Peritoneal dialysis related peritonitis due to Mycobacterium spp.: A case report and review of literature
- DOI
- 10.1016/j.jegh.2016.06.005How to use a DOI?
- Keywords
- Chronic kidney disease; Diagnosis; Tuberculous peritonitis
- Abstract
Mycobacterium tuberculous peritonitis is a less common cause of peritoneal dialysis related infection in developed countries. As both CAPD and APD are being used as renal replacement therapy in developing countries of South Asia, Mycobacterium tuberculous peritonitis are being reported. Any culture negative peritonitis should be investigated for this entity. In this manuscript, we report an index case and our experience with literature review of Mycobacterium tuberculous peritonitis. The diagnostic techniques, management and outcome are described.
- Copyright
- © 2016 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Case report
A 68-year-old male with diabetic nephropathy, hypertension, and Stage V chronic kidney disease, initiated on continuous ambulatory peritoneal dialysis (CAPD) in October 2012, presented 2 years later with cloudy fluid, effluent white blood cell count 680 cells/mm3 composed of 98% polymorphonuclear leukocytes and 2% lymphocytes. Peritoneal dialysis (PD) effluent gram stain, routine culture, acid-fast bacilli smear were negative. There was no exit site or tunnel infection. Chest X-ray was unremarkable. He was treated with IP ceftazidime and cefazolin for 8 days; Intra Peritoneal (IP) amikacin was added in place of cefazolin for 2 days along with IV metronidazole and oral fluconazole 150 mg on alternate days. On Day 9, in view of nonresolving peritonitis, the PD catheter was removed and catheter tip gram stain, culture, and acid-fast bacilli smear were negative. A peritoneal biopsy during catheter removal showed no granuloma and tuberculosis (TB) polymerase chain reaction (PCR) was negative. He was switched over to hemodialysis and succumbed to cardiac arrest the following day. Effluent fluid mycobacterial culture grew Mycobacterium tuberculosis on Lowenstein Jensen medium 5 weeks later (Fig. 1).
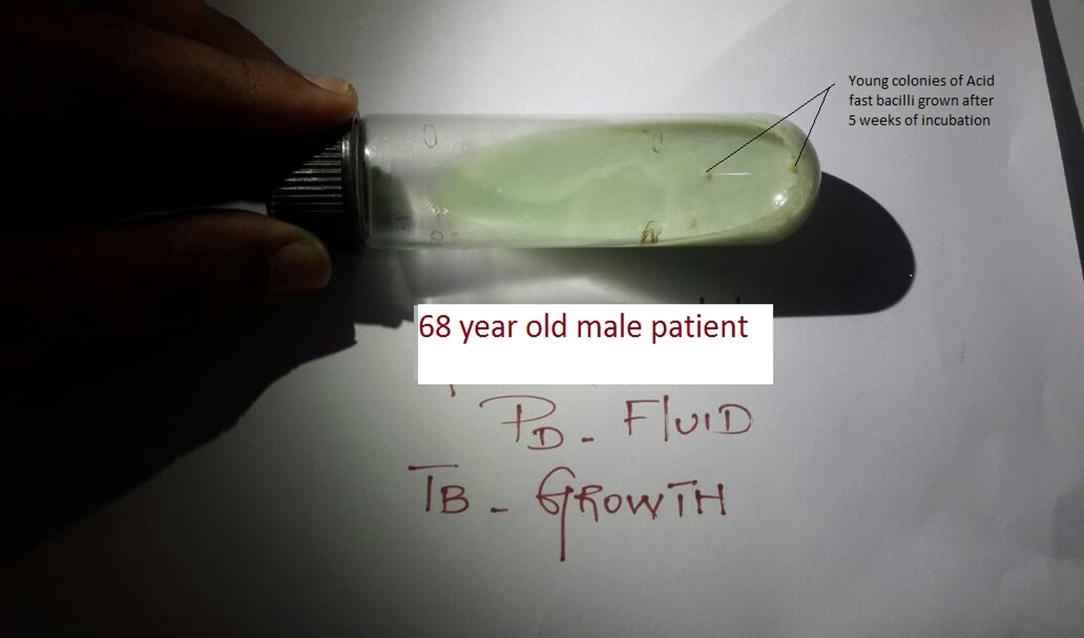
Mycobacterium tuberculosis grown on Lowenstein Jensen slant from peritoneal dialysis (PD) fluid of a 68-year-old patient. TB = tuberculosis.
