Treatment of White Coat HYpertension in the Very Elderly Trial (HYVET 2) - Feasibility of a Randomized Controlled Trial (Study Protocol)
- DOI
- 10.2991/artres.k.191106.001How to use a DOI?
- Keywords
- White coat hypertension; treatment; feasibility study; very elderly
- Abstract
The results of HYpertension in the Very Elderly Trial (HYVET) were crucial in providing evidence of benefit of the treatment of hypertension in those 80 years or older. Following a subsequent sub study analysis of the HYVET data there is a suggestion that 50% of patients in the main study had White Coat Hypertension (WCH), defined as clinic BP readings >140/90 mmHg and ambulatory BP readings <135/85 mmHg. Currently, definitive evidence in support of treatment for such individuals is not available. HYVET 2 has been designed in order to assess the feasibility of conducting a randomized controlled trial which might determine whether the treatment of WCH in the very elderly is clinically beneficial. One hundred participants aged ≥75 years diagnosed with WCH will be recruited from General Practices (GPs) in UK. Randomization will be 1:1 to a treatment arm (indapamide and perindopril) and control arm (no treatment) and follow up will be for 52 weeks. HYVET 2 will report on feasibility outcomes including participant recruitment, adherence and withdrawal rates, willingness of GPs to recruit and randomize patients and the frequency of a composite of cardiovascular events. Simple descriptive statistics will be presented.
- Copyright
- © 2019 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Cardiovascular disease (mainly stroke and coronary artery disease) remains an important global cause of morbidity and mortality and hypertension is a major contributing risk factor, particularly in older people [1,2]. The overall prevalence of hypertension in all adults in England, UK is 28.4% and this compares with a prevalence of 66.5% in patients over 75 years of age with trends covering 2003–2015 showing little change [2]. With an ageing population and the greatest expansion in population expected in the over 65 age group, hypertension will become a more prominent treatable and preventable cause of premature death [3].
There has been controversy regarding the appropriateness of treatment of hypertension in older people based on data from epidemiological studies, which suggest that there is a higher risk of death in patients over 80 years of age with lower Blood Pressure (BP) [4–6]. This notion has been challenged by data from the Syst-Eur and the HYpertension in the Very Elderly Trial (HYVET) studies which showed that antihypertensive treatment in persons 60 and 80 years or older respectively, is beneficial [7,8]. More recently, the SPRINT trial has demonstrated that lowering the BP to a target systolic BP of <120 mmHg is beneficial in patients aged over 75 years [9]. As a result, guidelines recommend BP control to reduce cardiovascular risk, irrespective of the age of the patient [10–12].
Current NICE guidelines for the diagnosis and treatment of hypertension recommend the widespread use of Ambulatory Blood Pressure Monitoring (ABPM) and Home BPM (HBPM) in order to exclude the presence of White Coat Hypertension (WCH), as the benefits of treatment are yet unproven [10]. WCH is defined as persistently raised clinic blood pressure readings (≥140/90 mmHg) in individuals who have normal HBPM or ABPM readings (<135/85 mmHg) and may affect up to 50% of the population, according to some studies [13,14]. Interestingly, up to 42% of patients who have WCH go on to develop sustained hypertension [13]. Evidence for a role for the active treatment of WCH has been contentious as some research results do not support protection against cardiovascular events [15]. However, a study by Franklin et al. [16] suggests that men and diabetics with untreated WCH are at increased risk. Furthermore, the HYVET ambulatory blood pressure sub study analysis estimates that 50% of patients in the main study may have had WCH [17]. It is therefore as yet unknown whether the patients with WCH benefit from treatment.
1.1. Aim
HYpertension in the Very Elderly Trial 2 is a feasibility study, which serves as a prelude to a definitive randomized controlled trial, in order to determine whether the treatment of WCH in the very elderly is feasible and outweighs any adverse events.
1.2. Outcomes
HYpertension in the Very Elderly Trial 2 focuses on the following outcomes:
- •
Estimation of the proportion of eligible patients that can be recruited from initial screening.
