Gut microbiota and vascular biomarkers in patients without clinical cardiovascular diseases
Russian Clinical Research Center for Gerontology, Pirogov Russian National Research Medical University, bld. 16, 1st Leonova Street, Moscow, 129226, Russian Federation.
Russian Clinical Research Center for Gerontology, bld. 16, 1st 15 Leonova Street, Moscow, 129226, Russian Federation.
Federal State Budgetary Establishment Endocrinology Research Centre, Moscow.
Laboratory of Bioinformatics, Scientific Research Institute for Physical-Chemical Medicine, bld. 1a, Malaya Pirogovskaya St, Moscow, 119435, Russian Federation.
Laboratory of Bioinformatics, Scientific Research Institute for Physical-Chemical Medicine, bld. 1a, Malaya Pirogovskaya St, 119435, Moscow, Russian Federation.
Department of Cardiology and Personified Medicine, RUDN-University, 6 Miklukho-Maklaya St., Moscow, Russian Federation, Laboratory of Cardiovascular Ageing, Russian Clinical Research Center for Gerontology, Pirogov Russian National Research Medical University, bld. 16, 1st Leonova Street, Moscow, 129226, Russian Federation.
“Research of Age and Age-associated Conditions” Department, National Research Centre for Preventive Medicine, bld. 10, Petroverigskiy Lane, Moscow, 101000, Russian Federation.
Department of Cardiology and Molecular Genetics, National Research Centre for Preventive Medicine, bld. 10, Petroverigskiy Lane, Moscow, 101000, Russian Federation.
- DOI
- 10.1016/j.artres.2017.02.007How to use a DOI?
- Keywords
- Gut microbiota; Arterial stiffness; Atherosclerosis; Low-grade inflammation
- Abstract
The aim of this research was to study the association between the gut microbiota composition and arterial wall properties. The study included 92 participants, men and women aged 25–76 years old without clinical manifestation of chronic diseases but with the possible presence of cardiovascular risk factors. Carbohydrate metabolism examination, duplex scanning of the carotid arteries with the measurement of the intima-media thickness (IMT), the carotid-femoral pulse wave velocity (PWV) measurement, and 16S rRNA (V3–V4 regions) sequencing of the gut microbiota were performed in all participants. Higher Serratia abundance was associated with the increased IMT and CRP levels. Blautia representation was associated with IMT. Higher Bacteroides representation was associated with higher pulse wave velocity in non-diabetic subjects. Although this study had some limitations, we have demonstrated that the composition of the gut microbiota was associated both with atherosclerotic and arterial stiffness markers.
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality and morbidity globally. The principal underlying mechanisms of CVD are the arterial wall stiffness and atherosclerosis.1 The key markers of these processes are the increased pulse wave velocity (PWV), the intima-media thickening, vascular stenosis and the presence of atherosclerotic plaques. Despite the obvious relevance of this issue and a huge amount of funding and research, the cause of the vascular wall damage is still the subject of debate. To date the gut microbiota is considered to be a new player in the pathophysiology of vascular wall changes. Microorganisms that inhabit us have a huge metabolic potential, the number of genes in metagenome exceeds the number of genes in the human genome. Microbiota affects the immune system, inflammatory processes and may either suppress or aggravate low-grade inflammation2 underlying the atherogenesis and other disorders.
Karlsson et al. in 2012 published a study that showed the differences of the gut microbiota in healthy people and in patients with symptomatic atherosclerosis. Greater abundance of Collinsella genus was more typical to the clinical group and Eubacterium, Roseburia and Bacteroides – for healthy subjects. Metagenome of patients with clinical atherosclerosis contained many genes involved in the peptidoglycan synthesis regulation which may be one of the causes of low-grade inflammation, while metagenome of healthy subjects contained more genes responsible for the anti-inflammatory agents and antioxidants production.3
In 2014 Jill Gregory et al. conducted an interesting experiment, they transplanted the gut microbiota from donor mice with atherosclerosis and without atherosclerosis to the recipient mice with the apolipoprotein E deficiency (predisposed to atherosclerosis) and with the gut microbiota suppressed by antimicrobial drug therapy. In mice, which were transplanted with feces from atherosclerotic mice, atherogenesis was much more pronounced than in the second group. Thus, during the transplantation mice were “infected” with atheroclerosis.4
Almost all studies that investigated the relationship between the blood vessels and gut microbiota were devoted to the atherosclerotic changes and occasionally to the arterial stiffness. Rossi et al. found that some of bacteria metabolites were associated with the PWV increase and were the “cardiovascular toxins”,5 afterward these data have been confirmed by Gulhan et al.6 However, there were no sequencing of the gut microbiota and metabolites were measured precisely (not as part of metabolome) in these studies.
