A unified mechanism for the water hammer pulse and pulsus bisferiens in severe aortic regurgitation: Insights from wave intensity analysis
- DOI
- 10.1016/j.artres.2017.12.002How to use a DOI?
- Keywords
- Pulsatile hemodynamics; Arterial tonometry; Aortic regurgitation; Water hammer pulse; Pulsus bisferiens; Physical exam
- Abstract
The carotid bisferiens pulse and the radial water hammer pulse are typical of severe chronic aortic regurgitation. Little is known about the mechanism of these classic cardiovascular signs identified on physical examination. We report the first characterization of these abnormal pulse patterns using wave intensity analysis (WIA) in a patient with severe aortic regurgitation. We demonstrate that an abnormally pronounced forward-traveling mid-systolic suction wave, which immediately followed the initial forward-traveling compression wave from ventricular contraction, explained these pulse patterns. This suction wave likely resulted from blood inertia, arising from a ventricle ejecting a very large stroke volume into a vasodilated arterial tree. Our report demonstrates a novel pulsatile hemodynamic mechanism that unifies the pathogenesis of the bisferiens pulse and the water-hammer pulse in severe aortic regurgitation.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
The bisferiens pulse in severe aortic regurgitation has been attributed to a Venturi effect that occurs in the ascending aorta in mid-systole due to the high flow produced by ventricular ejection.1 The water hammer pulse, characterized by a rapid upstroke and a sharp descent, is typical of severe chronic aortic regurgitation. Little is known about the mechanism of this classic cardiovascular sign identified on physical examination. Corrigan explained it by a sudden fall in the diastolic pressure in the arteries as a result of “arterial emptying” in diastole due to regurgitation of blood into the left ventricle.2,3 However, as pointed out long ago from the analysis of Sphygmograph recordings,3 the water hammer pulse is characterized by a sudden narrow peak in early systole followed by a sharp descent in mid-systole (i.e, during ventricular ejection) and a rather flat diastolic portion.3 Therefore, the “water hammer” is a systolic phenomenon which cannot be directly explained by the diastolic aortic regurgitant flow nor by continuous forward emptying to vasodilated distal microvascular beds. We report the first characterization of underlying pulsatile carotid and radial pulses using wave intensity analysis (WIA), in a patient with severe aortic regurgitation.
A 51 year-old male with a history of severe chronic aortic valve regurgitation from a previous episode of bacterial endocarditis with valvular perforation, presented with decompensated heart failure. Physical exam findings included a diastolic murmur that rapidly decreased in intensity in mid-diastole, as well as increased amplitudes of his carotid and peripheral pulses. His carotid pulse demonstrated two systolic peaks (pulsus bisferiens; Fig. 1A), whereas his radial pulse (Fig. 1D) demonstrated a sudden high-amplitude upstroke and descent (“water hammer” pulse). His echocardiogram was consistent with severe aortic regurgitation without stenosis, and demonstrated uninterrupted aortic systolic outflow.