- •
Exploration of different methods of identifying/recruiting patients.
- •
Determination of the willingness of General Practices (GPs) to recruit and randomize patients and the willingness of patients to be randomized.
- •
Recruitment rate.
- •
Adherence to the treatment protocol.
- •
Withdrawal from the trial.
- •
Expanding the opportunities for Patient and Public Involvement (PPI) in the research design and its subsequent conduct and dissemination.
- •
Ambulatory and home blood pressure monitoring and whether or not the patient achieves their target blood pressure.
- •
Composite of cardiovascular events and stroke.
2. MATERIALS AND METHODS
2.1. Design
This is a multi-center, open-label study assessing the feasibility of conducting a randomized controlled trial to treat WCH in the very elderly. It entails a 1:1 randomization of patients to a treatment arm with established antihypertensive drugs (Indapamide MR and Perindopril Erbumine) [18,19] and control arm (no treatment) which is the current standard of care (Figure 1). This design will gather preliminary information on the intervention and the feasibility of conducting a full-scale randomized controlled trial.
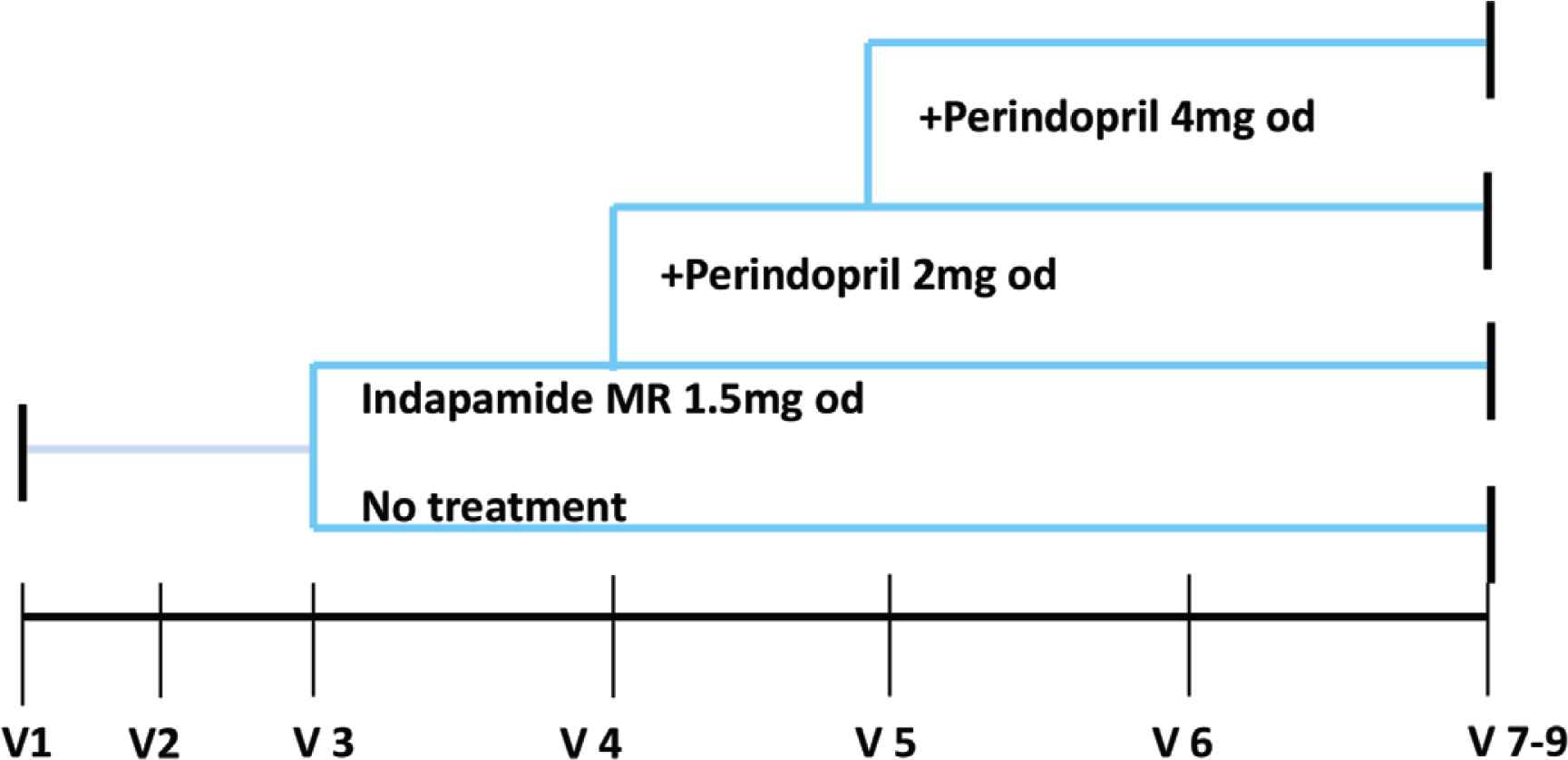
Treatment schedule.