In the present study, we have first examined the relationship between the gut microbiota composition and the markers of both athero- and arteriosclerosis.
Aim
To study the association between the gut microbiota composition and arterial wall properties in patients without clinical manifestation of cardiovascular disease.
Materials and methods
Patients from Moscow and Moscow Region (the Caucasian race) aged from 25 to 76 years old who had passed the preventive outpatient examination in the FGBI National Research Center for Preventive Medicine (Moscow) were included in the cross-sectional study.
The inclusion criteria were as follows:
Men and women over 25 without clinical manifestations of cardiovascular diseases (but with the possible presence of cardiovascular risk factors) and other noncommunicable diseases were included in the study. Participants were not treated with any medicine and signed informed consent to participate in the study.
The exclusion criteria were as follows:
Clinically evident atherosclerosis (coronary artery disease including history of myocardial infarction, stable or unstable angina, cerebrovascular disease including stroke, intermittent claudication, etc.) or any cardiovascular diseases (e.g valvular heart disease). Regular intake of any drug (including antibiotics (antimicrobial drugs) during the last 3 months). Type 1 diabetes and other specific types of diabetes, severe diabetic microangiopathy, a history of type 2 diabetes (except new-onset type 2 diabetes revealed by oral glucose tolerance test during the screening to this study). The presence of chronic liver and kidney disease, cancer, pregnancy, lactation, anemia (moderate and severe), infectious diseases, acute and chronic gastrointestinal diseases, abdominal surgery, diagnosed lactase deficiency, diagnosed allergic reaction to any type of food, history of organ transplantation, the oral cavity and dental diseases, refusal to participate in the study.
Ethical aspects
All the patients signed a legal informed consent form to participate in the study. The study protocol was approved by the local ethics committee FGBI Ministry of Healthcare, Russian Federation, minutes of the LEC, meeting number #8, 29 November 2011. Patient data privacy had been provided using the code identification numbers to correlate with patient records in the computer files.
Patients screening
All the patients underwent a careful clinical assessment during the screening. This baseline assessment included medical history, physical examination, height and weight measurements to calculate BMI, systolic and diastolic blood pressure measurement, complete blood count, biochemistry tests, urinalysis, 12-lead full-disclosure ECG recording (Schiller Cardiovit AT-10), the Bruce Treadmill Test (Intertrack, Schiller), specialists consultation (GP, cardiologist, stomatologist). Blood chemistry included glucose, creatinin, urea, uric acid, potassium, sodium, AST, ALT, bilirubin, total cholesterol (TC), triglycerides (TG), high and low density lipoproteins (HDL, LDL), and thyroid-stimulating hormone levels measurement. Persons with abnormalities in the blood tests, electrocardiogram, etc. were excluded from the study.
We also assessed the cardiovascular risks. They were as follows:
- •
Stage 1 arterial hypertension (systolic/diastolic BP level of 140/90–159/99 mmHg). Patients with stage 2 and 3 arterial hypertension (160/100 mmHg and higher) and hypertension that required antihypertensive treatment were excluded from the study;
- •
Dyslipidemia (total cholesterol>5.0 mmol/l and/or LDL>3.0 mmol/l and/or HDL<1.0 mmol/l for men and<1.2 mmol/l for women and/or triglycerides>1.7 mmol/l);
- •
Obesity (BMI≥30 kg/m2 and/or waist circumference ≥94 cm for men and ≥80 cm for women);
- •
Smoking (regardless of the number cigarettes and years of smoking);
- •
Fasting hyperglycemia (fasting glucose in venous blood plasma ≥6.1 and <7.0 mmol/l) and/or impaired glucose tolerance (IGT) (fasting venous plasma glucose<7 mmol/l, 2 h after OGTT≥7.8 and < 11.1 mg/dL) or new onset DM2 (fasting glucose ≥7 mmol/l or ≥ 11.1 mmol/l 2 h after the OGTT).
