Fetal programming and vascular dysfunction
- DOI
- 10.1016/j.artres.2017.11.005How to use a DOI?
- Keywords
- Cardiovascular risk; Vascular dysfunction; Epigenetic; Fetal programming; Preeclampsia; ART
- Abstract
Cardiovascular diseases are the main cause of mortality and morbidity in Western countries, but the underlying mechanisms are still poorly understood. Genetic polymorphisms, once thought to represent a major determinant of cardiovascular risk, individually and collectively, only explain a tiny fraction of phenotypic variation and disease risk in humans. It is now clear that non-genetic factors, i.e., factors that modify gene activity without changing the DNA sequence and that are sensitive to the environment can cause important alterations of the cardiovascular phenotype in experimental animal models and humans. Here, we will review recent studies demonstrating that distinct pathological events during the perinatal (transient perinatal hypoxemia), late foetal (preeclampsia), and early embryonic (assisted reproductive technologies) periods induce profound alterations of the cardiovascular phenotype in humans and experimental animals. Moreover, we will provide evidence that epigenetic modifications are contributing importantly to this problem and are conferring the potential for its transmission to subsequent generations.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Epidemiological observations in humans and studies in experimental animal models support the idea that CVD take their origins during the fetal or perinatal period. 20 years ago, David Barker, a British epidemiologist, was first to hypothesize that the embryonic, fetal, and early postnatal periods are characterized by a high phenotypical plasticity and that insults during these periods lead to alterations that predispose to cardio-metabolic disease during adulthood.1,2 These pioneering observations set the stage for a large number of epidemiological studies in this field and the term “developmental origins of health and disease” was coined to describe this concept. More recently, direct experimental evidence for this concept has been provided in humans and experimental animal models. Here, we will review recent studies demonstrating that pathological events during the perinatal (transient perinatal hypoxemia), the late fetal (preeclampsia) and early embryonic (assisted reproductive technologies, ART) period induce profound alterations of the cardiovascular and metabolic phenotype in humans and experimental animals and discuss underlying mechanisms.
Transient perinatal hypoxemia predisposes to exaggerated hypoxic pulmonary hypertension later in life
At birth, the transition from fetal to adult circulation is a delicate event, as gas exchange is no more carried out by the placenta but by the lungs.3 These functional and structural changes are particularly sensitive to noxious stimuli. In line with this concept, in normal rats, transient perinatal exposure to hypoxia during the first week of life predisposes to exaggerated hypoxic pulmonary hypertension when adult animals are re-exposed to hypoxia.4 To tests whether a similar long-term effect of perinatal hypoxia on the pulmonary vasculature exists in humans, we assessed hypoxic pulmonary vasoreactivity at high altitude (Capanna Margherita, 4559 m) in a group of young adults who had suffered from transient hypoxemia (persistence of the pulmonary hypertension (PTPH)9) during their first week of life and a group of age and sex-matched controls born without any perinatal problem.
We found that, similarly to what was observed in rats, the subjects having suffered of transient hypoxemia during their first week of life developed exaggerated pulmonary hypertension at high altitude compared to the controls5 (Fig. 1). This study represented the first demonstration that a transient insult during the perinatal period has major long-term consequences on the regulation of the vascular function in humans not only in the systemic but also in the pulmonary circulation.
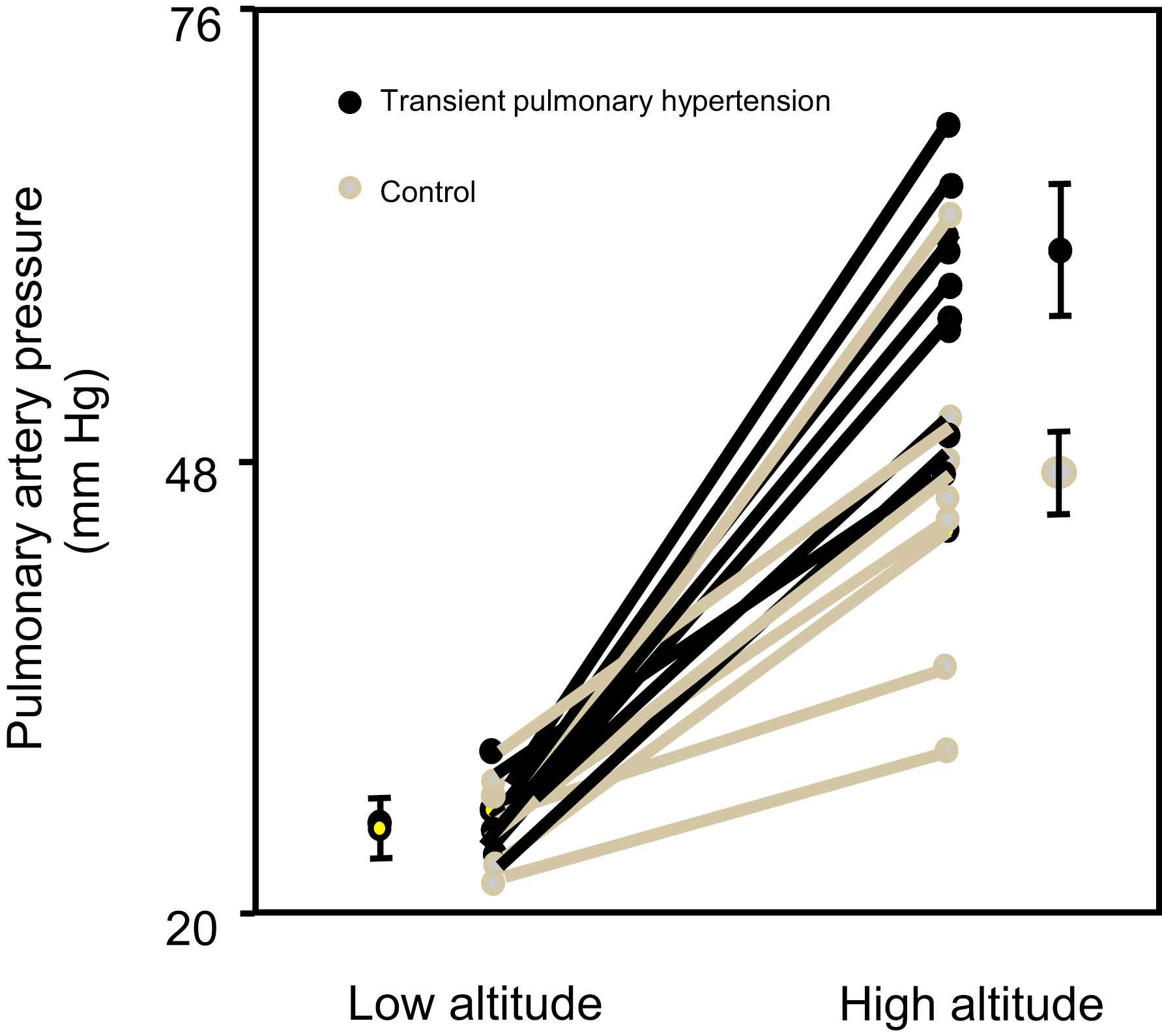
Transient pulmonary hypertension during the first week of life predisposes to hypoxic pulmonary hypertension later in life. Effects of high-altitude exposure (4559 m) on systolic pulmonary-artery pressure in participants with a history of transient perinatal pulmonary hypertension and in controls. Points with error bars = mean and SE for group (modified from Sartori et al., 1999).5
Preeclampsia in humans induces systemic and pulmonary vascular dysfunction in the offspring
Epidemiological studies have shown that offspring of preeclampsia are predisposed to premature cardiovascular disease,6,7 as evidenced, for example, by a roughly 3-fold increase of the risk for stroke. The underlying mechanisms are unknown.