A local PPI group has reviewed the patient facing documents for the trial and a lay member of the public sits on the Trial Steering Committee.
2.2. Trial Setting
HYpertension in the Very Elderly Trial 2 will be run from a number of GPs and will be supported by the Primary Care Clinical Research Network (CRN) in the Kent, Surrey and Sussex regions of the UK. Suitable GPs will be identified by the CRN and will then identify potentially suitable participants from their patient databases and will be responsible for the recruitment and follow up in accordance with the study protocol.
2.3. Population and Sample Size
One hundred participants will be randomized for this feasibility study after having been confirmed with WCH based on ABPM or HBPM. A sample size calculation has not been performed as HYVET 2 is a feasibility study and simple descriptive statistics will be presented, i.e. frequencies and percentages for binary and categorical variables and means and standard deviations for normally distributed variables and medians and interquartile ranges for skewed continuous variables. A more detailed statistical analysis plan will be agreed and signed off prior to final data analysis.
2.4. Inclusion Criteria
- •
Participants ≥75 years of age.
- •
Clinic sitting systolic BP ≥150 mmHg but <200 mmHg and a diastolic BP <110 mmHg.
- •
Established diagnosis of WCH - confirmed if the mean ambulatory day time systolic BP is <135 mmHg and diastolic BP is <85 mmHg (from at least 14 measurements) or from an average HBPM from BP readings twice a day for at least 5 days (ideally 7 days).
- •
Not prescribed drug therapy indicated for treatment of hypertension within the last 6 months.
- •
Capacity to consent.
- •
Ability to comply with the protocol and additional study visits and assessments.
2.5. Exclusion Criteria
- •
Contraindication to the use of Indapamide MR and Perindopril in accordance with the summary of product characteristics.
- •
Regular non-steroidal anti-inflammatory drug (NSAID) use. Regular use being defined by the local GPs with consideration to cardiovascular risk and blood pressure.
- •
Hypertensive emergency.
- •
Secondary hypertension.
- •
Postural hypotension (postural drop in systolic BP ≥20 mmHg or postural symptoms at screening).
- •
Any stroke or myocardial infarction in the previous 6 months.
- •
Heart failure requiring treatment with drugs having an antihypertensive effect.
- •
Previous documented evidence of gout.
- •
estimated Glomerular Filtration Rate (eGFR) <30 ml/min.
- •
Montreal cognitive assessment score (MoCA) <22.
- •
Life expectancy <1 year due to malignancy or chronic disease.
2.6. Recruitment
HYpertension in the Very Elderly Trial 2 will recruit initially from GPs based in the Kent, Surrey and Sussex CRN region of the UK. Each GPs will aim to recruit at least two patients over a 1-year period. This will allow accurate determination of the total number of GPs required for the definitive trial.