If any exclusion criteria were met, participants were excluded from the study at any time. This group was also described in earlier publication focused on diet and glucose metabolism.7
Basic methods
Assessment of the blood vessels
Subclinical atherosclerosis assessment was carried out by using the Q-LAB special application program (Philips, Eindhoven, The Netherlands) for duplex scanning of extracranial brachiocephalic arteries in B-mode with parallel ECG recording. The standards proposed by the experts of the European Society of Hypertension and the European Society of Cardiology (2003) were used to assess CCA IMT. IMT<0.9 mm was considered normal; increased thickness was 0.9–1.3 mm. IMT thickening of CCA>1.3 mm or a local increase in IMT of 0.5 mm or a 50% increase in nearby IMT was defined as atherosclerosis. Local IMT thickening>1. mm, which caused stenosis of the lumen but did not affect its internal anatomy, was considered as a plaque.8
PWV measurement was performed by using the SphygmoCor device (AtCorMedical, Australia). Pulse waves were recorded consistently with high precision applanation tonometer, which was superimposed on the proximal (carotid) artery and then, with a short interval, on the distal (femoral) artery with a minimum recording time of 10 cardiac cycles. The transit time was determined from the waveforms by using the intersecting tangent method. The path length was calculated by subtracting the distance between the carotid artery measurement site and sternal notch (carotid-notch), from the distance between the femoral artery site and the sternal notch (femoral-notch), all measured directly with a tape measure9:
ECG was simultaneously recorded. PWV value of 10 m/s and higher was considered as increased for all ages.9
Low-grade inflammation
Highly sensitive C-reactive protein (CRP) concentration was measured by using Sapphire-400 analyser, immunoturbidimetry method.
Assessment of the gut microbiota composition
The collected stool samples (2 ml) were frozen and stored at −20 °C and then thawed; the DNA was extracted from each sample; sequencing of the variable V3–V4 16S rRNA gene regions was performed (after the total DNA isolation and library preparation) by using an MiSeq Reagent Kit v2 (300 cycles) and MiSDefault (Illumina, San Diego, CA, USA) device according to the manufacturer’s recommendations.
Sequencing library preparation
The sequencing libraries were prepared using ‘16S Metagenomic Sequencing Library Preparation: Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System’ protocol (Part Number 15044223 Rev. B, http://ngs.biodiv.tw/NGSCore/wp-content/uploads/application%20forms/16s-metagenomic-library-prep-guide-15044223-b.pdf) using the Nextera XT Index Kit (Illumina) with a dual indexing strategy.
Bioinformatic processing
Quality reads filtering and taxonomic classification were performed using QIIME Software.10 Taxonomic composition of the samples was evaluated using reference-based approach according to the database of 16S rRNA gene sequences Greengenes v. 13.5 (http://greengenes.secondgenome.com/downloads/database/13_50 using RDP Classifier). As a result of the classification, read counts of operational taxonomic units (OTU, taxonomic unit classified to the genus, species or strain, determined by the 16S rRNA gene homology) were; the classifier output was transformed to the form of the OTU and genus relative abundance matrices. All statistical analyses were performed in R programming language (version 3.1.0). Statistical comparison of the groups of samples was performed using Mann–Whitney test (corrected for multiple comparisons using Benjamini–Hochberg method) and generalized linear models.11
UniFrac dissimilarity metric12 was used for the construction of multidimensional scaling (MDS) plots; ggplot2 package was used for the illustrations.
The horizontal line in the box-and-whisker plots marks the median; the rectangle lower and upper bounds represent the first and third quartiles respectively; ‘whiskers’ correspond to the distance between quartiles multiplied by 1.5. The values beyond the ‘whiskers’ are considered dropouts and are marked as points.
Results
The study included 92 subjects aged 25–76 years, the proportion of men was 29% (n = 28), women – 71% (n = 69). The average age was 52 ± 13 years. The most common risk factor was dyslipidemia identified in 85% of participants, abdominal obesity was diagnosed in 58%, hypertension 1° was detected in 37%, the new onset of type 2 diabetes was found in 23%. The last group was described in our previous publication.7
- 1.
Fecal microbiota analysis
An average of 1 02 582 ± 46 284 16S rRNA reads per sample was generated using Illumina MiSeq sequencing. The short- and low-quality reads (QV < 20), were removed and 1 02 581 ± 39 210 high quality reads (87 ± 2% of the initial amount) were included in the analysis. Out of those, 87.40 ± 7.4% was classified and 97.41 ± 0.9% was classified to the genus level. The dominant bacteria in all the samples were Bacteroidetes (12.7 ± 9.86%) and Firmicutes (57.09 ± 13.6%). About 50% of the total microbial abundance was represented by five genera: Blautia, Bacteroides, Prevotella, Faecalibacterium, and Clostridium. Blautia was the most dominant genus.