During preeclampsia, vasculotoxic factors are released into the maternal circulation by the diseased placenta. We speculated that these factors pass the placental barrier and leave a defect in the circulation of the offspring that predisposes to a pathological response later in life. To test this hypothesis, we assessed pulmonary-artery pressure and flow-mediated dilation of the brachial artery in 48 offspring of preeclampsia and 90 offspring of normal pregnancies born and permanently living at the same high-altitude location (La Paz, Bolivia, 3600 m).
The important new finding was that pulmonary-artery pressure was roughly 30 percent higher and flow-mediated dilation 30 percent smaller in offspring of mothers with preeclampsia than in control subjects (Fig. 2). Vascular dysfunction in the pulmonary and the systemic circulation was a robust finding, since we found a close inverse relationship between pulmonary-artery pressure and flow-mediated dilation (r = −0.6, P < 0.001).
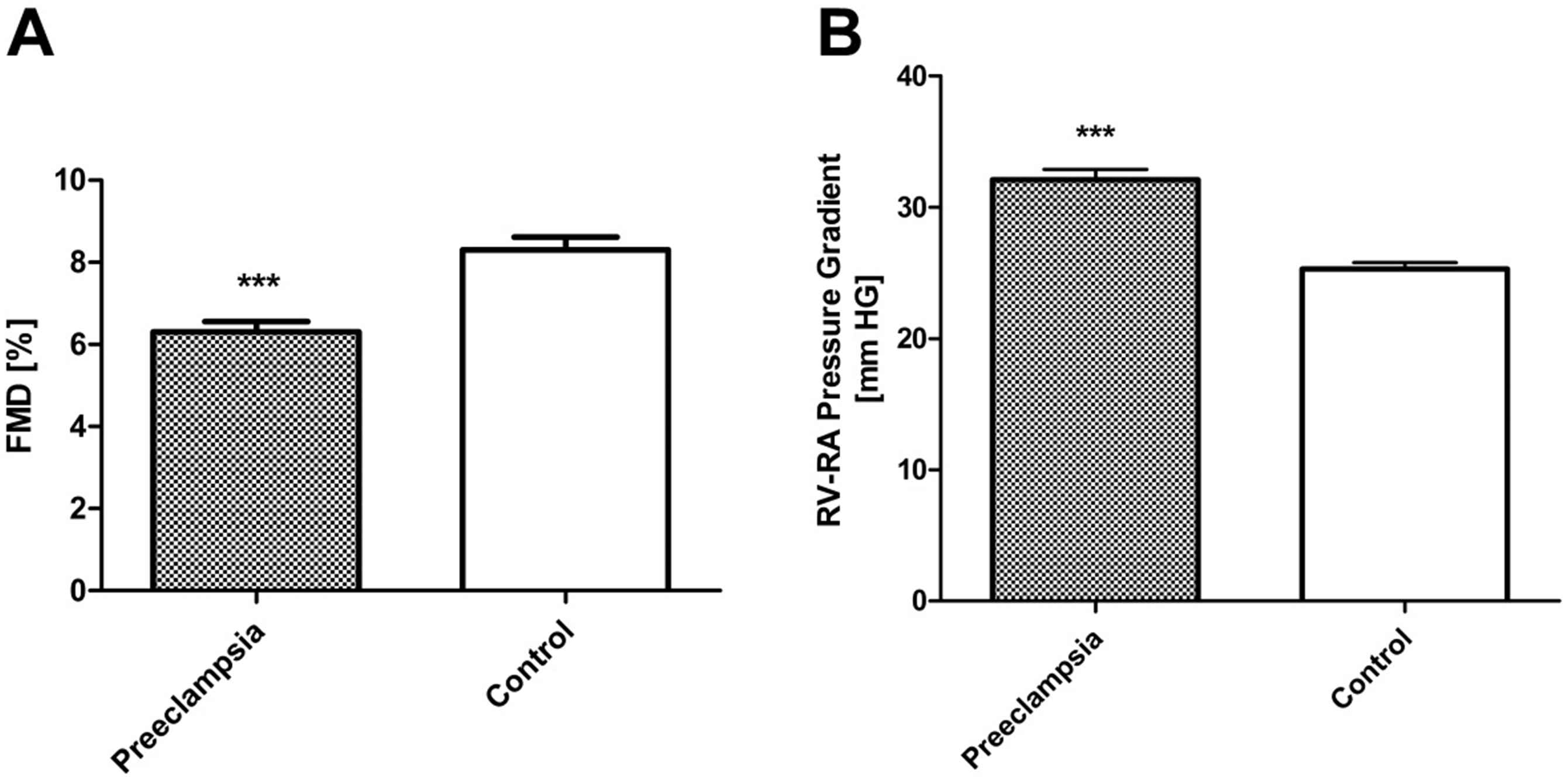
Preeclampsia induces vascular dysfunction in the systemic and pulmonary circulation of the offspring. Flow-mediated dilatation (FMD) of the brachial artery (A) and systolic pulmonary artery pressure (right ventricular-to-right atrial pressure gradient) at high altitude (3600 m) (B) in 48 apparently healthy offspring of preeclampsia and 90 matched control subjects; (***P ≤ 0.001 vs. controls) (modified from Jayet et al., 2010).14
The preeclampsia-induced vascular dysfunction has clinical consequences. Exaggerated hypoxic pulmonary hypertension is an important underlying mechanism of high-altitude pulmonary edema.8,9 Offspring of mothers with preeclampsia living at high altitude appear to be at risk for re-entry high-altitude pulmonary edema.5 Moreover, offspring of preeclampsia living at high altitude or suffering from disease states associated with chronic hypoxemia may be at risk for developing sustained pulmonary hypertension and right heart failure. Finally, defective flow-mediated dilation of the brachial artery in offspring of mothers with preeclampsia is related to endothelial dysfunction, because endothelium-independent dilation is normal in these subjects. Endothelial dysfunction in the systemic circulation represents a very early step in the development of cardiovascular disease.10,11 In line with this concept, epidemiological data show that the risk of arterial hypertension12,13 and stroke7 is increased in offspring of mothers with preeclampsia.