Eligible participants will be identified from the GPs electronic records, as well as those who have had ABPM and/or HBPM in the last 2 years and have not been prescribed drug therapy indicated for treatment of hypertension within the last 6 months. These patients will be invited to participate in the trial via a mail out from the GPs. The Patient Information Sheet will be sent out to potential participants along with a covering letter introducing the study. A follow-up phone call will be made to the potential participant to determine whether they are interested in participating in the trial or the participant may phone the local research contact if preferred. In addition, opportunistic recruitment will also be possible.
For those patients that are eligible but not randomized, anonymized information will be collected where appropriate to document why randomization did not occur on a HYVET 2 Screening Log. This is an important information for a feasibility trial.
2.7. Obtaining Informed Consent
Written informed consent forms will be personally signed and dated by the participant as well as the medical practitioner receiving consent. The person who provides the initial information should not be the same person who receives consent. If the participant is unable to sign the consent form, then an attempt to mark the document should be encouraged and a witness statement and signature provided by a carer (or equivalent) of the participant. If the participant loses capacity during the trial, the original consent endures the loss of capacity providing that the trial has not significantly altered. Once a participant has consented to the trial, they will be allocated a unique Participant Identification Number (PIN). These details will be logged on the Subject Identification Log. This document must remain at the investigator site at all times. Copies must not be sent to the Clinical Trials Unit.
2.8. Randomization and Group Allocation
After a medically qualified professional has checked and signed off that the participant is eligible for the study, they will be randomized to a trial arm. Randomization will be performed using the web-based system ‘Sealed Envelope’ (https://www.sealedenvelope.com/). The HYVET 2 Randomization Guide details how to log on to randomize a participant.
Simple randomization will be used, with a 1:1 allocation ratio. The allocation will be concealed. The randomization service providers generate a randomization list and upload it to the system. As each participant is entered onto the system by the site, the next randomization allocation is revealed. The next allocation in the sequence is concealed until irreversibly assigned to a participant.
Once randomization has been performed by the site, a randomization notification email detailing date of randomization, trial arm and PIN number will be sent to the randomizer and the trial management team.
Participants will be randomized to either arm of the study as described above.
2.9. Study Interventions
A summary of study interventions is highlighted in Table 1.
| Interventions | Visit 1 Screening | Visit 2 Baseline | Safety visit at 2 weeks (if on meds) | Visits 3 and 4 Follow-up | Visit 5 Follow-up | Visit 6 and 7 Follow-up | Visit 8 Follow-up | Visit 9 End of study follow up visit | Early termination visit |
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Week 4 ± 7 days | Weeks 12 and 20 ± 7 days | Week 28 ± 7 days | Week 36 and 44 ± 7 days | Week 52 ± 7 days | Week 60 ± 7 days | |||
| Informed consent | X | ||||||||
| Check eligibility | X | X | |||||||
| Randomization | X | ||||||||
| MoCA mental state examination | X | X | X | ||||||
| Frailty assessments ROCKWOOD and PRSIM-7 | X | X | X | ||||||
| BARTHELS Index | X | X | X | ||||||
| Demographics | X | ||||||||
| Past medical history | X | ||||||||
| Current medical conditions | X | X | X | X | X | X | X | ||
| Social history | X | ||||||||
| Concomitant medication | X | X | X | X | X | X | X | X | |
| Clinic BP1 | X | X | X | X | X | X | X | X | |
| 24 h ABPM2 | X | X | |||||||
| HBPM | X | X | X | X | X | X | X | ||
| Biochemistry bloods (U&E, uric acid, lipid profile)3 | X | X | X | X | X | ||||
| Urinalysis dipstick | X | X | X | X | |||||
| HbA1c | X | X | X | ||||||
| Hematology (FBC) | X | X | X | X | X | ||||
| 12-Lead ECG | X | X | X | ||||||
| Adverse events | X | X | X | X | X | X | X | X | |
| Fall assessment | X | X | X | X | X | X | |||
| Prescription/study intervention arm only) | X | X | X | X | |||||
| Prescription/on return to GPs care | X | X | |||||||
| Adherence checks (pill returns) | X | X | X | X | X | ||||
| Patient diary checks | X | X | X | X | X | X | X | ||
| Medication review (Intervention arm) | X | X | X | X | X | ||||
| Height | X | ||||||||
| Weight | X | X | X | ||||||
| Patient experience questionnaire | X | ||||||||
| Extra visit serum U&E check as required | |||||||||
Clinic BP sitting and standing.
