Nigella sativa Extract and Thymoquinone Regulate Inflammatory Cytokine and TET-2 Expression in Endothelial Cells
- DOI
- 10.2991/artres.k.191114.002How to use a DOI?
- Keywords
- Nigella sativa; TET-2; endothelial dysfunction; cytokine expression
- Abstract
Numerous natural compounds including Nigella sativa (N. sativa) demonstrate anti-inflammatory and anti-diabetic antiangiogenic properties. Lipopolysaccharide (LPS) mediated inflammation is regarded as an important contributor to the inflammation that is associated with the development of arteriosclerosis. In this study, it was hypothesised that N. sativa Extract (NSE) and its main active component Thymoquinone (TQ) could potentially inhibit LPS mediated inflammatory cytokine secretion and monocyte recruitment factors and monocyte in Human Vascular Endothelial Cell (HECV) lines. In addition the Ten-Eleven Translocation (TET-2) an epigenetic regulator, increasingly regarded has having a major role in both the regulation of cytokine secretion and in the development of atherosclerosis through its ability to inhibit the inflammasome Nod-like Receptor Protein 3 (NLRP3) and Interleukin-1β (IL-1β) secretion was investigated. NSE significantly inhibited the production of both IL-6 and -8 and both NSE and TQ inhibited the gene expression of vascular endothelial growth factor and monocyte chemotactic protein-1 in HECV cells. NSE and TQ inhibited the gene expression of NLRP3 and IL-1β and significantly upregulated the gene expression of TET-2 in the presence of LPS. To conclude, NSE and TQ attenuated inflammatory and monocyte recruitment response and also demonstrate a potentially important role in regulating both NLRP3 and TET-2 expression.
- Copyright
- © 2019 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Many natural compounds in particular flavonoids and polyphenols have been studied for their potential antiinflammatory actions. Nigella sativa (N. sativa) commonly known as black cumin is a spice, demonstrating anti-inflammatory, antidiabetic, antimicrobial and anticancer properties [1–3]. The spice is from the botanical Ranunculacea family and is found in North Africa, Southern Europe, India and the Middle East and has been used as herbal medicine in the treatment of several diseases including asthma, chronic headaches and skin disorders. Several compounds are present in Nigella seeds including Thymoquinone (TQ), thought to be its main active compound, as well as nigellicine, thymol and carvacol [4,5].
Inflammation underpins a wide range of diseases including atherosclerosis [6]. Atherosclerosis a specific type of arteriosclerosis, is a chronic inflammatory disease characterized by endothelial dysfunction, lipid deposition, smooth muscle cell proliferation, cell apoptosis, and local and systemic inflammation. The balance between proatherogenic inflammatory and atheroprotective anti-inflammatory responses involves complex interactions among vascular components and immune cells [7,8].
The recruitment of peripheral blood monocytes to the endothelium through their interaction with endothelial cells is major component in the initiation and progression of cardiovascular disease [9]. The activation of the endothelium, recruitment of inflammatory monocytes, macrophage accumulation, that induces and macrophage generation of inflammatory mediators, which also activate the endothelium [10,11]. Through these stages, the positive feedback loop is formed, which generates and promotes expansion of the atherosclerotic process. Endothelial cells secrete key promotors of angiogenesis such as Interleukin 8 (IL-8) [12], Monocyte Chemotactic Protein (MCP)-1 [13,14] a chemokine which has been reported to play a direct role in Vascular Endothelial Growth Factor (VEGF) which is a key factor in promoting vascular inflammation and inflammatory associated angiogenesis [15]. Evidence indicates that endothelial inflammatory activation promotes hypertension development [16].
Recently the Ten-Eleven Translocation (TET-2) an epigenetic modifier (involved in DNA methylation) has gained considerable attention, due to its role in the pathogenesis of diseases related to atherosclerosis. and cardiovascular diseases [17,18]. Its role in atherosclerosis has been linked to its ability to regulate the action of the inflammasome particularly Nod-like Receptor Protein 3 (NLRP3) and secretion of its regulated cytokine IL-1β. NLRP3 pharmacological inhibition has been shown to reduce blood pressure and angiogenesis [19]. The ability of natural products to modulate TET-2 expression and activity is not a well-researched area and the data presented from this study would add to the scientific knowledge of this gene.