Remarkably, the composition of the gut microbiota was quite rich, alpha diversity index was higher than the average in studies in Western countries (Fig. 1).13,14 Average Shannon index was 3.71 ± 0.56.
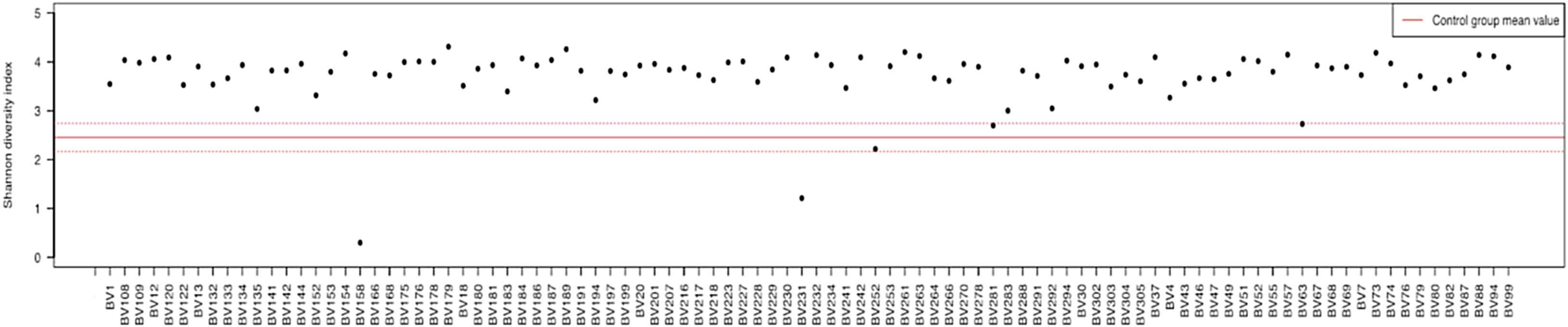
Diversity of the gut microbiota, Shanon (alpha diversity) index. Red line is the average alpha diversity±standard deviation in the Turnbaugh et al. study.14 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Multidimensional scaling chart depicting genera abundance is representing in Fig. 2.
- 2.
The gut microbiota composition and low-grade inflammation