ABPM to be conducted based on patient’s preference at visit 2 and 6 only.
Extra visits to be scheduled 2 weeks after the introduction or up titration of any study intervention.
Summary of study interventions
2.10. Clinic BP
Clinic BP measurement will be performed using an internationally validated automated oscillometric sphygmomanometer – e.g. Omron 705 cp-II.
- •
The participant should not have exercised, eaten or smoked for at least half an hour prior to taking blood pressure.
- •
The arm must be free of tight clothing and supported at heart level.
- •
The correct cuff size must be used, to avoid over or under estimating BP.
- •
BP will be measured in both arms at visit 1. Future BPs will be measured in arm with highest BP if there is 20/10 mmHg difference. The arm used must be documented.
- •
BP will be measured twice sitting and twice standing; (using an internationally validated automated device), after an initial rest of 5 min with 1 min between each reading.
- •
The participant will be asked not to talk during the procedure.
2.11. Home Blood Pressure Monitoring and Ambulatory Blood Pressure Monitoring
Participants with a mean sitting clinic systolic BP ≥150 mmHg but <200 mmHg and diastolic BP <110 mmHg from visits 1 and 2, will be offered a 24-h ABPM or HBPM. WCH is confirmed if the mean awake ambulatory systolic BP is <135 mmHg and mean awake ambulatory diastolic BP is <85mmHg (from at least 14 measurements) or from HBPM from BP readings twice a day for at least 5 days. If ABPM or HBPM identifies participants that have sustained hypertension (defined as average BP ≥135/85) they will be referred back to the GPs for standard clinical care and will excluded from the study.
Ambulatory Blood Pressure Monitoring will be performed by participants at screening visit 2 (Table 1). The participant will be asked to wear the ABPM for a period of 24 h at home, if tolerated. It will then be returned to the GPs practice on completion. For those who agree to be fitted with the ABPM a guidance leaflet will be issued detailing the management of the ABPM before, during and after the procedure.
Home blood pressure monitoring will be recorded for all participants in the week prior to follow-up visits (Table 1). The BP readings will be recorded twice a day for 5 days, morning and evening at 9 am and 6 pm ±2 h and documented in the HBPM chart provided. A guidance leaflet will be issued to all participants with instructions on how to record and document the HPBM.
The preferred option is that all participants undertake the ABPM; however, if participants do not wish to undertake an ABPM they will be asked to undertake only HBPM. About 24 h ABPM will be measured using a Diasys Integra II recorder (Novacor®, Cedex, France) or another internationally validated device as in the HYVET study [7]. HBPM will be measured by an internationally validated BP device.
2.12. Blood Tests
General practices will perform biochemical and hematological blood samples which will be analyzed using validated laboratory techniques and assays at local laboratories of the recruiting centers.
2.13. Electrocardiogram (ECG)
12-Lead ECGs will be performed using validated machines available at the recruiting centers.
2.14. Post Study Follow-up
Two years after the participant has stopped the treatment, follow-up information will be gathered from the GPs records for each participant, where available. This will include documenting whether the participant is alive or deceased and whether any cardiovascular outcomes have occurred. The participant is not contacted directly for this follow-up.
2.15. Withdrawal Criteria
Each participant has the right to withdraw at any time without giving reason but other reasons for withdrawal from the treatment might include postural hypotension and adverse events related to the drug treatment in the intervention arm. If the participant is withdrawn, then if at all possible an Early Termination Visit will be performed.
3. DATA ANALYSIS
Data will be exported from the MACRO by the data manager in Stata 14 format (or higher) and imported into Stata where descriptive analyses will be undertaken by the trial statistician.
