk of a diagnosis for specific delays in development. The present study employed a longitudinal cohort study design that allowed for the determination of the attributable risk of exposure to organic Hg from T-HBVs administered within the first 6 months of life. It was estimated that during the approximately one decade when T-HBVs were routinely recommended to all US children, 0.5–1 million infants were diagnosed with specific delays in development as a consequence of hepatitis B vaccination-related Hg exposure. Furthermore, in economic terms, the lifetime societal cost of such individuals to the United States may exceed $1 trillion. As a consequence, while routine childhood vaccination is an important public health tool to reduce the morbidity and mortality associated with infectious diseases, the results of the present study raise fundamental and potentially alarming questions about the adverse health and economic impacts of recommendations to routinely administer Thimerosal-containing childhood vaccines to infants worldwide.
Conflicts of interest
All of the investigators on the present study have been involved in vaccine/biologic litigation.
Acknowledgments
This study was financially supported by the Dwoskin Family Foundation and the Selz Foundation.
References
Cite this article
TY - JOUR AU - David A. Geier AU - Janet K. Kern AU - Brian S. Hooker AU - Paul G. King AU - Lisa K. Sykes AU - Mark R. Geier PY - 2015 DA - 2015/07/09 TI - A longitudinal cohort study of the relationship between Thimerosal-containing hepatitis B vaccination and specific delays in development in the United States: Assessment of attributable risk and lifetime care costs JO - Journal of Epidemiology and Global Health SP - 105 EP - 118 VL - 6 IS - 2 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2015.06.002 DO - 10.1016/j.jegh.2015.06.002 ID - Geier2015 ER -