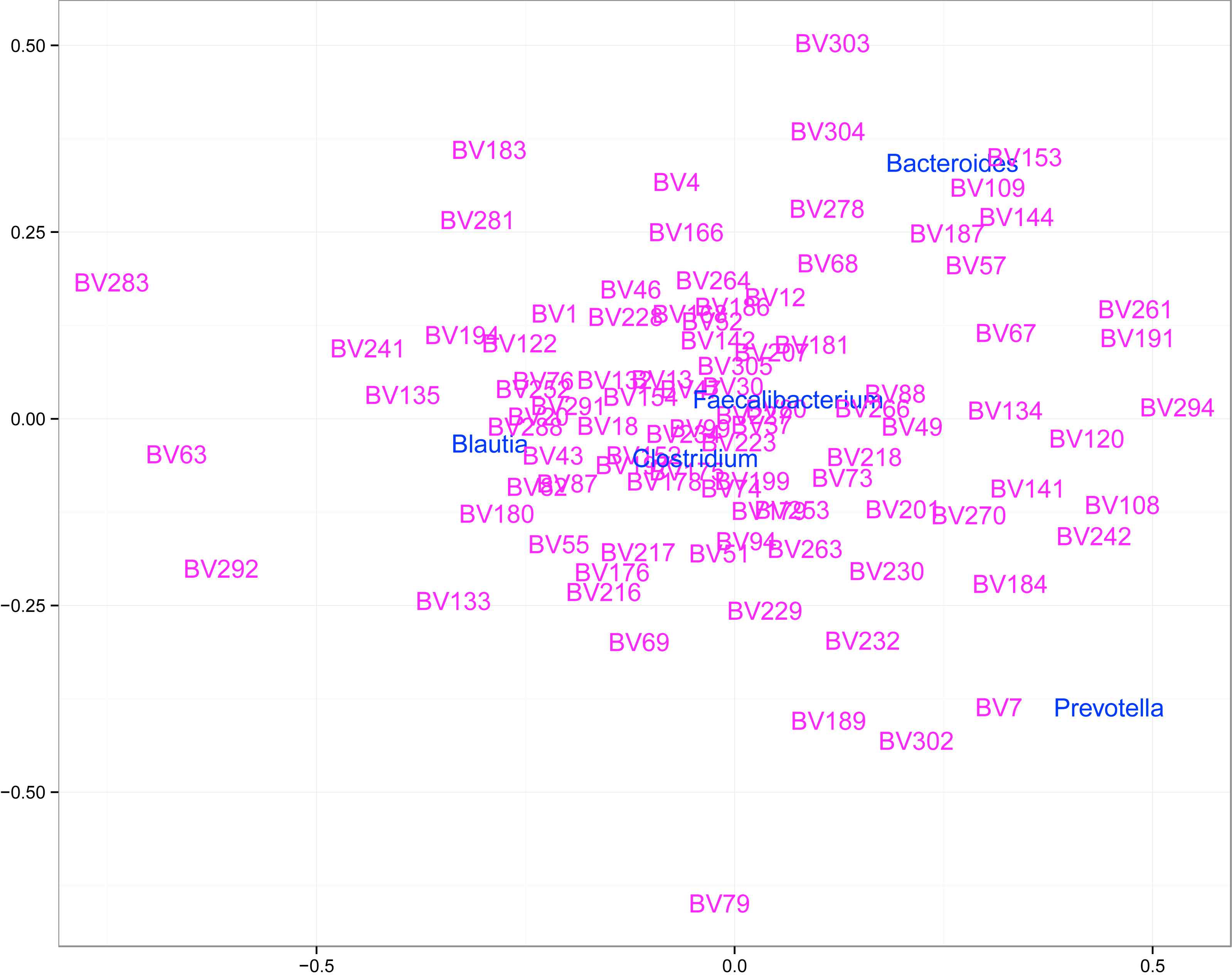
Multidimensional scaling chart of the samples depicting abundance of genera which comprised 50% of all units. The closer sample to the genus name, the higher abundance of this genus is in the sample.
The average CRP level among the participants was 3.50 ± 4.39 mg/l. High CRP levels were positively associated with Serratia (Fig. 3, p = 0.0001) genus. CRP level was ≥10 pg/ml in 24 participants.
- 3.
The gut microbiota composition and arterial wall properties
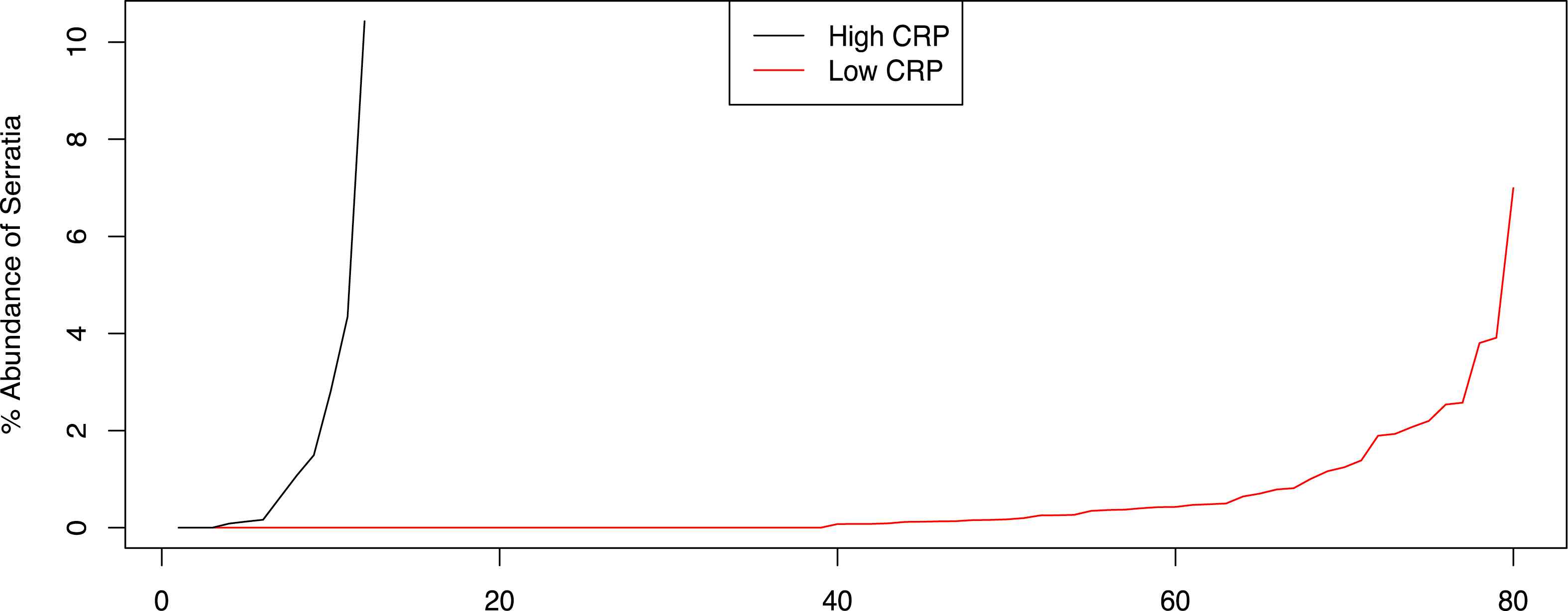
High CRP levels were positively associated with Serratia genus.