We have recently demonstrated that a naturally occurring glycosidic flavonoid, Vicenin-2 could regulate Lipopolysaccharide (LPS) reduced inflammatory cytokine secretion significantly increased TET-2 expression and NLRP3 expression in human macrophages [20]. Given the importance of endothelial cells in the regulation of arterial inflammation, we hypothesised that an extract of Nigella seeds and its major component TQ, could regulate inflammatory cytokine secretion and monocyte chemoattractant regulators in an immortalised human endothelial cell model. We further hypothesised that a methanolic extract of Nigella sativa seeds (Nigella sativa Extract; NSE) and TQ could both upregulate TET-2 and NLRP3 expression as a result of this modification. This study aimed to investigate the anti-inflammatory actions of NES and one of its constituent TQ in endothelial cells, was associated with the factors typically associated with endothelial inflammation and monocyte recruitment process. In addition we investigated if the extract and TQ could reduce expression of TET-2 and of NLRP3.
2. MATERIALS AND METHODS
2.1. Ethics
The Cardiff Metropolitan University Biomedical Sciences Ethics Panel granted the application for ethical approval (Project Reference Number: 9830).
2.2. Methanolic Extraction of N. sativa Seeds
About 40 g of N. sativa seeds (Bodrum, Turkey) were pulverised using a pestle and mortar and added to 200 mL of methanol and spun at 7500 for 15 min in 2 × 50 mL centrifuge tubes. The tubes were placed in a rotary mixer maintained at 25°C overnight. The tubes were centrifuged (6000 g) for 15 min at room temperature. The supernatant was collected, and the solvents evaporated in vacuo using a rotary evaporator and further subjected to N2 to obtain dry residue. The residue (extract) was reconstituted in Dimethyl Sulfoxide (DMSO) for increased solubilisation and termed NES. The extracts were stored at −20°C.
2.3. Tissue Culture and Reagents
The Human Endothelial Vascular Cell (HECV) line was purchased from the European Collection of Animal Cell Cultures (Salisbury, UK). HECV cells were grown in Dulbecco’s modified Eagle Medium (DMEM) (Gibco, Life Technologies, UK) (high glucose) supplemented with 2 mmol/L
2.4. Cell Viability Assay using Cell Titer Blue
A cell viability assay was conducted to establish the optimum concentrations for the HECV cells to work at. Cells were plated at 25,000 cells per well and cultured overnight until 80% confluence was achieved. The cells were then treated with either NSE or TQ at concentrations of 5, 2, 1, 0.5, 0.25, 0.125, 0.625 and 0.131 mg/mL, respectively. Control cells were treated with media only. The cells were then counted after both 6 h, 24 and 48 h incubation, 20 µL of Cell Titer Blue were added to each well and left for a further 1–4 h. Plates were read with a Tecan M200 (Tecan, Switzerland) infinite multi-detection reader using I-contro© software/excitation of 560 nm and emission of 590 nm set on optimal gain. Experiments were performed in triplicates to allow three sets of data to be obtained for statistical analysis.
2.5. Time Course
Human vascular endothelial cells were seeded at 106 cells per well in a 24-well plate. Cells were treated with either media (control), NSE, TQ, LPS (100 ng/mL), LPS with TQ and LPS with NSE respectively for 6 and 24 h time periods. After time periods were achieved, supernatants were collected, and mRNA was extracted using TRIzol® (Life Technologies, UK) for further analysis.
2.6. Determination of Interleukin-6 and -8 in Culture Supernatant using Enzyme-Linked Immunosorbent Assay
Enzyme-Linked Immunosorbent Assay (ELISA) assays for cytokines were undertaken as per manufacturer instructions (R&D Systems, UK). In brief, a 96-well ELISA plate was coated with diluted Capture Antibody and incubated overnight at room temperature. Each well was washed with Wash Buffer three times. Plates blocked by adding Block Buffer to each well and incubated at room temperature for a minimum of 1 h. The samples and standards in reagent diluent were added and incubated for 2 h at room temperature. Detection Antibody was added and diluted in reagent diluent, to each well. And then incubated for 2 h at room temperature. Streptavidin-HRP was added to each well, the plate was covered and then incubated for 20 min at room temperature. Substrate Solution TMB (3,3′,5,5′-tetramethylbenzidine) was added to each well and then incubated again for 20 min at room temperature. A stop solution (HCL) was then added to each well. The optical density of each well was then determined immediately, using a Tecan M200 infinite plate reader set to 450 nm.