2. Introduction
One of the infective complications of CAPD that causes failure of this modality requiring switch over to hemodialysis is peritonitis. This leads to technique failure, peritoneal membrane failure, hospitalization and increased mortality. Other causes for cloudy effluent include chemical peritonitis, eosinophilia of the effluent, malignancy, chylous effluent, and specimen from a dry abdomen [1]. Culture negative peritonitis may be another cause of cloudy fluid. Hence, the International Society for Peritoneal Dialysis (ISPD) guidelines indicate that culture negative peritonitis should not exceed 20% of episodes [1]. Culture negative peritonitis could also be due to prior use of antibiotics, low volume of effluent fluid, use of inappropriate media for culture, and rarely, the presence of unculturable organisms. Studies have shown that automated culture improves yield compared with conventional culture [2].
3. Peritonitis due to mycobacteria
Although an infrequent cause of peritonitis (<3%), Mycobacterial peritonitis is not uncommon in Southeast Asian countries. Special attention is required to diagnose Mycobacterial peritonitis which has been shown to have a higher incidence in developing countries in Asia and Africa [3]. The initial inflammatory response to peritonitis due to bacterial, mycobacterial, and fungal etiology is predominantly polymorphonuclear leukocytosis, thus making it difficult to diagnose. Peritonitis due to Mycobacteria may be due to M. tuberculosis or nontubercular Mycobacteria. Thus, routine testing for new infections in culture negative cases or for coinfection is vital. This may also lead to single agent therapy without full recovery [4].
4. Our experience
In 2014, we analyzed 92 bags with suspected peritonitis. Of these, 26 had bacterial growth. Tuberculous peritonitis was suspected in 15 patients. Of these, five patients (26%) were smear and culture positive (Table 1). Our rate of tuberculous peritonitis in 2014 was 6.52%. TB PCR (Gene Xpert, Cepheid, Sunnyvale, USA) was carried out for eight patients. Of these, four were positive. Of the four patients with positive PCR, smear and culture also was positive in two patients. Results of TB PCR correlated with culture in all but one case where the patient died due to a delay in diagnosis. This sample was also smear negative but culture positive.
| SL. No. | Patient demographics | Diagnostic modality | Day of presentation with peritonitis | Treatment outcome | Catheter removal done | Remarks |
|---|---|---|---|---|---|---|
| 1 | 65 y/female | Smear+ | 2 mo | 5 mo of ATT then patient lost to follow-up | Yes | Patient on PD for very small duration |
| 2 | 60 y/male | Smear+ | Recurrent peritonitis since the past 2 y with CoNS. On repeated courses of antibiotics | Expired after 12 wk on therapy | Removed and reinserted. | Recurrent peritonitis with CoNS |
| 3 | 28 y/female | Smear+ | Recent catheter insertion (2 mo prior). 1 episode of bacterial peritonitis 1 mo prior | Ongoing treatment | No | Patient doing well |
| 4 | 57 y/male | TB PCR positive | TB PCR of fluid +ve on several occasions over 4 years No growth on culture |
Patient expired on third episode of tuberculous peritonitis | No | Possibility of drug resistant TB in 2010 and 2011 but no culture and no gene Xpert then. Hence unconfirmed |
| 5 | 64 y/female | TB PCR positive | Clinically suggestive of TB | Expired 5 mo later | No | Presented with pain and tenderness. No clinical relevance of the diagnosis with TB |
| 6 | 68 y/male | Culture positive | Diagnosis made posthumously from PD fluid 5 wk after patient expired | No treatment since diagnosis was posthumous | Yes | Membrane biopsy culture negative |
ATT = Anti-tubercular therapy; CoNS = Coagulase Negative Staphylococcus spp; PD = peritoneal dialysis; SL = Serial Number; TB PCR = tuberculosis polymerase chain reaction.
Review of cases diagnosed with Tuberculous peritonitis in 2014.