Taken together our data demonstrate that in humans, a perinatal insult (transient perinatal hypoxia) as well as a fetal insult (preeclampsia) predispose the offspring to systemic vascular dysfunction and/or exaggerated hypoxic pulmonary hypertension later in life.5,14
Whether an earlier and/or shorter insult during gestation may have similar long-term consequences was not known.
Early embryonic insults; assisted reproductive technology (ART) causes premature vascular aging and arterial hypertension by an epigenetic mechanism
Assisted reproductive technology (ART) represents 5% of the births in industrialized countries.15 ART involves numerous manipulations of early embryos at a time when they may be particularly vulnerable to external disturbances. It, therefore, represents a unique model to study effects of environmental influences during the embryonic development on the individual’s susceptibility to disease later in life.
To this end, in 65 healthy ART children and 57 control children we assessed systemic and pulmonary vascular function. We found that flow-mediated dilation16 of the brachial artery (an early marker of endothelial dysfunction) was 25 percent smaller in ART than in control children. In contrast, endothelium-independent vasodilation was similar in the two groups, demonstrating that impaired flow-mediated dilation was caused by endothelial dysfunction in ART children. Carotid-femoral pulse wave velocity (a proxy of elastic arterial stiffness17) was significantly faster and carotid intima-media thickness (an early marker of atherosclerosis18,19) significantly greater in children conceived by ART than in controls. In addition, the systolic pulmonary-artery pressure at high altitude (3450 m) was 30 percent higher in ART than in control children19 (Fig. 3).
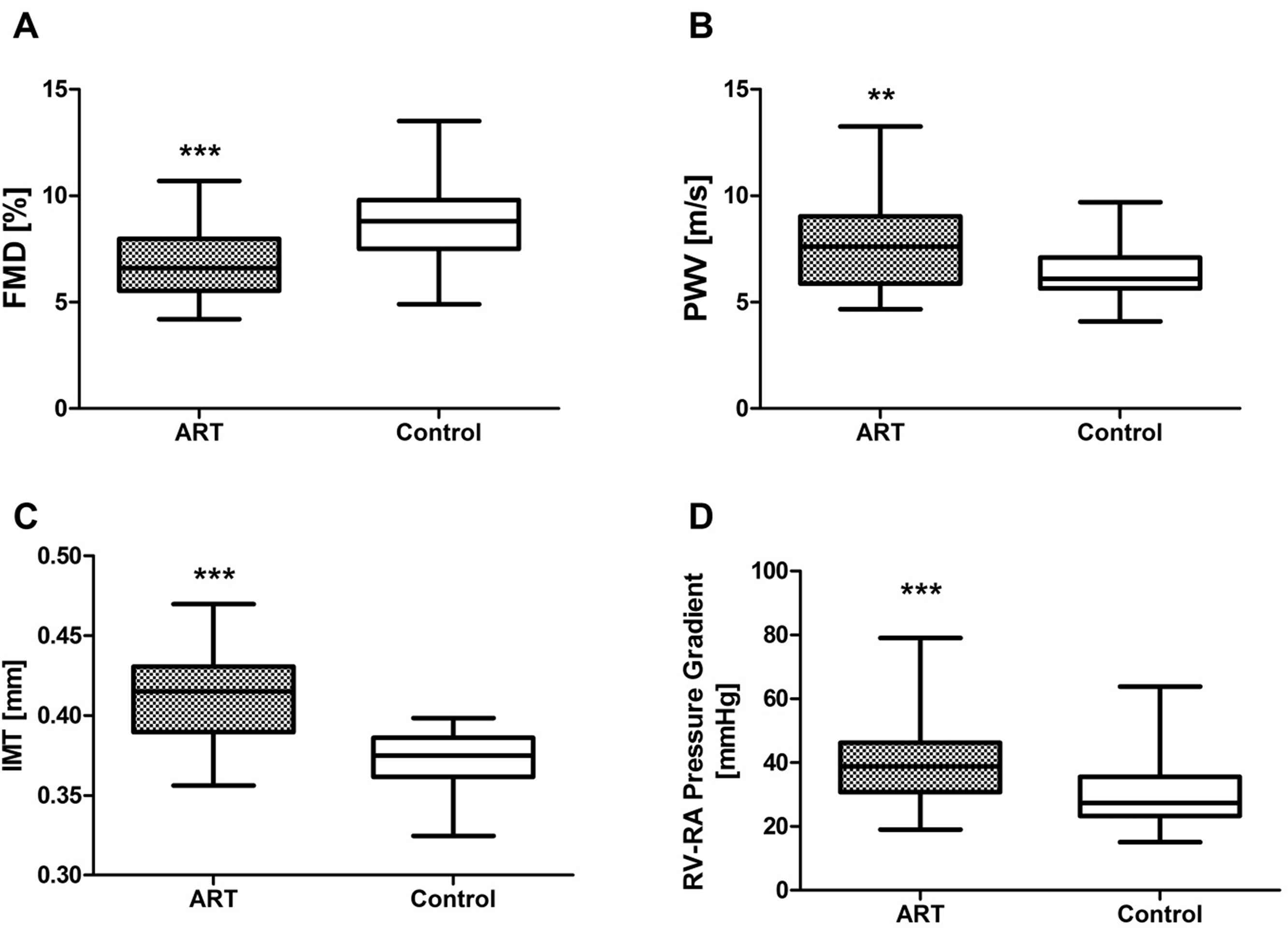
ART causes generalized vascular dysfunction in young apparently healthy children. Comparison of vascular function in 65 ART and 57 control children demonstrating decreased flow mediated dilation, FMD (A), increased pulse wave velocity, PWV (B), increased carotid intima media thickness, IMT (C) and increased systolic pulmonary-artery pressure at high altitude (D) in ART children; (**P < 0.01; ***P < 0.001 vs. controls) (modified from Scherrer et al., 2012).19
Interestingly, systemic and pulmonary vascular dysfunction was similar in children born after in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), and in children whose zygotes were kept frozen at the pronuclear stage for later transfer and those whose fresh embryos were transferred immediately.
