Arterial baroreflex sensitivity: Relationship with peripheral chemoreflex in patients with chronic heart failure
- DOI
- 10.1016/j.artres.2018.10.002How to use a DOI?
- Keywords
- Arterial baroreflex; Peripheral chemoreflex; Heart failure
- Abstract
Background: The purpose of this study was to evaluate the relationship between the breath-holding duration and the sensitivity of the arterial baroreflex, as well as the prognostic value of the breath-holding test in the assessment of decreased baroreflex sensitivity (BRS) in patients with chronic heart failure (CHF).
Methods: The study was conducted on 87 patients with CHF. In all participants, the breath-holding test was performed in the morning, while the single-breath carbon dioxide test was performed on the next day; the injection method was used to evaluate BRS. Other parameters, including pulse wave velocity (PWV), N-terminal pro-brain natriuretic peptide (NT-proBNP) level, and spirometry and echocardiography findings, were also evaluated.
Results: A significant correlation was found between BRS and the following two parameters: breath-holding duration and left ventricular ejection fraction (all p < 0.05) and a significant negative correlation between BRS and the following three parameters: age, NT-proBNP level, and PWV (all p < 0.05). After analysis, only the breath-holding duration remained significant in our model (R2 adjusted for the model, 0.55). The breath-holding duration was a good predictor of decreased BRS (<3 ms/mmHg), with a cut-off point of <34 s [area under curve, 0.876 (0.778–0.937, p < 0.0001)].
Conclusion: A reduced breath-holding duration is associated with decreased sensitivity of the arterial baroreflex. If the duration of the breath-holding test is <34 s, decreased BRS is predicted. This test can be effectively used for the initial assessment of the level of the reflex regulation of the cardiorespiratory system in patients with CHF.
- Copyright
- © 2018 The Authors. Published by Elsevier B.V. on behalf of Association for Research into Arterial Structure and Physiology.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
Introduction
Chronic heart failure (CHF) is still an acute problem worldwide, and the number of affected patients remains large, increasing morbidity and mortality. One of the main links of its pathogenesis is the imbalance of the autonomic nervous system and the impairment of the reflex regulation of the cardiorespiratory system.1–3 Increasing the activity of the sympathetic nervous system, reducing parasympathetic activity, and activating the renin-angiotensin system are the main links leading to the progression of the disease. At present, the generally accepted treatment measures are β-adrenergic sympathetic blockade and inhibition of the renin-angiotensin system,4,5 which significantly improves the results of treatment and reduces the risk of adverse cardiovascular events. Currently, several experimental and clinical studies indicate that the progression of heart failure leads to decreased arterial baroreflex sensitivity (BRS), which is an independent predictor of cardiac complications and an unfavourable outcome of the disease.3,6
Given this fact, the sensitivity of the arterial baroreflex is not only a prognostic marker and a marker of the heart failure severity, but also a potential therapeutic target, along with β-blockade and inhibition of the renin-angiotensin system. Recently, further data on baroreflex activation therapy (BAT) are accumulated. The effectiveness of this therapy has also been proven in experimental7,8 and clinical9,10 studies; however, BAT is not currently routinely performed.
The fundamental question is to whom BAT is indicated. The modern clinical methods for BRS assessment are complex and require special equipment; thus, the search for simple assessment methods can be considered as one of the priority research areas in this field.11
With the progression of CHF, there is an increase in the sensitivity of the peripheral chemoreflex, which is a consequence of a complex of causes.12 The decrease in the sensitivity of the baroreflex is largely a consequence of a change in the balance of the autonomic nervous system associated with chronic activation of peripheral chemosensitivity and possibly with a change in the vessels themselves.13,14 A previous study that investigated the relationship between the arterial baroreflex and peripheral chemoreflex demonstrated that the changes in these reflexes are interrelated and are inversely correlated.15
The breath-holding test (BHT) is a functional test that provides information on the functional state of the cardiorespiratory system. Despite the fact that the mechanism of the appearance of an imperative stimulus to inspiration has not been fully studied, it is known that in many respects, the breath-holding duration is a function of peripheral chemoreceptor sensitivity.16 Clinical studies have shown that the BHT provides information on the sensitivity of the peripheral chemoreflex to carbon dioxide (CO2) in both healthy subjects and patients with CHF.17
Thus, the purpose of this study was to evaluate the relationship between the peripheral chemoreflex sensitivity and the sensitivity of the arterial baroreflex, as well as the predictive value of the BHT in the assessment of decreased BRS in patients with CHF.