2.7. RNA Extraction and Determination of Gene Expression using Quantitative Real Time Polymerase Chain Reaction
RNA was isolated from cell monolayer using TRIzol (Life Technologies, UK) according to manufacturer’s guidelines. The RNA purity of samples was established using Nanodrop ND 1000 through measurement of the ratio of their absorbance at 260 and 280 nm and samples with a purity of >1.8 was preferred to be used in the quantitative Real Time Polymerase Chain Reaction (RT-PCR). RNA was converted to cDNA using high capacity cDNA reverse transcription kit (Applied Bio systems, Warrington, UK). Gene expression was analysed using Applied Bio Systems Fast 7500 Real-Time PCR System (Applied Bio systems, UK). Pre-designed probes used for GAPDH (Hs99999905_m1), MCP-1 (Hs00174265_m1) VEGF (Hs009000055_m1) IL-1β (Hs01555410_m1) TET2 (Hs00325999_m1) and NLRP3 (Hs00918082_m1) were purchased from Thermo Fisher Scientific (UK). The comparative CT method was used to calculate relative gene expression CT values were normalized against the housekeeping gene with intreated samples being used as the experimental controls.
2.8. Statistical Analysis
Graphs of data were expressed as a mean of independent triplicates ± standard deviation. Data analysis was conducted using the Microsoft Excel and Graph Pad Prism© programs. The one-way analysis of variance (ANOVA) test was used to find significant difference amongst the means. A multiple comparison test was used as a follow-up to calculate the comparisons between the different concentration groups to find significant differences between them. Values of p < 0.05 were considered significant.
3. RESULTS
3.1. The Effect of NSE and TQ on Cell Viability in Human Vascular Endothelial Cells
The viability of the HECV was expressed as a percentage of untreated cells. This was analysed by the Cell Titer Blue® assay at a 6, 24 and 48 h and is outlined in Figures 1 and 2. HECV viability when treated with a range of extracted NSE concentrations (0.015–5 mg/mL) demonstrated that cell viability was decreased from 0.5 mg/mL and upward with viability decreasing with increasing concentrations. Viability at 6 and 24 h show a proliferation of cells when treated with 0.25 and 0.125 mg/mL respectively, with 0.25 mg/mL being significantly higher than 0.125 mg/mL at 48 h, 0.25 mg/mL showed at least 90% viability of HECV cells whereas 0.125 mg/mL did not. Hence, 0.25 mg/mL was selected as the concentration to be used in further experiments.
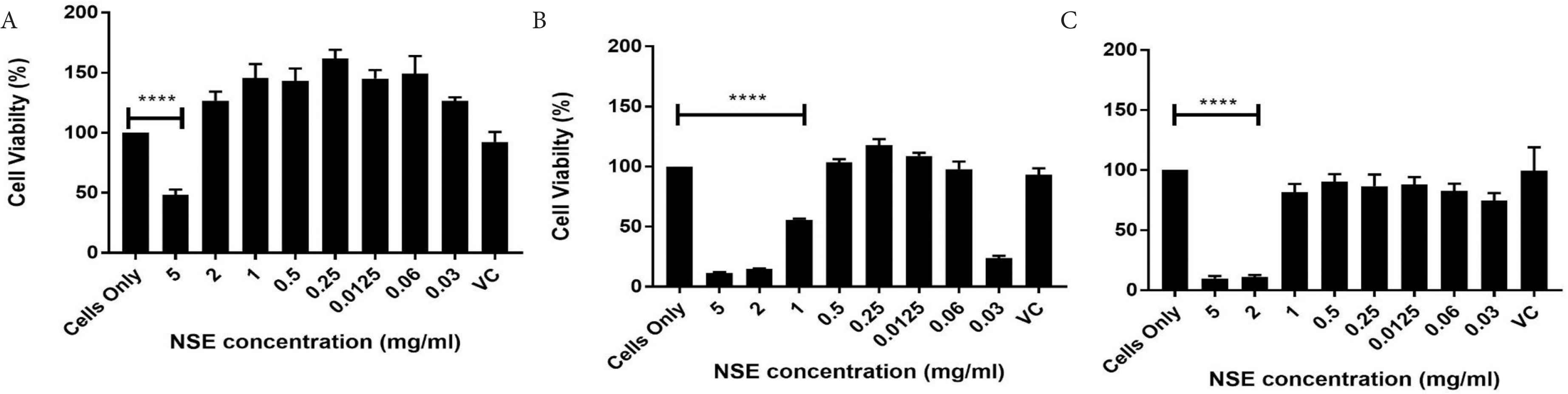
Effect of NSE on cell viability using Cell Titer Blue assay at (A) 6, (B) 24 and (C) 48 h. Data represents mean ± SD (n = 4) and data were analysed using one-way ANOVA with Tukey’s test for pairwise analysis, ****p < 0.001.