The average value of IMT was 0.76 ± 0.20 mm. IMT≥0.9 mm was diagnosed in 20 patients. The characteristics of patients with and without intima-media thickening are described in Table 1.
| Characteristic | IMT<0.9 mm | IMT≥0.9 mm | p | PWV<10 m/s | PWV≥10 m/s | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | St. deviation | Average | St. deviation | Average | St.deviation | Average | St. deviation | |||
| Age (years) | 49.92 | 12.14 | 62.75 | 6.68 | <0.001 | 50.07 | 12.32 | 59.26 | 10.02 | <0.001 |
| SBP (mmHg) | 123.77 | 15.90 | 136.95 | 16.09 | 0.0029 | 124.34 | 16.07 | 133.11 | 16.68 | 0.0274 |
| DBP (mmHg) | 77.66 | 9.76 | 79.75 | 8.76 | 0.3673 | 77.07 | 10.38 | 80.57 | 6.39 | 0.0570 |
| BMI (kg/m2) | 27.16 | 5.41 | 28.19 | 5.27 | 0.4505 | 27.02 | 5.00 | 28.69 | 6.03 | 0.2204 |
| Waist/hips ratio (sm) | 0.84 | 0.09 | 0.78 | 0.42 | 0.5077 | 0.84 | 0.09 | 0.81 | 0.37 | 0.6494 |
| IL-6 (pg/ml) | 11.02 | 25.85 | 5.33 | 5.16 | 0.0878 | 9.79 | 24.53 | 10.42 | 19.66 | 0.8989 |
| SCORE | 1.57 | 2.81 | 3.80 | 3.39 | 0.0123 | 133 | 1.68 | 3.92 | 4.63 | 0.0098 |
| Urea (mmol/l) | 5.09 | 1.58 | 635 | 2.33 | 0.0332 | 5.02 | 1.68 | 6.26 | 1.95 | 0.0068 |
| Fasting glucose | 5.48 | 1.26 | 6.75 | 2.01 | 0.0132 | 5.44 | 1.22 | 6.56 | 1.93 | 0.0103 |
| CRP (mg/l) | 2.96 | 2.74 | 5.44 | 7.71 | 0.1729 | 2.67 | 1.71 | 5.58 | 7.49 | 0.0611 |
| HbA1c (%) | 5.287 | 0.966 | 6.10 | 1.13 | 0.0069 | 5.23 | 0.72 | 6.04 | 1.46 | 0.0114 |
Comparative characteristics of participants with different IMT and PWV values.
The higher Blautia (p = 0.004) and Serratia (p = 0.009) representations were (Fig. 4), the greater the intima-media thickness was. The average value of the stenosis was 20 ± 20.12%. The carotid artery stenosis was directly associated with high abundance of Serratia (Table 2).