We performed a retrospective analysis on the old records that we held between 2000 and 2011 and found a total of 34 patients (20 male and 14 female) who had been diagnosed with tuberculous peritonitis. The average time for development of peritonitis was about 10 months. Thirty-eight percent (13) of patients were diabetic, 26% (9) were hypertensive, 23.5% (8) had cardiac disease, 6% (2) had a cardiovascular accident (CVA), and others had no comorbidities. Diagnostic tools such as PCR were used along with conventional culture techniques in all cases; molecular diagnostic techniques yielded positive results in 82.3% of the cases whereas only 20.5% cases were detected with smear and culture for acid fast bacilli. Only two patients had catheter removal due to tubercular peritonitis and no mortality was recorded [4].
Although PCR has shown us promising results, its application in routine diagnostics faces problems such as contamination and complicated procedure for sample preparation.
Amaro et al. [5] found that due to the complex cell wall structure of M. tuberculosis, the combined bead beating and enzymatic extraction method was the most efficient and easy method of mycobacterial DNA extraction.
It has been our experience that patients present with a coinfection of tuberculous peritonitis along with bacterial peritonitis or fungal peritonitis [4]. Hence, the risk factors for developing superimposed TB peritonitis include malnutrition, diabetes, partially treated TB, scars on chest X-ray, and patients who are immunosuppressed. M. tuberculous peritonitis may be diagnosed from culturing PD fluid or culture of peritoneal membrane when biopsied. Peritoneal biopsy culture while removing PD catheter for peritonitis or while implanting a new catheter may show a caseating granuloma or a fungus, and hence may be useful in the diagnosis of TB or fungal peritonitis as in our experience [6] (Fig. 2). In the case discussed above, the peritoneal biopsy showed no granuloma which may be a sampling bias. However, the PD fluid grew TB bacilli after 5 weeks of incubation. Sampling error of the peritoneal biopsy may lead to false negatives. Early diagnosis and therapy improve the outcome, minimizing membrane damage and ultrafiltration failure. This also improves nutritional status, thereby reducing morbidity and mortality. TB peritonitis may be also due to nontubercular Mycobacterium as is described in ISPD guidelines [1].
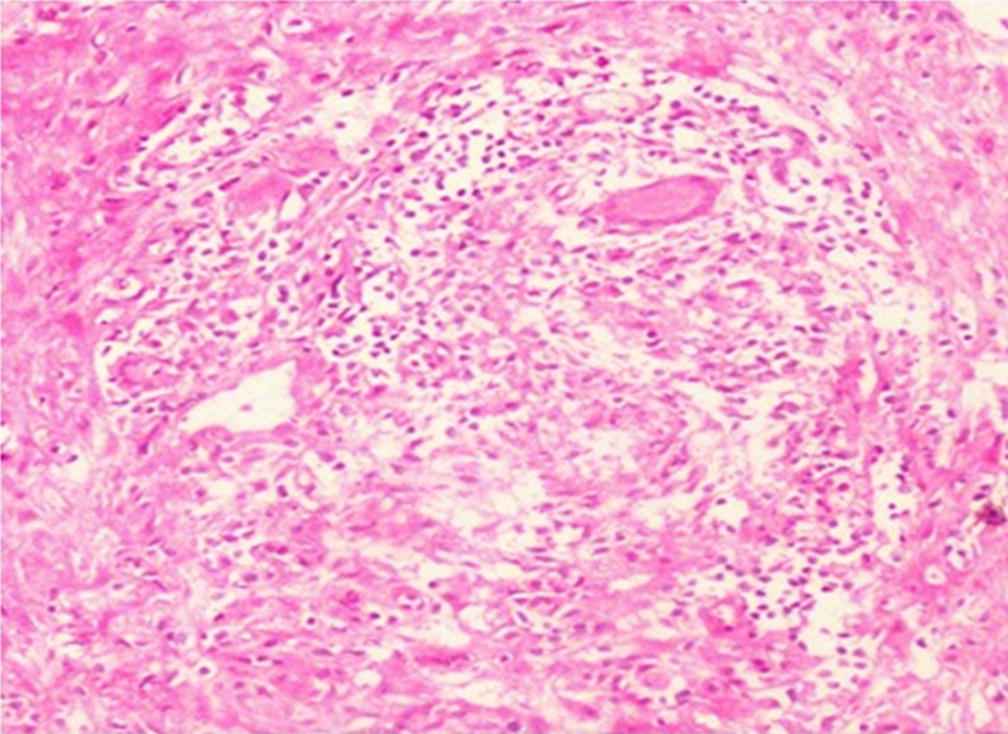
Hematoxylin and eosin 100× showing granulomatous inflammation with ill-defined epithelioid cell granuloma and Langhans’ cell multinucleated giant cell.