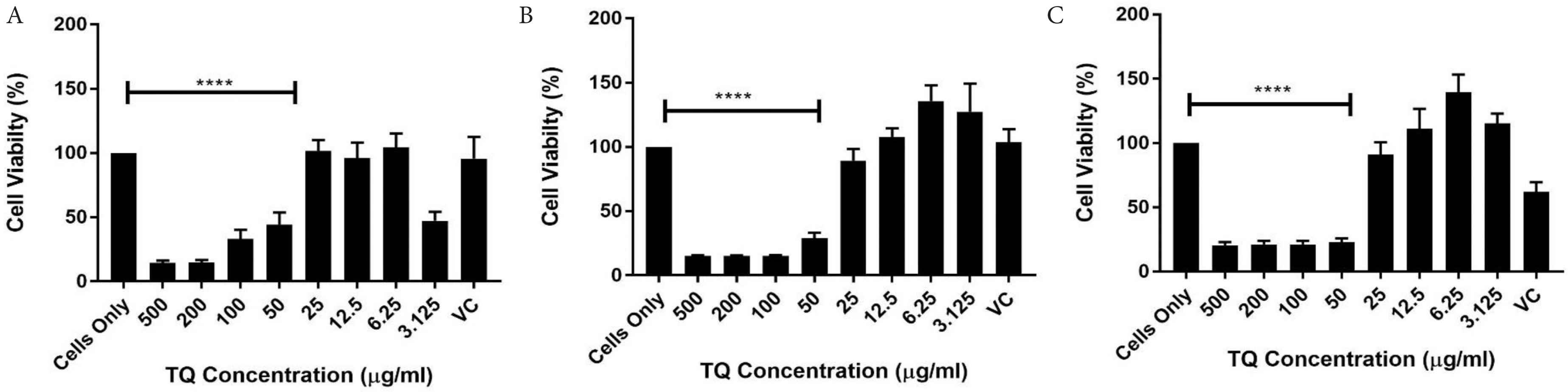
Effects of TQ on cell viability using Cell Titer Blue assay at (A) 6, (B) 24 and (C) 48 h. Data represents mean ± SD (n = 4) and data were analysed using one-way ANOVA with Tukey’s Test for pairwise analysis. TQ, thymoquinone, ****p < 0.001.
With TQ being reported to be the most potent compound in N. sativa, it is expected that less of it is required to induce an effect of cells, therefore much lower concentrations were used in the cell viability test, and concentrations of 500 µg/mL all the way down to 3.125 µg/mL were chosen for this experiment. As expected, the higher concentrations from 50 µg/mL induced a cytotoxic effect on the HECV cells at 6 and 24 h respectively, while 25 µg/mL also showed a less 90% viability at 48 h. About 3.125 µg/mL did not induce a positive effect at 6, 24 and 48 h. These results correlate with other studies who use similar concentrations of TQ. A recent study by Ismail et al. [5], used 46 µM of TQ in a hepatocellular carcinoma cell line, which relates to the 6.25 µg/mL/38.1 µM used in the future experiments in this study.
3.2. IL-6 and -8 Production in HECV Cells Treated with NSE and TQ
IL-6 and -8 are factors that are both produced by endothelial cells. IL-6 is a cytokine that can act as either a pro- or an anti-inflammatory cytokine [21]. IL-8 on the other hand is a cytokine that is purely a proinflammatory cytokine. This experiment examined both extracted NSE and its main active constituent TQ on HECV cells using ELISA. HECV cells were treated with either media only (control), NSE, TQ, LPS only, LPS with TQ and LPS with NSE for 24 h. As shown in Figure 3A, there was no significant difference between cells only and the cells treated with NSE and TQ alone. Stimulation with LPS showed a significant increase in IL-6 protein quantification, whereas co stimulation with LPS and NSE showed a significant reduction in IL-6 protein levels at 24 h. There was a reduction in IL-6 levels when the cells where treated with LPS and TQ; however, this was not found to be statistically significant.