Methods
Study population
The study was conducted on 87 patients with CHF under NYHA functional class II-III who were treated in the Department of Cardiology in our hospital. Patients with respiratory diseases, central nervous system diseases, and mental disorders were excluded in the study. Permission of the Ethics Committee of Kuban State Medical University was obtained for the study in February 2017; all participants signed an informed consent before inclusion to the investigation.
Measurements
Peripheral chemoreflex sensitivity
The BHT was performed as follows18: the voluntary breath-holding duration was assessed three times, with 10-min intervals of normal resting breathing. After inspiration of an atmospheric air volume equal to 2/3 of ±15% vital lung capacity (controlled using spirometry), the participants were asked to hold their breath; the duration of voluntary apnoea was measured from the beginning of the voluntary inspiration until reflex contractions of the diaphragm were noted on the researcher’s palm. The mean value of the duration of the three samples was calculated.
The single-breath CO2 (SB-CO2) test was performed on the next day as follows.19 The participants’ nose was clamped using a soft grip. Breathing through the mouth was monitored using a mouthpiece connected to a pneumatic respiratory valve separating the inhaled gas mixture from the exhaled air. The inspiratory port was connected to a T-shaped valve in such a way that ventilation was conducted from either a rubber bag or a 2-L tank, which was filled after each inhalation with a gas mixture containing 13% CO2 or atmospheric air. After a brief period of eupnoea (approximately 5 min) in the expiratory phase, the T-shaped valve was switched to breathing a mixture with high CO2 content; the next breath was taken using this mixture. Subsequently, the valve was switched to atmospheric air. On average, 10 breaths of the hypercapnic mixture were taken with intervals of 2 min of breathing room air. The respiratory rate and tidal volume were estimated breath-to-breath with the calculation of minute ventilation (MV). The CO2 fraction in the exhaled mixture was measured using a sidestream gas analyser (Nihon Kohden, Japan). As a control, the average MV was calculated from the data of the last five breaths before breathing the hypercapnic mixture. Similarly, the average feedback of end-tidal CO2 fraction (FetCO2) was determined during these breaths and used as the control FetCO2. The ventilation response to a hypercapnic stimulus was determined as the average of the two highest rates of MV (during the first 20 s after the stimulus; breaths beyond this time-point were excluded to minimise the contribution of central chemoreception). Post-stimulus FetCO2 was also assessed during these cycles. The ventilation response to breathing a hypercapnic mixture was calculated using the following formula: [post-stimulus MV − control MV]/[(post-stimulus FetCO2 − control FetCO2) × (Patm − 47)], where Patm represents the atmospheric pressure in mmHg and 47 is the saturated water vapour pressure in mmHg. The median of all 10 episodes was considered to indicate the sensitivity of the peripheral chemoreflex and was expressed in L/min/mmHg.
Baroreflex measurement
The injection method was used in all patients. Interbeat interval responses to vasodilator-induced hypotension provided measurement of BRS. Graded boluses of nitroglycerin 3 μg/kg were injected via the right internal jugular vein.20 The invasive systolic blood pressure (ISBP) for each beat and corresponding RR intervals with a one-beat delay were recorded using a record module (Nihon Kohden MU-651K; Japan) until the ISBP decreased to the bottom most level. BRS was defined as the change in the RR interval in ms/mmHg-change in the ISBP. The BRS values were calculated using the method by Parmer et al.,21 and the formula was as follows: BRS (ms/mmHg) = (RR interval before decompression − RR interval after decompression)/(ISBP before decompression − minimum ISBP after decompression). The BRS level of <3 ms/mmHg was accepted to indicate a reduced BRS and as an independent predictor of complications in patients with CHF.22
Pulse wave velocity (PWV)
Carotid-femoral PWV was assessed using the SphygmoCor device. The distance from the sternal notch to the carotid pulse site and distance from the sternal notch to the umbilicus and then to the femoral pulse site were measured. With the use of a probe, pressure waveforms at the carotid and femoral sites were captured sequentially, and the time between the QRS complex and onset of the pulse wave was calculated. The PWV was derived by applying a previously described and validated methodology that uses an ECG-gated signal and anthropometric distances.23
Other measurements
Spirometry, arterial blood gas analysis, N-terminal pro-brain natriuretic peptide (NT-proBNP) level assessment, blood pressure and heart rate assessment, and echocardiography were performed in all patients after admission to the department.