5. Review of literature
Mycobacterium spp. remains a significant cause of morbidity and mortality worldwide, with over 3 million deaths per year. Large pools of latently infected cases, difficult to diagnose, and long duration of treatment represent challenges in controlling this organism.
End stage renal disease patients are at an increased risk of developing TB due to their deficit in cell-mediated immunity. Furthermore, dialysis effluent which is bioincompatible with increased glucose concentration with nonphysiological pH, and osmolality can impair phagocytic function and lymphocytic activity of the peritoneum due to organisms even with a smaller inoculums size [7].
Talwani and Horvath [8] studied Mycobacterial peritonitis literature from 1976 to 1999 and identified 52 cases associated with CAPD; 13% of these were from the USA and 54% were diagnosed in the Asian subcontinent. According to their analysis, TB was considered a statistically significant predictor for death in these patients. Further, delay in treatment by 4 weeks caused a significant difference in the outcome of the patients, including mortality. Duration of treatment varied between 6 months and 12 months [8]. In our experience, two diabetic patients on CAPD who had treatment for <12 months duration had recurrence of tuberculous peritonitis. This highlights the importance of treating tuberculous peritonitis for at least 12 months.
Akpolat [9], noted that most of the cases of tuberculous peritonitis had been reported from Hong Kong, closely followed by Turkey and India. The authors concluded that early diagnosis and timely initiation of antitubercular drugs was the key to effective management of tuberculous peritonitis [9].
Abraham et al. [4] conducted a prospective study of 155 patients who were initiated into CAPD until 2001. In their cohort, four patients (2 men and 2 women) developed tuberculous peritonitis. These patients had been on PD for a duration of 2–84 months. The authors concluded that although TB peritonitis constituted a mere 1–2% of all peritonitis episodes, in culture negative peritonitis, a high index of suspicion of TB is required. The various methodologies for diagnosis include Ziehl–Neelsen stained smear, culture, and PCR [4]. The yield by smear and culture is very low and hence a more sensitive methodology may be needed to arrive at the diagnosis.
Another interesting paper by Vadivel et al. [7] compared the incidence of TB among immigrants and native Americans and Europeans. They found that the incidence of tuberculous peritonitis in immigrants from endemic areas like the Indian subcontinent increased to 13% as compared with 1–6% in native people. They concluded that all efforts should be made to rule out TB peritonitis in patients with prolonged failure to thrive or relapsing peritonitis with negative bacterial cultures, and in febrile patients with nonresolving peritonitis.
A working group from China made a retrospective study of patient files spanning 10 years, i.e., 1996 to 2006. The diagnosis of TB was made with comparison of smear, culture, and histopathological findings. They concluded that since tuberculous peritonitis presents with nonspecific and protean manifestations, the differential diagnosis has to be considered in the context of culture negative peritonitis or culture positive peritonitis not responding to appropriate antibiotics [10].
6. Challenges for diagnosis
Diagnosis of tuberculous peritonitis in PD has always been an Achilles heel. Diagnosis is based on microscopy which has 50% sensitivity over culture [3]. Most countries do not even have an infrastructure to diagnose TB. A need for a simple, rapid, robust, and dynamic test for detecting resistance is essential. Although powerful molecular diagnostic platforms are emerging, they are fraught with problems. Delays in sample transport, cost of procuring and maintaining equipment, and trained personnel for handling the equipment are just a few of the issues involved in diagnosis of TB [3].
The various tests available for diagnosis include microscopy (Ziehl–Neelsen staining and auramine-rhodamine staining), culture on solid base media like Lowenstein Jenson slopes, liquid culture on Middlebrook broth, automated culture on Mycobacteria growth indicator tube (MGIT) etc., PCR for IS6110 gene or manual nucleic acid amplification technique, Line probe assay (Hain Lifescience, Germany) for TB by Hain, real time PCR on various platforms using single nucleotide polymorphism, gene Xpert MTB (Mycobacterium tuberculosis)/RIF (Rifampicin Resistance) assay, sequencing, molecular beacons assay, loop-mediated isothermal amplification, tuberculin skin test/Mantoux test, among others [11].