Effect of NSE, TQ and/or LPS (100 ng/mL) on (A) IL-6 and (B) IL-8 protein expression. HECV cells were treated with media only, TQ, NSE only and/or LPS for 24 h. Cytokine production were quantified by ELISA. Data represents mean ± SD (n = 3) and data were analysed using one-way ANOVA with Tukey’s Test for pairwise analysis. TQ, thymoquinone; ELISA, enzyme-linked immunosorbent assay; HECV, human vascular endothelial cell, *p < 0.05.
The experiment was repeated with IL-8 quantification being the focus. As seen in Figure 3B, there was no significant difference between cells, only and the cells treated with either NSE or TQ with the IL-8 being at basal levels. There is a significant increase when the cells are stimulated with LPS and a significant decrease when the cells are treated with LPS and NSE. The decrease seen when treated with LPS and TQ; however, was not found to be statistically significant.
3.3. NSE and TQ Inhibits the Gene Expression of VEGF in HECV Cells
Endothelial cells such as HECV express factors such as VEGF and MCP-1 as regulators of inflammation and angiogenesis. VEGF is a growth factor that is strongly associated with both the angiogenesis and inflammatory response cascade. This experiment examined both the extracted NSE and its main active constituent TQ on HECV cells using RT-PCR analysis. HECV cells were treated with either media only (control), NSE, TQ, LPS only, LPS with TQ and LPS with NSE for 24 h. As shown in Figure 4, there was no significant difference between cells only and the cells treated with NSE and TQ alone. Stimulation with LPS showed a significant increase in VEGF expression, whereas co stimulation with LPS and either TQ or NSE showed a significant reduction in VEGF mRNA expression (p < 0.05) at 24 h.

Effect of NSE, TQ and/or LPS (100 ng/mL) on VEGF production at 24 h. HECV were treated with media only, TQ, NSE only and/or LPS. Gene expression at the mRNA of VEGF was determined by qRT-PCR. Data represents mean ± SD (n = 3) and data were analysed using one-way ANOVA with Tukey’s Test for pairwise analysis. LPS, lipopolysaccharide; TQ, thymoquinone; HECV, human vascular endothelial cell; qRT-PCR, quantitative real time polymerase chain reaction; VEGF, vascular endothelial growth factor, ***p < 0.01.
3.4. NSE and TQ Inhibits Gene Expression of MCP-1 in HECV Cells
Human vascular endothelial cells also express MCP-1 as a regulator of inflammation and endothelial monocyte activation. This experiment examined both the extracted NSE and its main active constituent TQ on HECV cells using RT-PCR analysis. HECV cells were treated with either media only (control), NSE, TQ, LPS only, LPS with TQ and LPS with NSE for 24 h. As shown in Figure 5, there was no significant difference between cells only and the cells treated with NSE and TQ alone. Stimulation with LPS showed a significant increase in MCP-1 expression, whereas co stimulation with LPS and either TQ or NSE showed a significant reduction in MCP-1 mRNA expression at 24 h. These results suggest both NSE and TQ modulate antiangiogenic responses in endothelial cells.