Statistical analysis
All data showed normal distribution (Kolmogorov–Smirnov test) and were thus presented as means ± standard deviations. The correlations among the variables were tested using univariate (Pearson’s linear correlation coefficients) and multivariate models. The significance level was set at p < 0.05. An ROC analysis was also performed to evaluate the predictive value of the BHT in comparison with the SB-CO2 test.
Results
Patient demographic and clinical characteristics
The characteristics of the study population are reported in Table 1. Eighty-seven patients were included in the study.
| Parameter | Value |
|---|---|
| Age, y | 63 ± 7 |
| Weight, kg | 77.4 ± 5.3 |
| Height, cm | 174 ± 7 |
| BMI, kg/m2 | 22.8 ± 2.1 |
| Breath-holding duration, s | 40 ± 10 |
| BRS, ms/mmHg | 4.3 ± 1.5 |
| SB-CO2, L/min/mmHg | 0.39 ± 0.12 |
| LVEF, % | 34 ± 6 |
| NYHA class | 2.4 ± 0.7 |
| NT-proBNP level, pg/mL | 462 ± 164 |
| PWV, m/s | 11.4 ± 1.4 |
| Heart rate, min−1 | 76 ± 13 |
| SBP, mmHg | 137 ± 25 |
| DBP, mmHg | 82 ± 13 |
| SaO2,% | 98.9 ± 1.0 |
| PaCO2, mmHg | 34.5 ± 1.4 |
| PaO2, mmHg | 89.1 ± 4.2 |
| VC, % predicted | 95.6 ± 4.1 |
| FVC, % predicted | 93.1 ± 2.7 |
| FEV1, % predicted | 96.3 ± 1.9 |
| FEV1/FVC, % predicted | 0.94 ± 0.03 |
| Breathing rate, min−1 | 15.2 ± 3.4 |
| Medical therapy | |
| ACE-I/ARB, % | 100 |
| β-blockers, % | 100 |
| Aldosterone antagonists, % | 84 |
| Loop diuretics, % | 39 |
| Thiazide diuretics, % | 32 |
| Statins, % | 84 |
BMI – body mass index, BRS – baroreflex sensitivity, SB-CO2 – single-breath carbon dioxide, LVEF – left ventricular ejection fraction, NT-proBNP – N-terminal pro-brain natriuretic peptide, PWV – pulse wave velocity, SBP – systolic blood pressure, DBP – diastolic blood pressure, SaO2 – oxygen saturation, PaCO2 – arterial carbon dioxide partial pressure, PaO2 – arterial oxygen partial pressure, VC – vital capacity, FVC – forced vital capacity, FEV1 – forced expiratory volume in the first second, ACE-I/ARB – angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Demographic and clinical characteristics of the study population (n = 87).
Correlations between BRS and selected variables
To assess the relationship between the sensitivity of the arterial baroreflex and other parameters, a correlation analysis was performed. A significant correlation was found between BRS and the following two parameters: breath-holding duration and left ventricular ejection fraction (LVEF) (all p < 0.05) and a significant negative correlation between BRS and the following three parameters: age, NT-proBNP level, and PWV (all p < 0.05), (Fig. 1). The other parameters were not related to BRS (Table 2).