Parent-related factors, such as hormonal stimulation to induce hyperovulation in the mother or infertility, could be responsible for long-term health problems in the offspring. This does not appear to be the case, since vascular function was normal in children who were conceived naturally following hormonal stimulation of the ovulation in the mother and vascular function in sterile and fertile parents was normal and comparable. There existed also no relationship between maternal age and vascular dysfunction in the progeny.
Most importantly, among 5 pairs of siblings, one being conceived by ART, the other naturally, flow-mediated dilation was significantly smaller and pulmonary-artery pressure was significantly higher in the siblings who were conceived by ART than in those who were conceived naturally.
Recent evidence suggests that ART not only causes premature vascular aging, but also alters cardiac function. Gratacos et al. showed that ART is associated with in utero cardiac remodeling that persists in 6 month old infants.20 We subsequently demonstrated that cardiac alterations persist into adolescence, as evidenced by larger right ventricular end-diastolic volume and diastolic dysfunction at high altitude.21
In summary, these studies show for the first time that in humans, ART per se causes marked premature alterations of the cardiovascular phenotype in the offspring that are detectable already during childhood and are expected to result in premature cardiovascular morbidity and mortality. In the pulmonary circulation, these alterations predispose to exaggerated hypoxic pulmonary hypertension already during childhood. With regard to the systemic circulation, information on hard cardiovascular endpoints (stroke, myocardial infarction, heart failure) is not yet available, given the young age of the ART population in humans (the first ART children was born in 1978). However, already now there is evidence that ART-induced altered vascular regulation leads to increased arterial blood pressure (and even established arterial hypertension) in adolescents.22 Moreover, systemic vascular dysfunction in ART children is of similar magnitude as the one described in offspring of mothers with preeclampsia and in children suffering from type 1 diabetes,14,23,24 two diseases associated with a well-known increase of premature cardiovascular morbidity and mortality.25
Epigenetic regulation of gene expression and fetal programming
Cardiovascular diseases are the main cause of mortality and morbidity in Western countries, but the underlying mechanisms are still poorly understood. Genetic polymorphisms, once thought to represent a major determinant of cardiovascular risk, individually and collectively, only explain a tiny fraction of phenotypic variation and disease risk in humans. It is now clear that epigenetic, i.e., a set of mechanisms that regulate gene expression without modifying the DNA sequence and that are sensitive to the environment can cause important alterations of the cardiovascular phenotype in experimental animal models and humans.
DNA methylation is the most extensively studied epigenetic regulatory mechanism. Methylation at the 5′ position of cytosine occurs in 60–90% of CpG dinucleotides within the vertebrate genome and is associated with stable variation in gene expression. Methylation of CpG-rich clusters, termed CpG islands, is generally associated with transcriptional repression, whereas hypomethylation is associated with transcriptional activation.26,27 Acting mainly on promoters, these covalent changes in DNA and histone structure affect the extent to which the transcription machinery is able to access specific regions of the DNA over extended periods of time.
Epigenetic regulatory mechanisms are characterized by 4 important features. First, environmental insults may cause dysmethylation of gene promoters and alter histone acetylation. Second, these epigenetic changes alter gene expression and, in turn, the synthesis of its bioproduct. Third, these modifications are maintained during cell division, may persist throughout the life span of the individual,28–30 and can be transmitted to the next generation, although the mechanism for epigenetic inheritance is not yet well understood. Forth, despite being generally very stable, epigenetic alterations are potentially reversible, for example by the administration of histone deacetylase inhibitors (HDI)31 (Fig. 4).
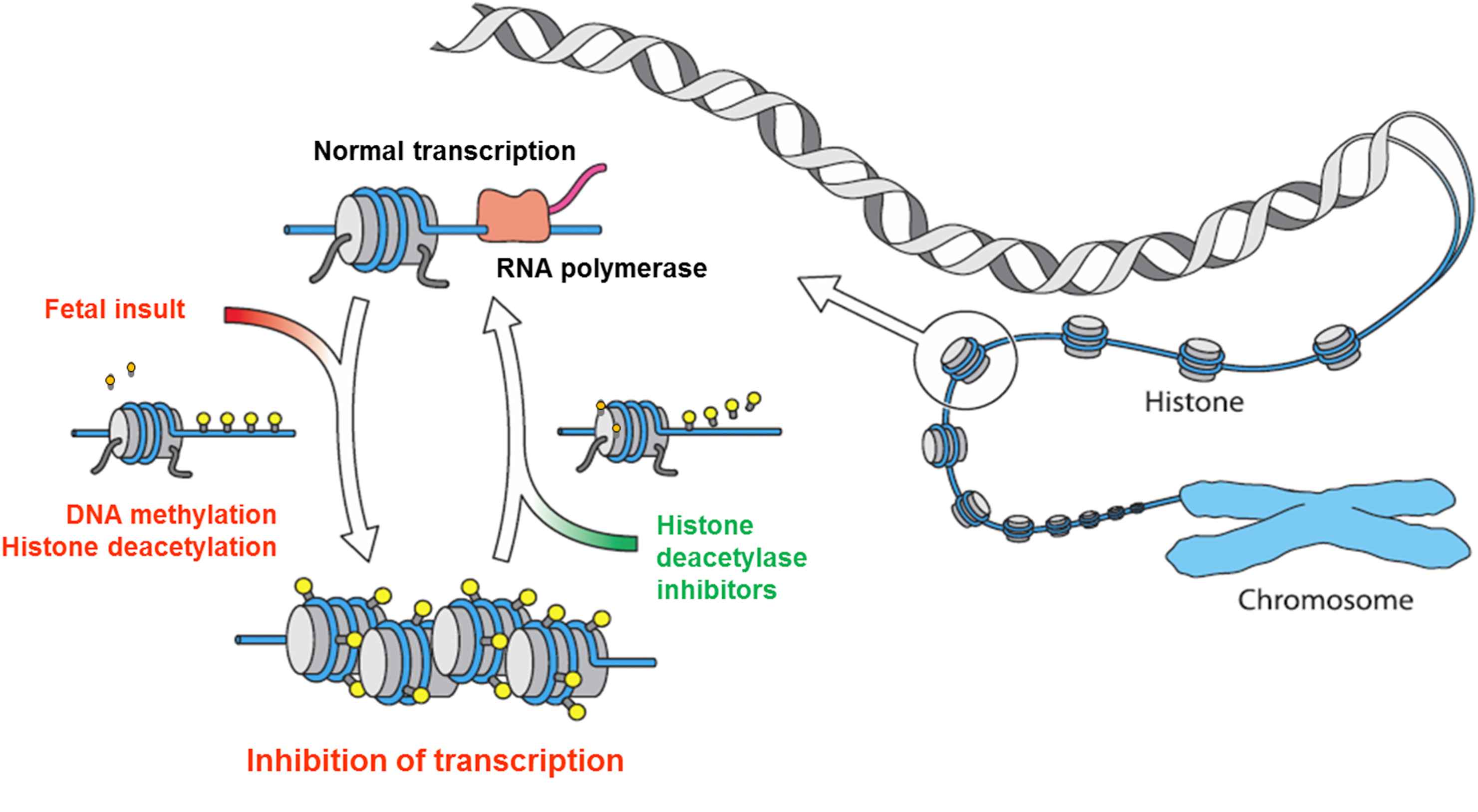
Fetal/perinatal insults induce epigenetic alterations (DNA methylation, histone deacetylation) that alter the transcription and function of the gene. Despite being stable and maintained during cell division throughout the life span, epigenetic alterations can be reversed by pharmacological agents (e.g. histone deacetylase inhibitors) resulting in normal transcription and function of the gene.