As this is a feasibility trial, simple descriptive statistics will be presented i.e. frequencies and percentages for binary and categorical variables and means and standard deviations for normally distributed variables and medians and interquartile ranges for skewed continuous variables. A more detailed statistical analysis plan will be agreed and signed off prior to final data analysis.
Patient flow through the trial will be shown in a CONSORT diagram in accordance with the CONSORT extention for pilot and feasibility studies (Figure 2).
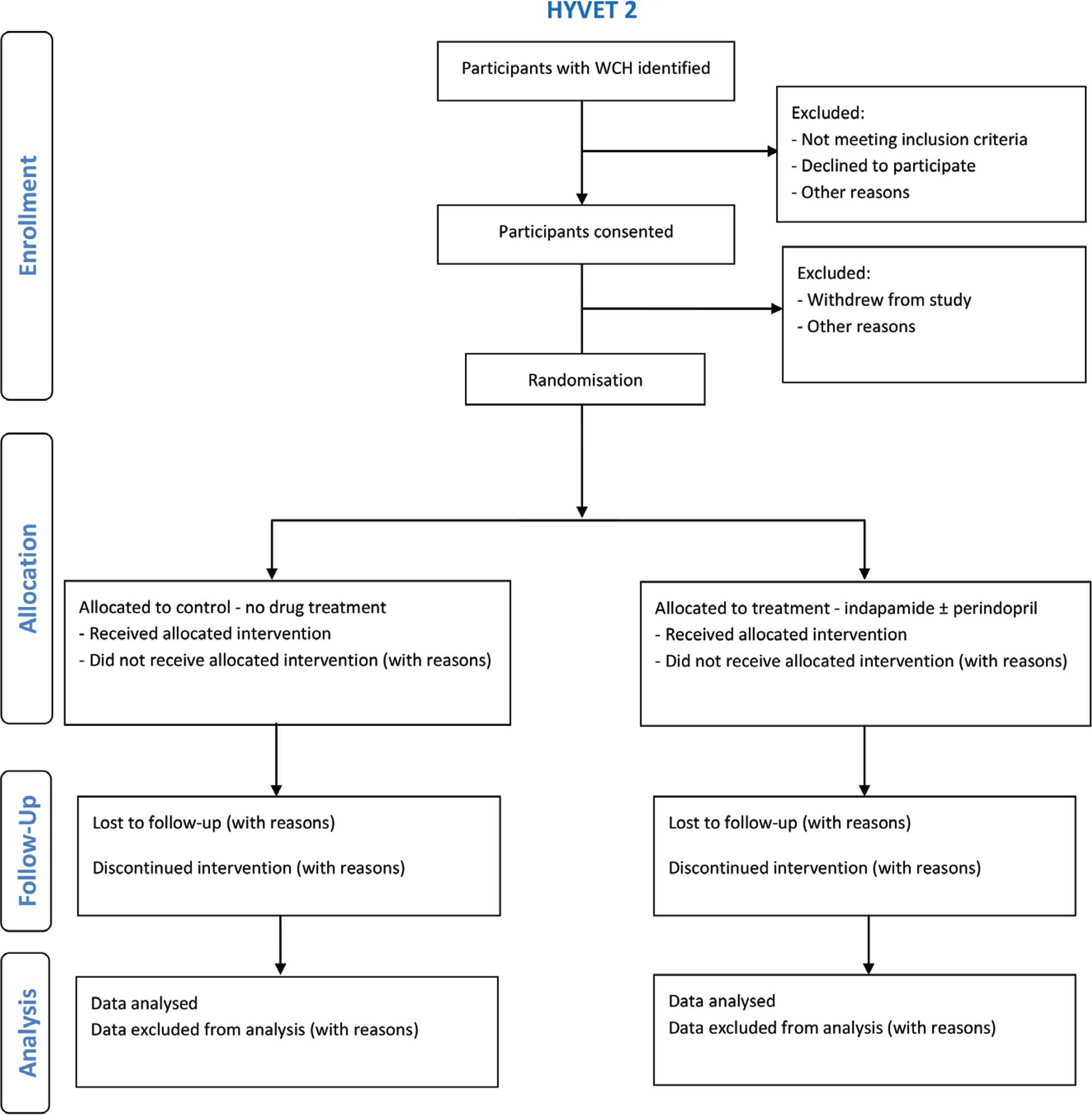
Participant flow through the HYVET 2 feasibility study.