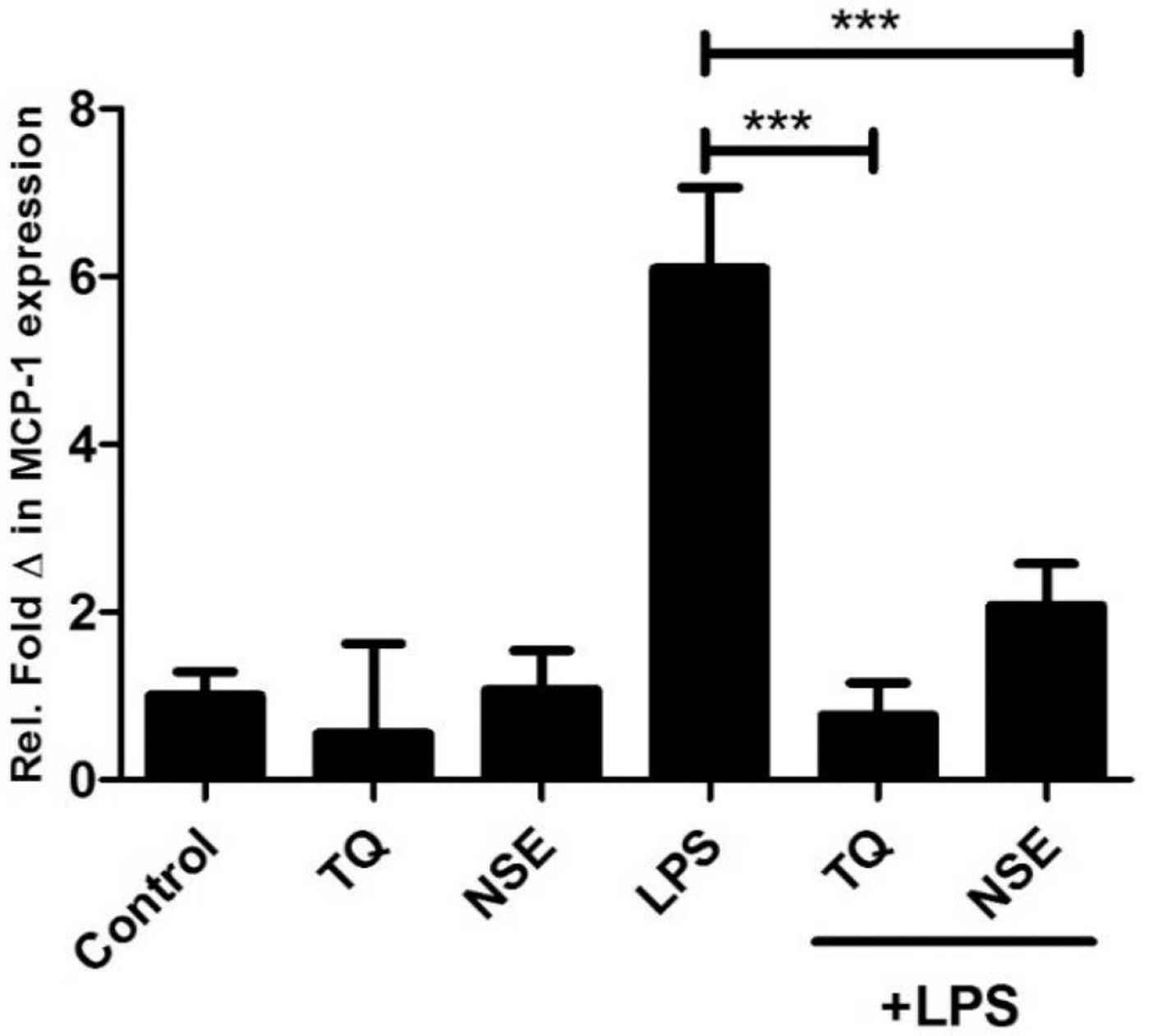
Effect of NSE, TQ and/or LPS (100 ng/mL) on MCP-1 gene expression at 24 h. HECV cells were treated with media only, TQ, NSE only and/or LPS for 24 h. Gene expression at the mRNA of the MCP-1 was determined by qRT-PCR. Data represents mean ± SD (n = 3) and data were analysed using one-way ANOVA with Tukey’s Test for pairwise analysis. LPS, lipopolysaccharide; TQ, thymoquinone, MCP-1, monocyte chemotactic protein; HECV, human vascular endothelial cell; qRT-PCR, quantitative real time polymerase chain reaction.
3.5. NSE and TQ Inhibits the Gene Expression of NLRP3 and IL-1β and Upregulates LPS Mediated Expression of TET-2
The NLRP3 inflammasome is an essential mediator of host immune responses through the activation of cytokines such as IL-1β [22]. The NLRP3 inflammasome activity is also regulated by epigenetic regulators proteins such as TET-2. The effect of TQ and NSE on the expression at mRNA level of NLRP3, TET-2 and IL-1β was determined. Figure 6A shows that both NSE and TQ significantly downregulates the expression of NLRP3 in LPS stimulated HECV.