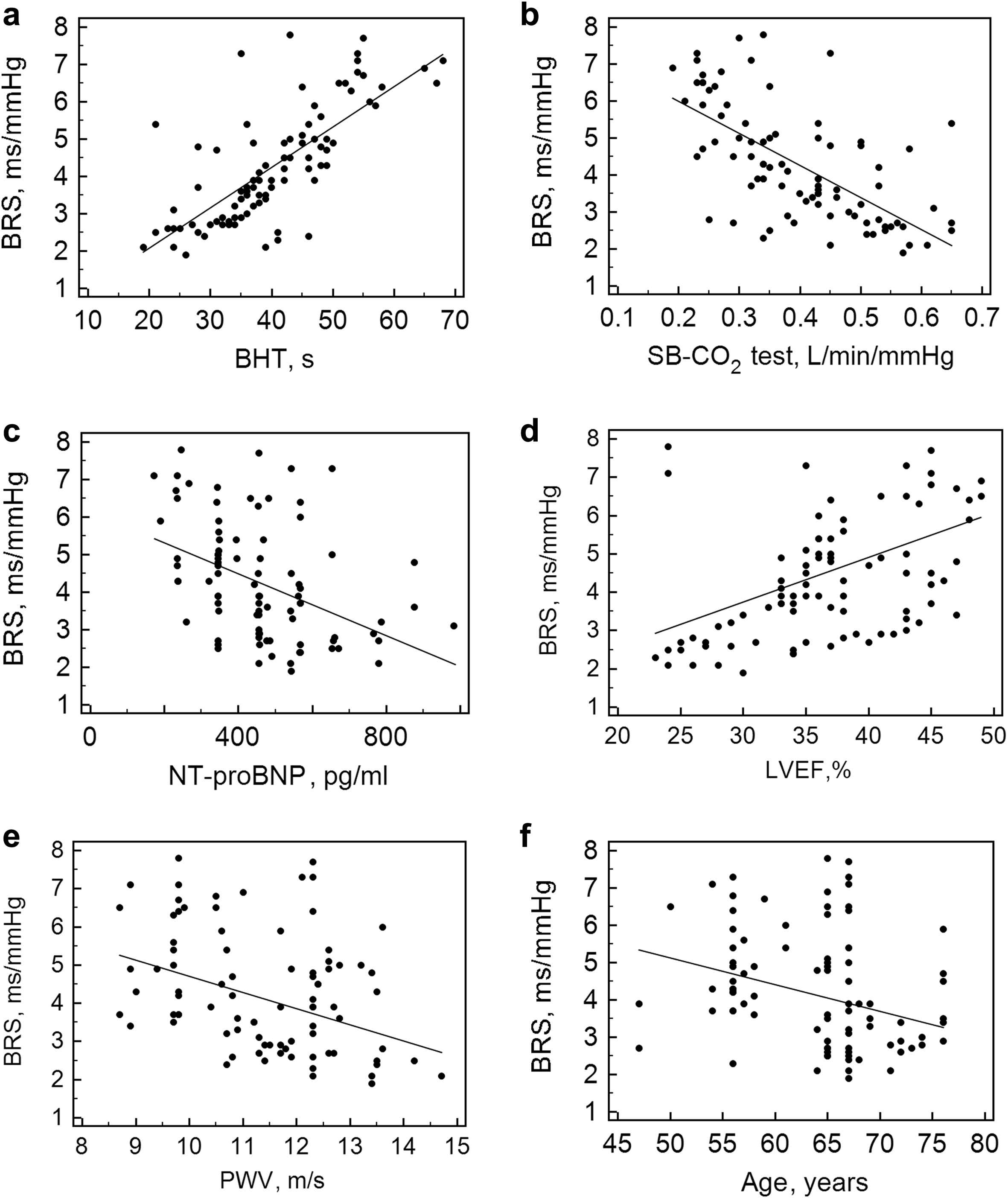
Correlation between BRS and breath-holding test (a), single-breath carbon dioxide test (b), N-terminal pro-brain natriuretic peptide (c), left ventricular ejection fraction (d), pulse wave velocity (e), age (f). BRS – baroreflex sensitivity, SB-CO2 test – single-breath carbon dioxide test, BHT – breath-holding test, NT-proBNP – N-terminal pro-brain natriuretic peptide, LVEF – left ventricular ejection fraction.
| Parameter | R | p |
|---|---|---|
| Age | −0.27 | 0.012 |
| Sex | 0.12 | 0.26 |
| BMI | 0.16 | 0.61 |
| BR | 0.05 | 0.24 |
| Height | 0.21 | 0.2 |
| Weight | −0.11 | 0.42 |
| NT-proBNP | −0.4 | 0.0001 |
| PetCO2 | −0.19 | 0.13 |
| SaO2 | −0.07 | 0.23 |
| GFR | 0.17 | 0.34 |
| SBP | 0.23 | 0.15 |
| DBP | 0.31 | 0.29 |
| Initial RR interval | −0.08 | 0.21 |
| PWV | −0.35 | 0.0009 |
| LVEF | 0.46 | <0.0001 |
| BHT | 0.74 | <0.0001 |
| SB-CO2 test | −0.63 | <0.0001 |
BMI – body mass index, BR – breathing rate, NT-proBNP – N-terminal pro-brain natriuretic peptide, SaO2 – oxygen saturation, GFR – glomerular filtration rate, SBP – systolic blood pressure, DBP – diastolic blood pressure, PWV – pulse wave velocity, LVEF – left ventricular ejection fraction, BHT – breath-holding test, SB-CO2 test – single-breath carbon dioxide test.