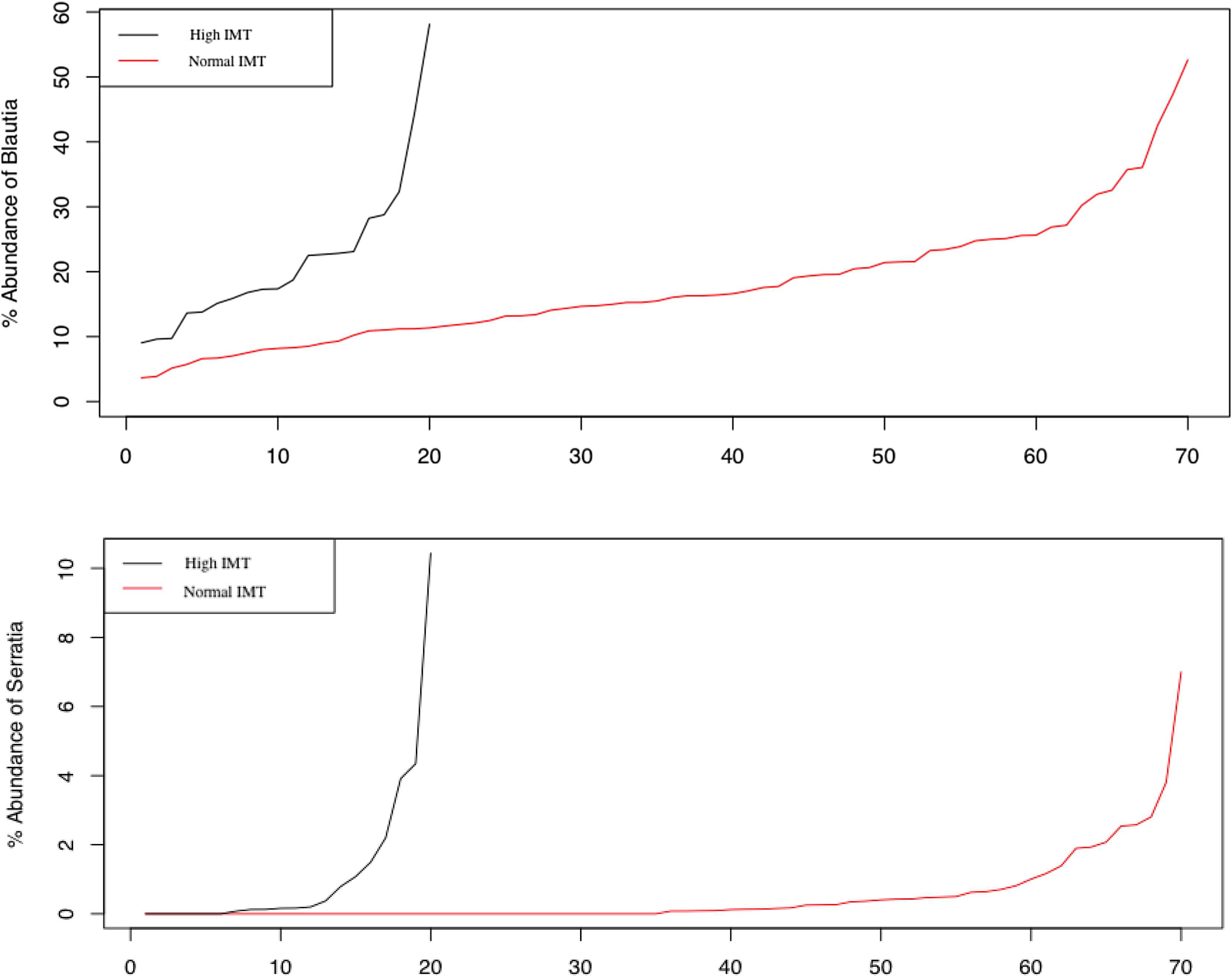
Representation of Blautia and Serratia genera in individuals with normal and thickened intima-media complex.
| Vascular wall characteristic | Estimation | St. error | z value | p value | Genus |
|---|---|---|---|---|---|
| IMT≥0.9 mm | 0.2109 | 0.0555 | 3.8019 | 0.004 | Blautia |
| IMT≥0.9 mm | 0.9009 | 0.2586 | 3.4842 | 0.009 | Serratia |
| Stenosis (maximal, right and/or left) | 0.0251 | 0.006 | 41569 | 0.0018 | Serratia |
| PWV≥10 m/s | 0.3735 | 0.0782 | 4.7747 | 0.0001 | Bacteroides |
Arterial wall characteristics associated with Blautia, Bacteroides, and Serratia genera abundance.