Importantly, periods that are particularly vulnerable to external disturbances (i.e. embryogenesis, the late fetal and the perinatal period) are characterized by very intense methylation activity. These periods are increasingly recognized as critical windows for fetal programming. Recent studies have provided evidence that epigenetic modifications of DNA may represent the link between early life exposition and a particular pattern of gene expression characterizing fetal programming.
Role of epigenetic mechanisms in ART-induced alteration of the cardiovascular phenotype
Owing to the difficulty of studying underlying mechanisms in healthy children, we turned our attention to ART mice. Consistent with our data in humans, we found that ART mice also display premature vascular aging and arterial hypertension. The fact that these observations were made in offspring of healthy and fertile mice further strengthens the concept that the alterations of the cardiovascular phenotype are caused by ART per se and are not related to other putative parental confounding factors.32
To test for epigenetic mechanisms, we studied a) the methylation of genes in the vasculature of ART and control mice; and b) assessed whether male ART mice transmitted the vascular dysmethylation and dysfunction to the next generation and whether HDI administration prevented this transmission. We found that the methylation of imprinted genes and of the promoter of the endothelial nitric oxide synthase (eNOS) gene was altered in arterial tissue of ART mice. Promoter DNA methylation regulates eNOS expression in endothelial cells in vitro.33 In line with this concept, eNOS expression in the carotid artery was decreased in ART mice compared to control animals. Decreased eNOS expression appeared to be of functional importance, because NOx plasma concentration was lower in ART than in control mice (Fig. 5).
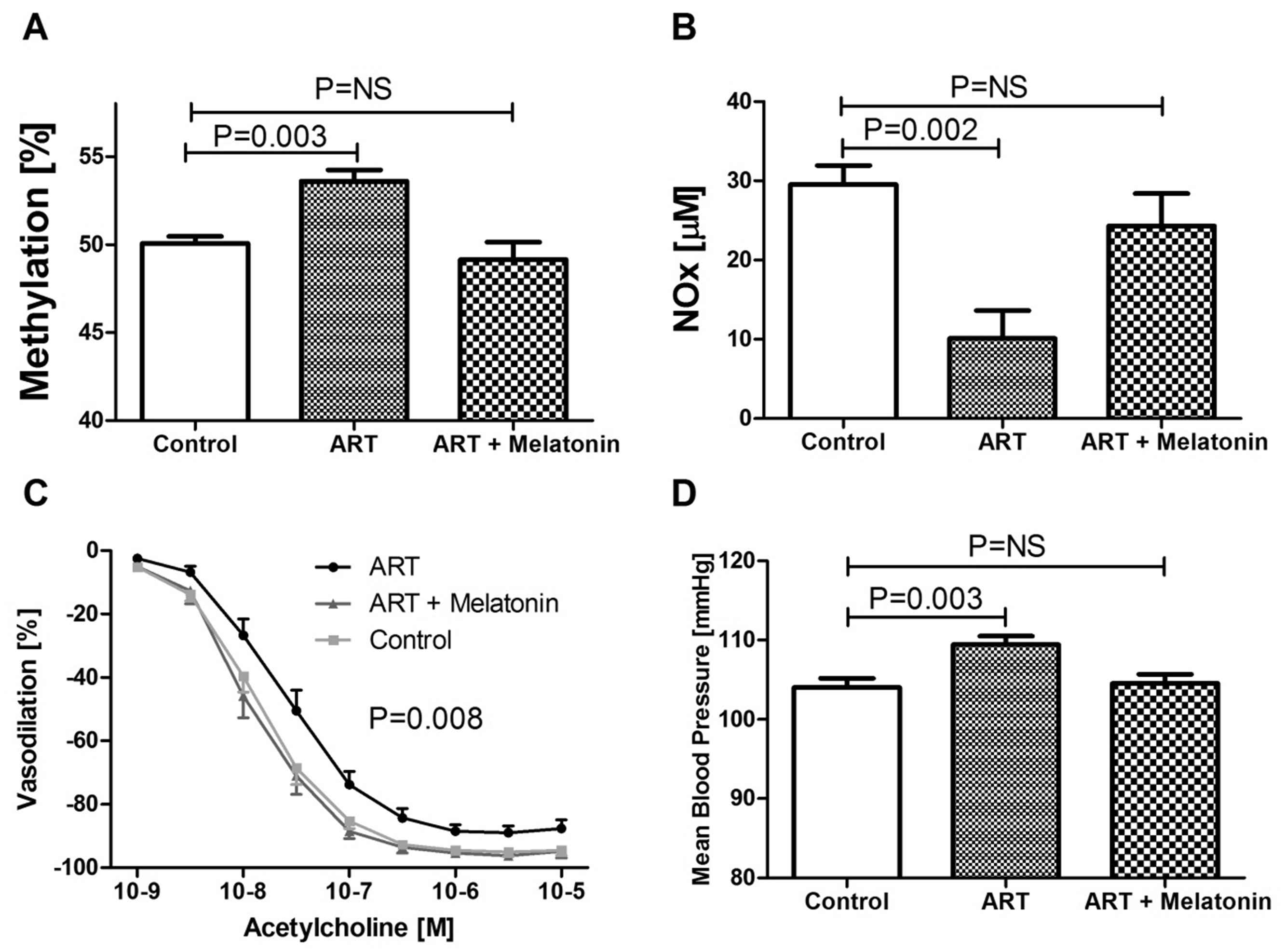
Addition of melatonin to the culture media prevents the alteration of the epigenetic and cardiovascular phenotype in ART mice. Effects of addition of melatonin on the methylation of the promoter of eNOS gene (A), plasma NOx bioavailability (B) and acetylcholine-induced vasodilation of the mesenteric artery (C) and arterial blood pressure (D) in ART mice (modified from Rexhaj et al., 2015).19,40
There was evidence for transgenerational transmission of these alterations of the epigenetic and cardiovascular phenotype, because vascular dysmethylation, endothelial dysfunction and arterial hypertension in offspring of male ART mice was comparable to the one observed in their fathers. In line with this concept, butyrate administration to male ART mice normalized the epigenetic and cardiovascular phenotype and prevented the transmission of the altered phenotype to the progeny.32
In summary, these data provide strong evidence that in mice, ART-induced alteration of the cardiovascular phenotype is mediated by an epigenetic mechanism involving altered methylation of the eNOS gene leading to decreased vascular expression and function of this major regulator of cardiovascular function in animals and humans.34,35
To find possible triggers for the epigenetic alterations in ART, we examined more in detail the in vitro fertilization and embryo culture processes. We investigated whether epigenetic alterations were dependent upon the duration of embryo culture. We found that reducing the time spent in the culture medium, by transferring embryos in the 2 cell stadium rather than as blastocysts did not have any detectable favorable effect on the cardiovascular phenotype of ART mice, suggesting that the noxious effects take place very early during the ART process. Thus, shortening of the culture time does not appear to offer advantages with regard to ART-induced alteration of the phenotype and other approaches are needed.