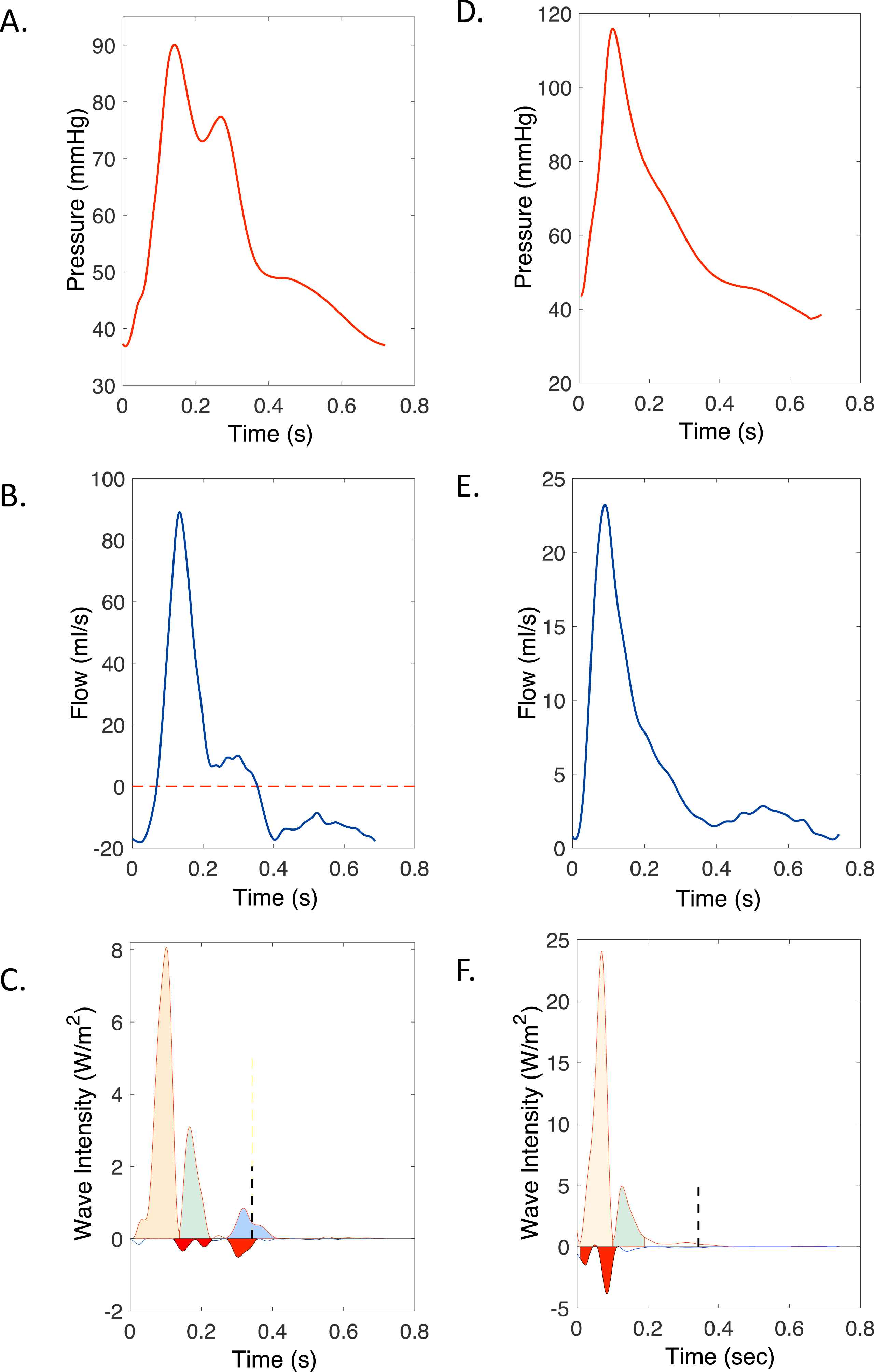
Carotid (A–C) and radial (D–F) pressure (top panels, A and D), flow (middle-panels, B and E) and wave intensity (C and F). In C and F, forward-traveling waves are plotted as positive intensity, whereas backward-traveling waves are plotted as negative intensity. The vertical dashed lines in C and F mark the duration of ventricular ejection after correcting for wave transmission delays to the respective measurement sites. The carotid pulse is bisferiens, due to an abnormal forward-traveling suction wave in mid-systole (green area in C), which follows the normal forward compression wave (orange area) generated by ventricular contraction. The radial pulse (D) has a “water hammer” configuration, with a sharp upstroke and downstroke. As demonstrated by WIA (F), the mid-systolic expansion wave transmitted from the central circulation to the radial artery (green area) is responsible for the sudden radial pressure descent after its initial peak, resulting in its “water hammer” configuration. Note that the pressure drop occurs well within systole. The red areas in the carotid and radial wave intensity represent wave reflections from the head and hand, respectively. (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article.)