The average value of PWV was 10.9 ± 2.6 m/s. PWV of more than 10 m/s was observed in 55 participants. PWV was associated with age, SBP, levels of urea, glucose, and glycated hemoglobin (Table 1). There were no significant differences in microbiota composition at the first examination in patients with different PWV. After the separation of patients and studying the relationship between PWV and microbiota composition in subjects without type 2 diabetes, we used the generalized linear models and false discovery rate (testing correction) for conducting multiple comparison. It has been found that the representation of Bacteroides was significantly higher in non-diabetic subjects with PWV≥10 m/s (p = 0.0001, Table 2).
Discussion
In our study we have shown the differences in the composition of the gut microbiota not only in patients with atherosclerotic vascular lesions but also with vascular wall rigidity.
After sequencing, we found that in residents from Moscow and Moscow region dominated phyla were Firmicutes and Bacteroidetes. Similar results were obtained in foreign studies.15 The gut microbiota diversity index was quite rich (Fig. 1). Shannon index takes into account not only species richness but also the uniformity (equitability of species according to their abundance). Rich diversity of the gut microbiota shows its stability as an ecosystem. The selected patients did not have severe disorders or diseases. Although we did not find any differences in the diversity between the groups with subclinical atherosclerosis or rigid vessels and with intact vascular wall.
The most represented genera in the samples were Blautia, Bacteroides, Prevotella, Faecalibacterium, and Clostridium. Blautia genus (Blautia coccoides in particular) was the most abundant one.
In this study Blautia was associated with IMT. Blautia coccoides belongs to a class of gram-positive anaerobic bacteria (Clostridia, Firmicutes phylum). Not long ago, after a phylogenetic analysis of these bacteria, Clostridium coccoides were renamed to Blautia coccoides gen. nov.16 According to the Tuovinen et al. study, Blautia coccoides genus activates TNFa and cytokines secretion, and interleukin-8 secretion to an even greater extent than the lipopolysaccharide of gram negative bacteria.17 Also we had shown in the previous study that these bacteria were associated with glucose intolerance and type 2 diabetes mellitus.7
The amount of Serratia was greater in patients with higher CRP levels, IMT, and stenosis of the arteries. Serratia is a genus of Gram-negative, facultatively anaerobic, opportunistic pathogens of the Enterobacteriaceae family. These bacteria contain lipopolysaccharide (LPS) also known as endotoxin. Its release occurs during the physiological microorganisms destruction and membrane components synthesis. LPS is presented in blood at physiological concentrations required to maintain the normal functioning of the immune system and increase nonspecific resistance against infections and tumors.18 Increased concentration of LPS triggers the low-grade inflammation and this leads to endothelial dysfunction and other inflammation related conditions.
Bacteroides genus (belongs to Bacteroidetes phylum) was more abundant in donors with high PWV. Moreover, in this analysis, we excluded patients with initial stage of type 2 diabetes, and thus eliminated the influence of other factors. Interestingly, the number of these bacteria in the gut has been laid by some researchers as the basis for determining the enterotypes or “fecotypes”. The “Bacteroides fecotype” was associated with the so-called “Western diet” rich in animal proteins and fats.19,20 Bacteroides is also a genus of gram-negative bacteria and some species of this genus are opportunistic human pathogens. According to the recent study, Bacteroides have genes, which triggers an inflammatory response.21 Thus, we have shown some possible links between the gut microbiota, inflammation and vascular walls. However this concept requires further clarification.
Conclusion
Establishing whether any structural or functional configurations of the gut microbiota are causally related to a given physiologic or disease phenotype is challenging. We have tried to recruit a group of individuals without clinical atherosclerosis or arterial stiffness to analyze the interrelations between the gut microbiota composition and early vascular changes. These findings support the concept that the microbiota might provide novel strategies for early diagnosis and prevention of cardiovascular diseases.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
Cite this article
TY - JOUR AU - Daria Kashtanova AU - Olga Tkacheva AU - Anna Popenko AU - Lilit Egshatyan AU - Alexander Tyakht AU - Dmitry Alexeev AU - Yulia Kotovskaya AU - Ekaterina Plokhova AU - Sergey Boytsov PY - 2017 DA - 2017/03/21 TI - Gut microbiota and vascular biomarkers in patients without clinical cardiovascular diseases JO - Artery Research SP - 41 EP - 48 VL - 18 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.02.007 DO - 10.1016/j.artres.2017.02.007 ID - Kashtanova2017 ER -