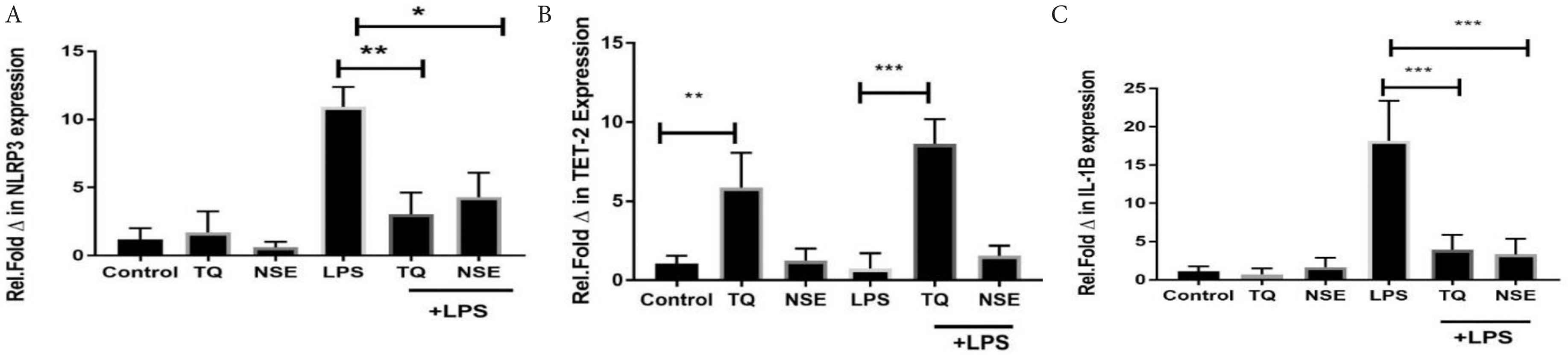
Effect of NSE, TQ and/or LPS (100 ng/mL) on (A) NLRP3, (B) TET-2 and (C) IL-1β mRNA expression. HECV cells were treated with media only, TQ, NSE only and/or LPS for 24 h. Gene expression at the mRNA of the NLRP3, TET-2 and IL-1β were determined by qRT-PCR. Data represents mean ± SD (n = 3) and data were analysed using one-way ANOVA with Tukey’s Test for pairwise analysis. LPS, lipopolysaccharide; TQ, thymoquinone; NLRP3, nod-like receptor protein 3; TET-2, ten-eleven translocation; HECV, human vascular endothelial cell; qRT-PCR, quantitative real time polymerase chain reaction.
Due to the growing interest in the role of TET-2 in its potential of regulating the NLRP3 inflammasome, its LPS expression was also explored in the presence of NSE and TQ. As can be seen in Figure 6B, TQ increases significantly the expression of TET-2 mRNA in the absence of any stimulus by LPS. Upon stimulation of the HECV cells with LPS, there was a significant decrease of TET-2 mRNA expression. When the LPS stimulated cells were treated with TQ and NSE, a significant increase was observed with the TQ treated cells; however, there was no significant difference between that of the LPS only cells and the LPS and NSE treated cells at 24 h.
4. DISCUSSION
This study investigated the effect of NSE and its main active compound TQ on inflammatory and monocyte recruitment factors in HECV and established that both NSE and TQ significantly inhibited VEGF and MCP-1 gene expression in LPS-induced HECV cells. Furthermore, both compounds were shown to downregulate the secretion of IL-6 and -8, responses associated with both inflammation and monocyte recruitment to the endothelium. The study also for the first time demonstrated that both the NSE and TQ could regulate both TET-2 and NLRP3 expression.
Lipopolysaccharide treatment of endothelial cells led to an increase in IL-6 and -8 protein expression. NSE was found to significantly attenuate this inflammatory response indicating the potential of the extract in preventing inflammation in endothelial cells. LPS-induced inflammation produces a microenvironment similar to that of what can be expected in disease whereby release of major proinflammatory and proangiogenic cytokines can be studied [23]. Gram-negative bacterial endotoxin (LPS) has been invoked in the pathogenesis of many diseases-not only as a trigger for septic shock, once it is most cited role, but also as a contributor to atherosclerosis, obesity, chronic fatigue, metabolic syndrome, and many other conditions. For instance, there is an increasing evidence that changes in the gut microbiota and the secretion of LPS is associated with changes in blood pressure [24]. Endothelial cells in conjunction with monocytes can induce vascular inflammation as well as tissue remodelling and adaptation by secreting inflammatory cytokines chemokines and transforming into inflammatory macrophages. A multitude of adhesion molecules promote the infiltration and accumulation of monocytes into vasculature in hypertension. All these facets offer the possibility to nutraceutical target of endothelial cells may represent novel therapeutic ways to treat hypertension, attenuate hypertension-associated end organ damage or prevent the development or worsening of high blood pressure.