We assessed the pulsatile hemodynamic phenomena underlying our patient’s pulse patterns via WIA, a novel technique applied to arterial hemodynamics, in which wavefronts are assessed with simultaneous measurements of local pressure and flow (Fig. 1) in order to determine the intensity, direction (forward vs. backward) and type (compression vs. suction) of waves traveling in arteries.4 WIA is a time-domain method in which waves are regarded as small wave incremental fronts. Measured arterial waveforms are decomposed into successive wavefronts, which act to increase or decrease pressure or flow, and travel either forward or backward. Waves can be characterized as forward or backward-traveling of the compression or suction type, based on their simultaneous effect on pressure and flow. Forward-traveling waves change pressure and flow in the same direction (increase or decrease), whereas backward-traveling waves change pressure and flow opposite directions (either increase pressure and decrease flow, or decrease pressure and increase flow). Compression waves increase pressure, whereas suction waves decrease pressure. Therefore: (a) forward-traveling compression waves increase pressure and flow; (b) forward-traveling suction waves decrease pressure and flow; (c) backward-traveling compression waves increase pressure and decrease flow; (d) backward-traveling suction waves decrease pressure and increase flow.
Doppler ultrasound and arterial applanation tonometry allow for the measurement of time-resolved flow velocity and pressure waveforms,5 which can be obtained at the same location (such as peripheral arteries) for the assessment of forward- and backward-traveling waves via WIA.6 High-fidelity carotid and radial applanation tonometry and pulse-waved Doppler interrogation of flow velocities in both arteries were performed in the supine position, in immediate sequence at each arterial site (carotid and radial). Radial recordings were done in the extended upper extremity, with the artery at approximately the level of the heart.
Carotid WIA (Fig. 1C) demonstrated an early systolic forward-traveling compression wave from ventricular ejection (orange area) followed by a prominent, highly abnormal forward-traveling mid-systolic suction wave (green area). A small forward-traveling end-systolic suction wave (blue area) was present, as normally observed. However, in contrast to the normal wave intensity pattern, in which wavefronts beyond the duration of ventricular ejection are totally absent, the patient demonstrated an early diastolic forward-traveling suction wave (blue area), consistent with forward transmission of early diastolic ventricular suction from ventricular relaxation as a result of severe valve incompetence.
Radial WIA demonstrated a forward-traveling mid-systolic suction wave (Fig. 1F), which immediately followed the initial forward-traveling compression wave from ventricular contraction, and was responsible for the sudden decrease in radial pressure after its initial peak, leading to the “water hammer” pulse. Therefore, our patient’s bisferiens pulse and water hammer pulse seem to have been associated with the same hemodynamic phenomenon: a mid-systolic suction wave traveling forward in both the carotid and radial arteries. The aortic valve was not stenotic, implying that it is unlikely that the observed effect can be explained by a true Venturi effect (which is associated with local fluid acceleration due to a local narrowing). A plausible explanation, however, is that the deceleration wave is the result of blood inertia, arising from a ventricle ejecting a very large stroke volume into a vaso-dilated arterial tree with a very low peripheral resistance. In these conditions, the buffer function of the aorta is not sufficiently recruited, leading to large blood volume displacements and associated inertial forces.
In summary, our report demonstrates a novel pulsatile hemodynamic mechanism that unifies the pathogenesis of the bisferiens pulse and the water-hammer pulse in severe aortic regurgitation.
- WIA
wave intensity analysis
Conflict of interest statement
J.A.C. has received consulting honoraria from Bristol-Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Ironwood Pharmaceuticals, Sanifit, Vital Labs and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb, Microsoft and CVRx Inc., and device loans from AtCor Medical. J.A.C. is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. Other authors have no disclosures.
Funding information
JAC is supported by
References
Cite this article
TY - JOUR AU - Julio A. Chirinos AU - Scott R. Akers AU - Jan A. Vierendeels AU - Patrick Segers PY - 2017 DA - 2017/12/21 TI - A unified mechanism for the water hammer pulse and pulsus bisferiens in severe aortic regurgitation: Insights from wave intensity analysis JO - Artery Research SP - 9 EP - 12 VL - 21 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.12.002 DO - 10.1016/j.artres.2017.12.002 ID - Chirinos2017 ER -