Melatonin administration to women has been shown to significantly improve the success rate of ART, but the mechanism is unknown.36 Interestingly, melatonin is involved in epigenetic regulation of gene expression37,38 and has antioxidant properties.39 We speculated that addition of melatonin to the culture media may have favorable effects on the cardiovascular phenotype in ART mice. We found that addition of melatonin normalized the methylation pattern in the preimplantation embryo, and prevented the altered methylation, expression and function of the eNOS gene in vascular tissue, and, in turn, prevented mesenteric artery endothelial dysfunction and arterial hypertension in adult ART mice40 (Fig. 5).
Epigenetic alteration of the eNOS synthesis predisposes ART-mice also to glucose intolerance and insulin-resistance
Studies in genetically engineered animals and in humans indicate that eNOS not only plays a key role for cardiovascular homeostasis, but also for the regulation of glucose homeostasis.41,34 In mice, eNOS knockout animals display insulin resistance and arterial hypertension, whereas eNOS heterozygous mice are predisposed to exaggerated high-fat diet (HFD)–induced insulin resistance and arterial hypertension.34,42 In eNOS-deficient mice, impairments of insulin stimulation of muscle blood flow and substrate delivery to skeletal muscle and stimulation of intrinsic skeletal muscle glucose uptake contribute to insulin resistance.34,42 We speculated that a similar mechanism contributes to insulin resistance in ART mice. We therefore assessed glucose tolerance, insulin sensitivity, and insulin stimulation of muscle blood flow in vivo together with intrinsic skeletal muscle glucose uptake in vitro in ART and control mice. To accentuate potential differences of glucose homeostasis between the two groups, assessments were performed in animals fed a normal chow (NC) or challenged with a HFD during 8 weeks.43
To characterize the effects of ART on glucose tolerance, we performed intraperitoneal glucose tolerance tests (IPGTTs). Figure 6 A shows that in NC-fed animals, glucose tolerance was similar in the two groups. HFD, as expected, impaired glucose tolerance in both groups. The important new point was that glucose intolerance was significantly more severe in ART than in control mice.
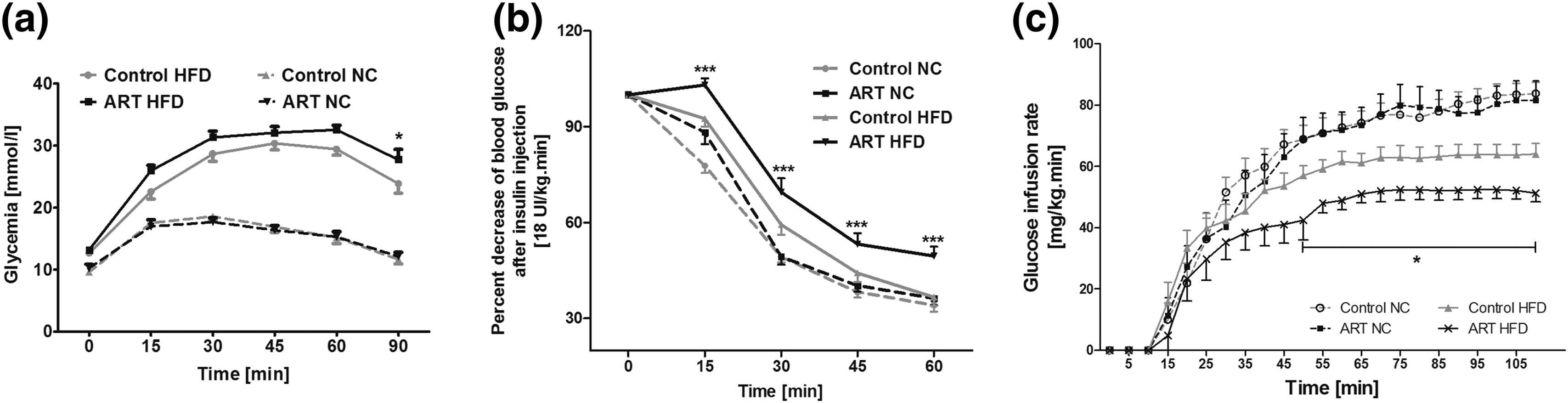
ART predisposes to glucose intolerance and insulin resistance in mice. Plasma glucose concentration during IPGTTs (a), decrease of the plasma glucose concentration during IPITTs (b), and GIRs during hyperinsulinemic euglycemic clamp studies (c) in 12-week-old male mice conceived by ARTs or naturally conceived control (Ctrl) mice fed with a NC diet or HFD for 8 weeks. Data represent mean ± SD for at least eight mice per group. (a) *P < 0.05 ART HFD vs control HFD. (b) ***P < 0.001 ART HFD vs all the others groups. (c) *P < 0.05 ART HFD vs all the others groups (modified from Cerny et al., 2017).43
To assess the effects of ART on insulin sensitivity, we performed intraperitoneal insulin tolerance tests (IPITTs) and hyperinsulinemic clamp studies. The insulin-induced decrease of plasma glucose concentration was comparable in NC-fed animals, but significantly smaller in ART than in control mice (Fig. 6B).