NSE and TQ’s effect on endothelial cell secreted monocyte recruitment factors was demonstrated through a significant reduction of both VEGF and MCP-1 expression. In addition, VEGF is widely accepted to promote angiogenesis itself a mediator of atherosclerosis and has been the target of multiple therapies to inhibit angiogenesis [25,26]. Likewise, previous studies have reported the proangiogenic properties of MCP-1. Niu et al. [13] found that this chemokine promoted angiogenesis by MCP-1-induced protein and its suppression therefore supressed MCP-1 expression.
Moreover, the data presented here that both NSE and TQ significantly inhibited the gene expression of NLRP3 and the proinflammatory cytokine IL-1β a potent pro-inflammatory cytokine that is associated with atherosclerosis. Additionally this study demonstrated that NSE and TQ significantly increased the expression of TET-2. In a recent study by Fuster et al. [18], showed that TET-2-deficient macrophages exhibited an increase in NLRP3 inflammasome-mediated IL-1β secretion, and pharmacological inhibition of NLRP3 inhibits hypertension development [19]. The importance of mutations such as TET-2 in the pathogenesis of atherosclerosis was reported recently by Jaiswal et al. [27,28] and its potential importance as a novel target in atherosclerosis. In their study Hassan et al. [20] proposed the anti-inflammatory actions of another naturally occurring glycosidic flavonoid vicenin-2 (V2) in phorbol myristate acetate (PMA)-differentiated THP-1 cells (dTHP-1) and that its anti-inflammatory actions where associated with upregulating of TET-2. This study supports the hypothesis that the anti-inflammatory properties of other naturally occurring compounds can be associated with the upregulation of TET-2 in endothelia cells. Whether this can definitely be linked to the upregulation of TET-2 will require further investigation and in multiple cell lines. However, our data is supportive of a role in TET-2 regulation by naturally derived compounds in endothelial cells as well as in monocytic cells.
This study did not investigate the mechanism of the action of either NSE or TQ. The anti-inflammatory actions of NSE could possibly be linked to the suppression of the protein complex; the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB). This protein complex has been mainly considered as a proinflammatory pathway due to its activation of proinflammatory cytokines such as the interleukin-1 family. A study by Elkady et al. [29] founded that an extract of NSE suppressed the NF-κB protein in a human colon cancer cell line. In this study it would have proved useful to investigate this protein complex as well as other protein complexes in endothelial cells to fully understand the mechanism of action that NSE has on the endothelial cells.
5. CONCLUSION
This study presents significant anti-inflammatory and monocyte recruitment properties of NSE and its main active component TQ in endothelial cells in vitro, through their ability to reduce secretion and expression the cytokines IL-6 and -8 and both VEGF and MCP-1. The study also presented a novel data on their ability to upregulate the epigenetic regulator TET-2 and inhibiting NLRP3 and IL-1β. These reductions would suggest that these compounds could potentially play a role in the prevention of arteriosclerosis in part through the regulation of blood pressure. Further studies should be carried out on in multiple cell lines to confirm their beneficial effects and if these effects are also observed in vivo.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
JA contributed in investigation, study conceptualization, formal analysis, methodology writing (draft), validation. CW contributed in study conceptualization and writing (draft), project administration, supervision, funding acquisition, resources. NH and SG contributed in investigation, methodology, validation. KM contributed in writing – review & editing, supervision, visualization, resources.
FUNDING
That the work was funded internally by
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Jason Amartey AU - Samuel Gapper AU - Nurudeen Hussein AU - Keith Morris AU - Cathryn E. Withycombe PY - 2019 DA - 2019/12/02 TI - Nigella sativa Extract and Thymoquinone Regulate Inflammatory Cytokine and TET-2 Expression in Endothelial Cells JO - Artery Research SP - 157 EP - 163 VL - 25 IS - 3-4 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.191114.002 DO - 10.2991/artres.k.191114.002 ID - Amartey2019 ER -