Apart from the feasibility outcomes stated above no other primary outcome is specified and secondary outcome, subgroup and adjusted analyses are not applicable. Furthermore, neither interim analysis nor early stopping criteria are planned.
There will be a large degree of uncertainty around the proportion of Serious Adverse Reactions (SARs) as it is anticipated that these would be rare. To illustrate this, SARs attributable to the Investigational Medicinal Product (IMP) listed on the patient’s information leaflet are presented with likely frequencies [18,19]. With only 50 patients receiving IMP in this study, the 95% confidence interval around the event rate will be so wide that we would be most unlikely to detect an exaggerated event rate, this being the basis of a stopping rule.
4. LIMITATIONS OF STUDY
Currently WCH is a condition that is not treated; hence, a major issue will be to convince eligible participants to be part of the study. Older patients often already take multiple medication, so adding more medication might be challenging. Locating patients from GPs databases will also be an issue given the fact that WCH is a condition is not treated and often not documented as a diagnosis. To address these issues we have on our team general practitioners with expertise in primary care systems which will help identify eligible patients. The study is conducted with support from trained research nurses who will explain the benefits versus adverse effects to patients in detail. Primary care CRN are part of the study and are also helping to address the above issues.
Another limitation is that the pharmacological treatment (indapamide ± perindopril) chosen for this study is not strictly in line with the NICE guidelines for the treatment of hypertension. The reasoning behind this treatment regimen is that it was used in the HYVET trial and has been demonstrated to be effective for the treatment of hypertension in the very elderly. This approach also allows for comparison of treatment effects in HYVET versus HYVET 2. In an attempt mitigate this issue, research investigators have visited trial sites in order to explain the rationale and are readily available to discuss any queries.
5. ETHICS, DISSEMINATION AND IMPACT
Ethics approval has been acquired from Regional Ethics Committee Westminster, London (Reference No: 18/LO/0104) for the trial protocol, informed consent forms and other relevant documents e.g. advertisements and GPs information letters. It is registered on ISRCTN (ISRCT No: 13127656). Substantial amendments that require review by REC will not be implemented until the REC grants a favorable opinion for the trial. All correspondence with the REC will be retained in the Trial Master File/Investigator Site File.
Results of this study will be presented at conferences such as the British and Irish Hypertension Society and the European Society for Hypertension. We also intend to submit articles to peer reviewed journals. In addition, results will be disseminated to participants, patients and the general public through the PPI and relevant community groups.
CONFLICTS OF INTEREST
NB, CB and CR were part of the HYVET research team.
AUTHOR CONTRIBUTIONS
MO, KA, SB, NB, SJ, RQ, JP, CB and CR were responsible for study conceptualisation and funding acquisition. NP and CM are responsible for project administration, data curation and formal analysis. All authors contributed to preparation of the original draft of this manuscript.
FUNDING
Dunhill Medical Trust.
ACKNOWLEDGMENTS
We acknowledge the support provided by the CTU Brighton.
ETHICS
This trial has full research ethics approval.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Michael Okorie AU - Khalid Ali AU - Stephen Bremner AU - Nigel Beckett AU - Stephen Jackson AU - Richard Quirk AU - Colin McAlister AU - Nicky Perry AU - John Potter AU - Christopher Bulpitt AU - Chakravarthi Rajkumar PY - 2019 DA - 2019/11/18 TI - Treatment of White Coat HYpertension in the Very Elderly Trial (HYVET 2) - Feasibility of a Randomized Controlled Trial (Study Protocol) JO - Artery Research SP - 19 EP - 25 VL - 25 IS - 1-2 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.191106.001 DO - 10.2991/artres.k.191106.001 ID - Okorie2019 ER -