Similarly, while in NC-fed animals, the glucose infusion rate (GIR) necessary to maintain euglycemia during the steady-state phase of hyperinsulinemic euglycemic clamp studies was comparable in ART and control mice, when fed with a HFD, the GIR needed to maintain euglycemia was roughly 20% lower in ART than in control mice, demonstrating insulin-resistance in ART-mice compared to controls (Fig. 6C).
To test whether ART alters insulin stimulation of skeletal muscle perfusion and substrate delivery, we measured hindlimb muscle blood flow during clamp studies. In NC-fed animals, insulin stimulation of muscle blood flow was similar in the two groups, whereas in HFD-fed animals, this effect was significantly impaired in ART compared with control mice (Fig. 7A).
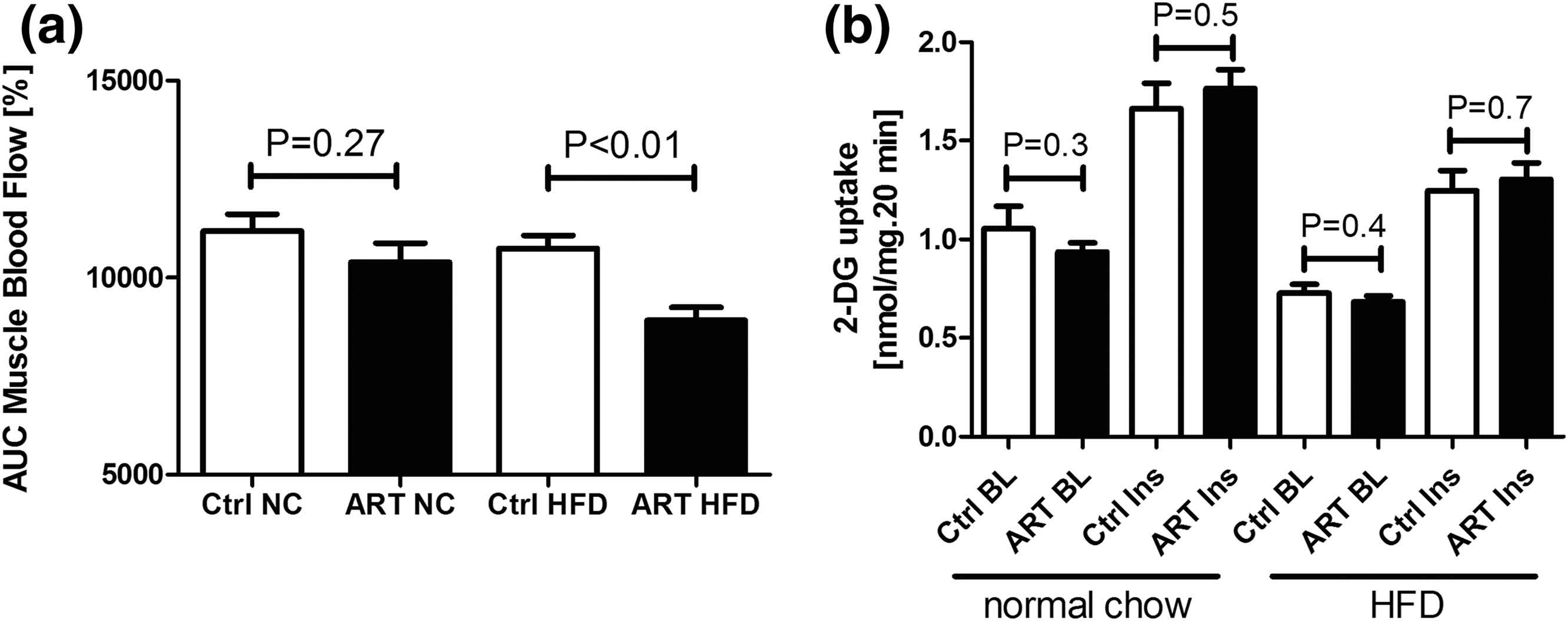
ART-induced insulin resistance is related to impaired insulin-mediated muscle blood flow and substrate delivery rather than impaired intrinsic skeletal muscle glucose uptake. AUC of insulin stimulation of muscle blood flow (a) during 90-min hyperinsulinemic euglycemic clamp studies. (b) Basal (BL) and insulin-stimulated (Ins) 2-deoxyglucose (2-DG) uptake in soleus muscle in vitro harvested from 12-week-old male mice conceived by ARTs or naturally conceived control (Ctrl) mice fed with a NC diet or HFD for 8 weeks. Data represent mean ± SD for at least seven mice per group (modified from Cerny et al., 2017).43
To study the effects of ART on muscle glucose uptake in the absence of confounding effects of muscle perfusion, we measured glucose uptake in isolated skeletal muscle preparations. The basal and insulin-stimulated intrinsic skeletal muscle glucose uptake in vitro was comparable in NC-fed and HFD-fed ART and control mice. Consistent with these results, NOS activity was significantly lower in ART mice than in control mice, whereas in skeletal muscle tissue NOS activity was comparable in all groups (Fig. 7B).43
Taken together these data demonstrate that, when fed a NC diet, ART mice had normal insulin-glucose homeostasis. When challenged with a HFD for 8 weeks, however, ART mice developed glucose intolerance, and insulin resistance. Insulin-resistance appeared to be primarily related to impaired endothelium-dependent insulin stimulation of blood flow and substrate delivery to skeletal muscle tissue rather than altered intrinsic skeletal muscle glucose uptake.
Altered glucose metabolism and insulin resistance have recently been reported in humans conceived by ART under energy balanced and HFD overfeeding conditions,44 suggesting that a similar mechanism may be operational in humans. Moreover, there is evidence for decreased vascular NO bioavailability in ART children.45 We speculate that, under a metabolic stress, such as the one represented by a Western-type diet, this alteration may facilitate the development of insulin resistance, glucose intolerance, and obesity in the human ART population.