Correlations between baroreflex sensitivity and selected variables in the subjects with chronic heart failure.
Multivariate regression
The multivariate regression model included all the variables that appeared to be significantly correlated with BRS in the univariate models, with the exception of those that were highly interrelated (arbitrary cut-off: r = 0.70). For this reason, of the two tests characterizing the peripheral chemosensitivity, only the BHT was included in the model (correlation coefficient with the SB-CO2 test, 0.81; p < 0.0001). After analysis, only the breath-holding duration remained significant in our model (Table 3) (R2 adjusted for the model, 0.55).
| Independent variables | Coefficient | Standard error | T | p |
|---|---|---|---|---|
| (Constant) | −2.24 | |||
| Breath-holding duration | 0.1 | 0.01 | 6.3 | <0.0001 |
| LVEF | 0.03 | 0.02 | 1.5 | 0.12 |
| PWV | −0.001 | 0.001 | −1.5 | 0.12 |
| NT-proBNP | −0.09 | 0.09 | −1.1 | 0.27 |
| Age | −0.01 | 0.02 | −0.9 | 0.38 |
NT-proBNP – N-terminal pro-brain natriuretic peptide, PWV – pulse wave velocity, LVEF – left ventricular ejection fraction.
Multivariate regression analysis.
Predictive value of the chemoreflex sensitivity test in the prediction of decreased BRS
To assess the predictive value of the BHT in determining decreased BRS, an ROC analysis was performed. The BRS level of <3 ms/mmHg was considered to indicate decreased BRS (24 patients). The cut-off point for the breath-holding duration was <34 s; the sensitivity and specificity for this point were 75% and 91%, respectively. The area under the curve was 0.876 (0.778–0.937, p < 0.0001). Further, the BHT showed a greater AUROC than the SB-CO2 test [0.8 (0.81–0.89)] (Fig. 2); however, the difference was insignificant.
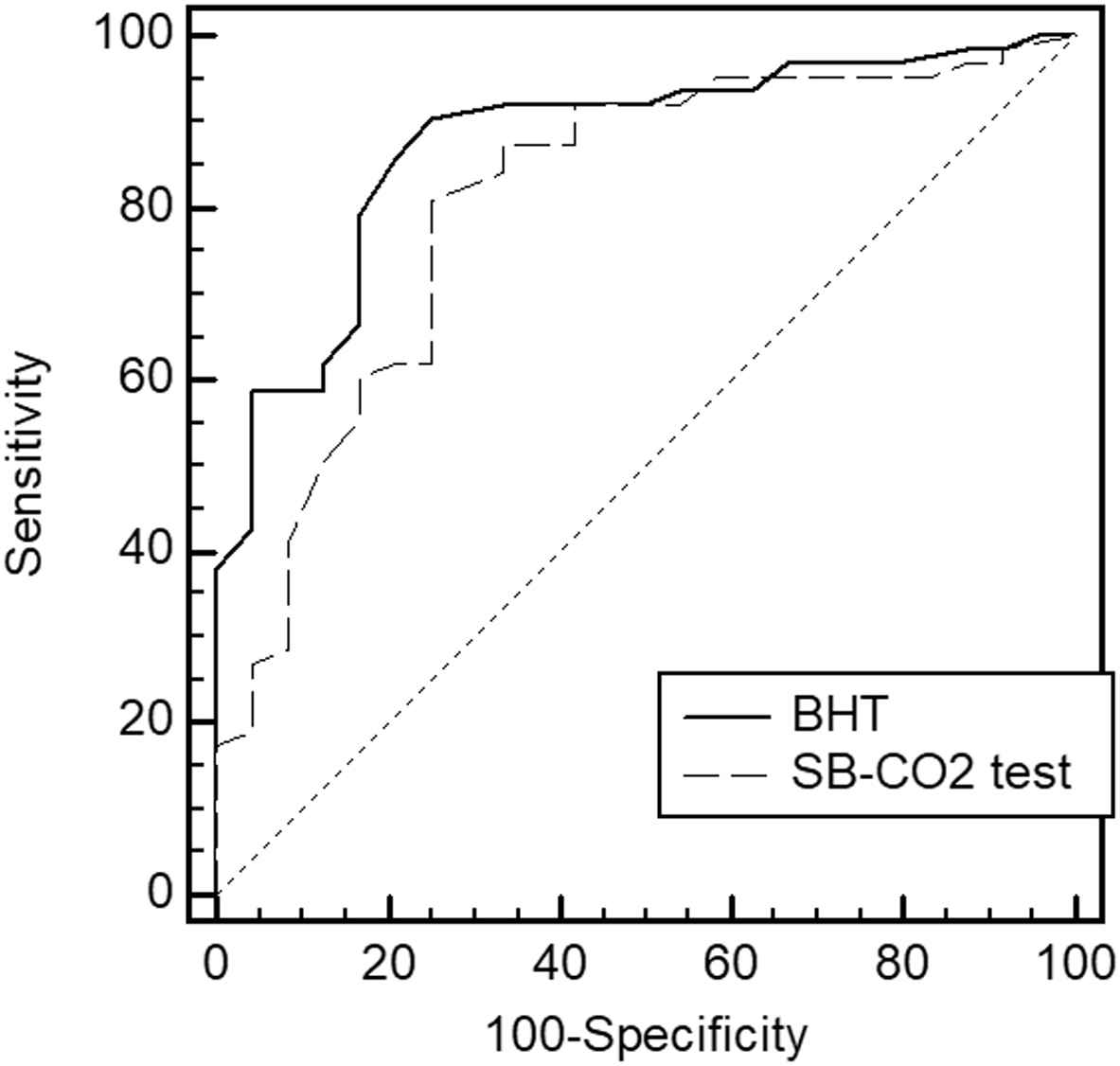
Predictive values of the BHT and SB-CO2 tests for a decreased baroreflex sensitivity. BHT – breath-holding test, SB-CO2 test – single-breath carbon dioxide test.
Discussion
The main findings of our study are that the breath-holding duration is an independent predictor of arterial BRS in patients with CHF and that the BHT may be useful in the initial assessment of baroreflex function.
Baroreceptor-heart rate reflex sensitivity assesses the integrity of the carotid and aortic baroreceptors in response to changes in blood pressure. Abnormalities in baroreceptor function are intrinsic to the pathophysiology of heart failure.24 Experimental25 and clinical26 studies have shown that carotid sinus baroreceptor function is diminished in heart failure. The site within the baroreflex arcade are not clearly identified. A significant number of studies have been devoted to investigate