Ziehl–Neelsen stained smears and culture on solid media have been in use for a while. However, these techniques lack sensitivity and cannot detect resistance. Studies have now found that liquid media like Middlebrook broth are better than solid media like Lowenstein Jensen media, since they contribute significantly to improve patient management. Automated culture systems like MGIT (Bactec, Becton Dickinson, USA) have been developed, with the advantages of using liquid culture, thereby reducing the turnaround time along with drug sensitivity testing, which is made simple and accurate. The MGIT makes use of modified Middlebrook 7H9 broth along with sensors. Hence, the principle of fluorometry is safe and accurate and has been recommended by the World Health Organization [11].
However the landscape of TB diagnosis is changing world over thanks to the roll out of gene Xpert, genotype line probe assay, and other nucleic acid based technologies. The involvement of the public sector has been crucial in developing countries to improve diagnosis. Gene Xpert has changed the face of TB diagnosis (pulmonary and extrapulmonary) world over. It is based on the nucleic acid amplification technique. It additionally adds information about rifampicin resistance [12].
The line probe assay is DNA hybridization based and adds to information on first line drug resistance [13]. Diagnosis of latent TB can be made with the QuantiFERON gold assay (Qiagen, Germany) and through proteomic technology. However, the usefulness of these tests for diagnosis of active TB is questionable. Moreover, they have not been proven useful in extrapulmonary samples such as pleural fluid and PD fluid. Hence, diagnosis of smear negative extrapulmonary TB remains challenging [11].
7. Treatment and management
The ISPD guidelines are useful for guiding therapy for tuberculous peritonitis in CAPD. However, increased duration of therapy as suggested will help avoid recurrence. TB peritonitis remains a treatable condition with no real consensus on the removal of the PD catheter [13]. ISPD recommends reinsertion of the catheter within 6 weeks if removal was indicated [1].
Drugs used are isoniazid and rifampicin, which need to be given for 12–18 months, along with pyrazinamide and ofloxacin for the first 3 months. Oral pyridoxine (50–100 mg/d) should be given daily. Rifampicin, however, is found only in low levels in dialysis fluids due to high molecular weight, high protein binding capacity, and lipid solubility, and hence may need to be given via the intraperitoneal route [1,14].
Data regarding extrapulmonary multidrug resistant (MDR) TB is limited, with few cases being described in large case cohorts of MDR TB. The prevalence of primary MDR TB in India was about 2–3% and 5–15% in previously treated cases. These patients run a risk of treatment failure due to poor drug penetration at the site of infection, inaccessibility for surgical intervention, and lack of drug sensitivity testing data from most laboratories. The emergence and spread of MDR TB can be prevented by prompt diagnosis and effective therapy. Treatment for MDR-TB lasts for 2 years. Second line drugs include injectables like streptomycin, kanamycin, capreomycin and drugs like ofloxacin, moxifloxacin, levofloxacin, ethionamide, cycloserine, and Para-aminosalicylic Acid (PAS) [15].
Dialysis effluent after an adequate dwell time and appropriate length of therapy (usually 2 weeks) shows clearing of the organism which is better confirmed by nucleic acid based tests [4]. Adequate attention should be paid to the adjunctive role played by nutrition (energy requirements, protein requirements, and macronutrient and micronutrient deficiencies) in the success of treatment for TB. With the emergence of multidrug resistance and extensive drug resistance, treatment options become narrow. ISPD has not issued guidelines on the same.
8. Conclusion
Tuberculous peritonitis is an underdiagnosed condition due to nonavailability of diagnostic techniques, lack of standardization of the techniques available, and reduced index of suspicion. Hence, it is imperative that early diagnosis and appropriate therapy for not less than 12 months with a multidisciplinary team approach holds the key to better treatment and outcomes in these patients.
Conflict of interest
There are no conflict of interest.
References
Cite this article
TY - JOUR AU - Anusha Rohit AU - Georgi Abraham PY - 2016 DA - 2016/07/18 TI - Peritoneal dialysis related peritonitis due to Mycobacterium spp.: A case report and review of literature JO - Journal of Epidemiology and Global Health SP - 243 EP - 248 VL - 6 IS - 4 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2016.06.005 DO - 10.1016/j.jegh.2016.06.005 ID - Rohit2016 ER -