Long-term consequences of ART-induced alteration of the cardiovascular and metabolic phenotype
The consequences of these epigenetic-induced vascular and metabolic alterations on long-term morbidity and mortality are unknown. Therefore, we compared the life-span of ART and control mice fed with normal chow or a high-fat diet. We found that when challenged with a high-fat diet, the lifespan of ART mice was roughly 25% shorter than in control mice32 (Fig. 8).
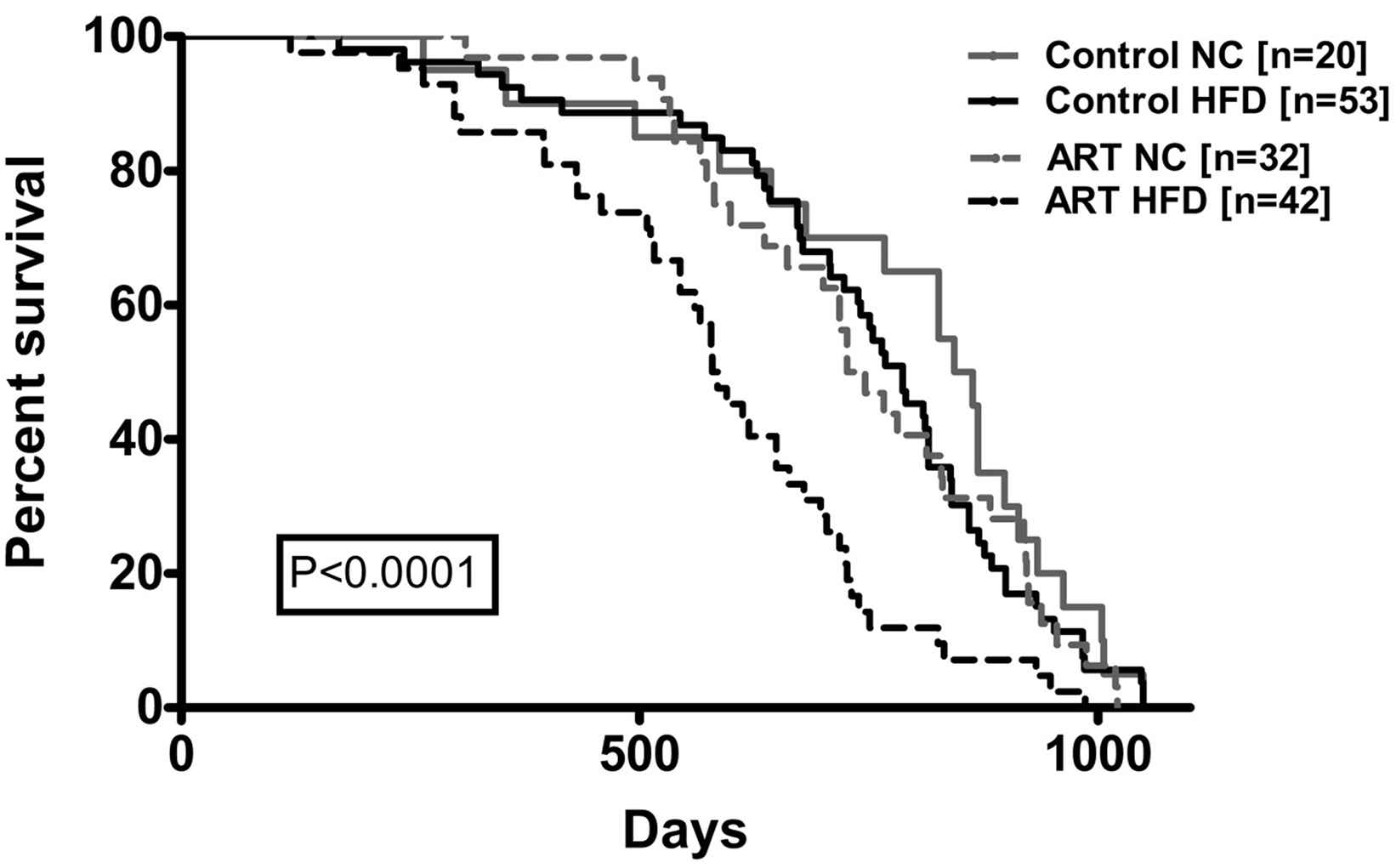
Marked shortening of the life span in ART mice challenged with a high fat diet. In ART mice, high fat diet resulted in a highly significant shortening of the life span by roughly 25% compared to control mice (modified from Rexhaj et al., 2013).32
Conclusion
Cardiovascular and metabolic diseases are the main cause of mortality and morbidity, but the underlying mechanisms are still poorly understood.46 Genetic variants associated with an altered cardiovascular phenotype in humans, individually and collectively, only explain a tiny fraction of phenotypic variation and disease risk. Phenotypic alterations that are unrelated to changes in DNA sequence may offer an attractive alternative explanation. Progress in reproductive medicine, obstetrics and neonatology has resulted in an increasing number of apparently healthy individuals born after having experienced unique environmental pressure in their early life. Abundant epidemiologic and increasing direct experimental evidence shows that this undeniable medical success comes at the cost of marked alterations of the phenotype resulting in premature morbidity and mortality.47 Here we provide evidence that pathological events occurring during three distinct critical periods (perinatal, fetal and embryonic) very early in life, cause similar major alterations of the cardiovascular and metabolic phenotype in experimental animal models and humans that increase cardiovascular risk later in life. Studies in animal models demonstrate that epigenetic alterations represent an important underlying mechanism linking these early life pathological events with the altered cardiovascular and metabolic phenotype and cardiovascular risk. New technologies that enable the rigorous assessment of epigenetic changes and their phenotypic consequences may provide the basis for explaining how fetal programming contributes importantly cardiovascular and metabolic morbidity and mortality in humans.
Last but not least, altered early life conditions appears to represent a new, significant but underestimated; cardiovascular risk factor. There is, therefore, an urgent need to recognize this concept; this may be particularly important for the increasing number of children born with ART or ART-related interventions (preimplantatory genetic diagnosis, social freezing).
Conflict of interest
The authors have nothing to disclose.
References
Cite this article
TY - JOUR AU - T.A. Meister AU - E. Rexhaj AU - S.F. Rimoldi AU - U. Scherrer AU - C. Sartori PY - 2017 DA - 2017/12/13 TI - Fetal programming and vascular dysfunction JO - Artery Research SP - 69 EP - 77 VL - 21 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.11.005 DO - 10.1016/j.artres.2017.11.005 ID - Meister2017 ER -