the sensitivity of the arterial baroreflex in patients with CHF. In most of these studies, the average sensitivity level was below 6 ms/mmHg and varied depending on the design of the study and the characteristics of the patients. Thus, in the study by Yoshikawa et al.,27 the average sensitivity level in patients with CHF with an average LVEF of 26 ± 9% was 4.3 ± 3.7 ms/mmHg, which corresponds to the results obtained in the present study (4.3 ± 1.5 ms/mmHg). Nevertheless, the greater variance in the values may be because of the fact that in their study, patients with conditions under NYHA classes I-IV were included. Similar BRS levels were obtained by Mortara et al.28 and Davies et al.29 in a similar cohort of patients (3.9 ± 4.0 ms/mmHg and 4.4 ± 4.4 ms/mmHg, respectively). In general, all studies showed that the sensitivity of the baroreflex in patients with CHF is reduced, with approximately 1/3 showing a critical decrease in the BRS level below 3 ms/mmHg, which is an independent predictor of unfavourable outcomes of heart failure and mortality.22
Although the mechanisms of BRS impairment are not completely known, it is well known that activation of chemoreceptors results in baroreflex impairment in heart failure with reduced ejection fraction. The peripheral chemoreceptors are a key link of arterial blood gas, pH, and cardiovascular regulation.30 The carotid bodies are the main peripheral chemoreceptors31,32 and play an important role in the control of cardiorespiratory function at rest and during exercise. They respond to changes in arterial PO2, PCO2, pH, glucose level, and blood flow.33 The activation of type I carotid bodies glomus cells triggers a reflex response that increases pulmonary ventilation, arterial blood pressure, heart rate, and sympathetic activity.34 Several studies have demonstrated that the peripheral chemoreflex function is altered in heart failure.35,36 In CHF, it has been shown that the CB-mediated chemoreflex is over sensitised and plays a fundamental role in the progression of the disease.37,38 Del Rio et al.39 showed that CB denervation in rats with CHF completely restored normal BRS. Taken together, this evidence strongly suggests that hyperactivation of the peripheral chemoreceptors promotes baroreflex control impairment38 and that both may contribute to disease progression/maintenance in HFrEF. Our results demonstrate that the sensitivity of the peripheral chemoreflex (assessed using both the SB-CO2 test and BHT) is associated with BRS and that the breath-holding duration is an independent predictor of BRS. This indicates one of the main roles of the impairment of peripheral chemoreception in the reduction of BRS.
Existing studies on the function of arterial baroreception in patients with CHF demonstrated its relationship with age, NYHA class, and natriuretic peptide level.40,41 The observed relationship between these variables and BRS is not surprising, since they reflect the degree of heart failure progression and hence the level of impairment of the reflex regulation of the cardiorespiratory system. In our study, age, LVEF, and NT-proBNP level were significantly correlated with the sensitivity of the baroreflex, but were not included in the final model according to the multivariate regression analysis. Baroreceptors are stretch receptors, located in the adventitia of the aorta and carotid arteries; thus, the physical properties of the vascular wall are important determinants of the sensitivity of the arterial baroreflex. Previous studies have shown that an increase in vascular stiffness is associated with a decrease in BRS in patients with hypertension42 and chronic kidney disease.43 The correlation between PWV and BRS, similar to the findings of the abovementioned studies, confirms this relationship. Nevertheless, this parameter was not included in the final model. Probably, the impairment of the reflex regulation of the cardiorespiratory system during the progression of CHF is a more significant factor in the reduction of BRS than arterial stiffness. The association between LVEF and BRS in CHF is controversial - from a strong correlation28 to its complete absence,44 which is apparently connected with the nonlinear nature of this relationship.
The duration of breath-holding was 40 ± 10 s in our study in patients with CHF. Over the past 100 years, a lot of studies have been conducted to study the duration of the maximum inspiratory breath-holding in different diseases and in different laboratory conditions.16 In general, most studies suggest that healthy people without prior training are able to hold their breath for a maximum of 1–2 min, however, they also showed significant variability of this parameter between subjects, but low inter-subject variability, which reflects the good reproducibility of the test. For example, Feiner et al. reported a breath-holding of 107 ± 34 s in healthy young volunteers without comorbidities, while the variation was from 36 to 150 s.45 More recent studies have shown the duration of breath-holding in young subjects more than 60 s.46,47 Analysis of the results allowed the authors to conclude that the sensitivity of peripheral chemoreceptors is the main predictor of the breath-holding duration.16 It is obvious that in conditions of chronic heart failure, in which peripheral chemosensitivity increases, the duration of breath-holding should be less than that we observed earlier, as we observed in this study.
Our ROC analysis demonstrated that both the BHT and SB-CO2 test showed a good similar prognostic value in predicting reduced arterial BRS. However, the BHT is undoubtedly a simpler clinical tool and does not require special equipment; nevertheless, despite its simplicity, it shows good reproducibility in patients with CHF.17 The cut-off point was 34 s; thus, with a duration of a voluntary apnoea of <34 s, decreased BRS can be predicted. These data correlate with those of a previously published study in which the breath-holding duration of <38 s was associated with a greater incidence of cardiac events in patients with CHF during the perioperative period.48
In conclusion, a reduced breath-holding duration is associated with a decreased sensitivity of the arterial baroreflex. If the breath-holding duration is <34 s, decreased BRS is predicted. The BHT can be effectively used for the initial assessment of the level of the reflex regulation of the cardiorespiratory system in patients with CHF.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was funded by
References
Cite this article
TY - JOUR AU - Nikita Trembach AU - Igor Zabolotskikh PY - 2018 DA - 2018/10/22 TI - Arterial baroreflex sensitivity: Relationship with peripheral chemoreflex in patients with chronic heart failure JO - Artery Research SP - 9 EP - 15 VL - 24 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2018.10.002 DO - 10.1016/j.artres.2018.10.002 ID - Trembach2018 ER -
