Concomitance of atherosclerotic lesions in arteries of the lower extremities and carotid arteries in patients with abdominal aorta aneurysm
- DOI
- 10.1016/j.artres.2016.08.003How to use a DOI?
- Keywords
- Abdominal aorta aneurysm; Atherosclerosis; Carotid arteries stenosis
- Abstract
Atherosclerosis was considered the main cause of abdominal aorta aneurysms. Many researchers suggest the presence of the association between these two entities. The aim of this paper was to evaluate the presence of atherosclerosis in the aorta and the arteries of the lower extremities, as well as stenoses and occlusions of carotid arteries in patients with abdominal aorta aneurysm.
One-hundred and eighty patients with abdominal aorta aneurysm (23 women and 157 men), aged 50–86 years old (mean 67.28 ± 9.95) were qualified for the study. Each patient had ultrasound of abdominal aorta, arteries of the lower extremities and extracranial carotid arteries. The patients were divided into two groups: A – isolated abdominal aorta aneurysm (AAA-92 patients), B – AAA with atherosclerotic lesions in the abdominal aorta and/or the arteries of the lower extremities (88 patients), in which four different localizations of atherosclerotic lesions were distinguished.
The statistically significant differences between the groups were observed in the prevalence of carotid arteries stenosis (CCA + ICA). No correlation was found between the aneurysm diameter and the stenosis grade of the extracranial segments of the carotid arteries.
The presented results confirm the common occurrence of atherosclerotic lesions in aorta and/or arteries of the lower extremities and carotid arteries in patients with abdominal aorta aneurysm, yet their etiology is different. Such patients represent a heterogeneous group in respect to the presence of carotid arteries stenoses.
- Copyright
- © 2016 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Atherosclerosis was for many years considered the main cause for the formation of abdominal aorta aneurysms.1 According to the theory, which prevailed until recently, the basic role in the pathological aortic dilatation was attributed to the aging of the blood vessels. Such assumption was supported by clinical observation of patients with chronic aortic diseases and the examinations of pathologically changed vessels’ segments. The stiffness of atherosclerotic aorta and processes taking place in its wall were to play the fundamental role in its degeneration and strength reduction. One of the rationales for such observation was the common presence of atherosclerotic plaques in the intima of the aortic aneurysm. Their formation and evolution were considered to influence the natural course of the aneurysm leading to the weakening of the wall and raising the risk of rupture.2 An association between the raised elastase activity and elastin degradation and the severity of atherosclerotic plaque formation was suggested.3,4 The above mentioned phenomena led to the development of the atherosclerotic theory of abdominal aorta aneurysm formation.5,6 The studies carried out in the recent years did not, however, confirm the previous theory of atherosclerotic-based formation of AAA, indicating the morphological and immunological dissimilarities of both pathologies.7,8 The identification of the gene responsible for the hereditary form of AAA, being one of the most important finding of modern medicine in the recent years, seems to confirm such thesis.9,10
Aim
The aim of this paper was to evaluate the presence of atherosclerotic lesions in the aorta and/or the arteries of the lower extremities, as well as stenoses and/or occlusions of carotid arteries in patients with abdominal aorta aneurysm.
Methods
The study was carried out in the Department of Vascular, General and Oncological Surgery, Copernicus Memorial Hospital, Lodz, Poland. One-hundred and eighty patients with abdominal aorta aneurysm (23 women and 157 men), aged 50–86 years old (mean 67.28 ± 9.95) were qualified for the study. Each patient had a color Doppler ultrasound of the abdominal aorta, the arteries of the lower extremities and extracranial carotid arteries performed. At the time of the study almost every patient with AAA + AS (84 out of 88) received best medical treatment for this condition (ASA + statin), whereas only approx. 50% of the patients with AAA only received ASA.
The patients were divided into two groups according to the ultrasound findings and observed flow disturbances:
- GROUP A (AAA) –
isolated abdominal aorta aneurysm, 92 patients, including 14 women (15.2%) and 78 men (84.8%), mean age 67.43;
- GROUP B (AAA + AS) –
the presence of atherosclerotic lesions in the abdominal aorta or the arteries of the lower extremities concomitant to AAA, 88 patients, including 9 women (10.2%) and 79 men (89.8%), mean age 67.13.
The extracranial segments of both carotid arteries (CCA + ICA) were assessed in each patient using 2-D presentation. The flow velocities were measured and spectral analysis (duplex Doppler) and color flow imaging were performed as well. The quantitative evaluation of atherosclerotic lesions in the assessed carotid arteries segments (CCA + ICA stenoses) was performed according to the NASCET guidelines, including the analysis of the alterations in flow spectrum as well, to confirm the stenosis grade. Once each stenosis was automatically calculated, each artery was assigned to one of the following groups:
- a –
no atherosclerotic lesions (0%),
- b –
low-grade stenosis (1–29%),
- c –
medium-grade stenosis, hemodynamically insignificant (30–49%),
- d –
medium-grade stenosis, hemodynamically significant (50–69%),
- e –
high-grade stenosis (70–99%),
- f –
artery occlusion (100%).
Statistics
The obtained results were subjected to statistical analysis using Excel and Statgraphics Plus v. 5.0 software. The patients’ structure estimated according to the observed features was determined using percentages. The studied group of patients was described using stratum weights for the qualitative features. For quantitative features the arithmetic mean (Xm) was calculated as an average value and measure of dispersion – standard deviation (SD) and minimal and maximal values were presented. Test for two means was used to compare the calculated arithmetic means: a) for large sample size (n1, n2 > 30), b) for small sample size (n1, n2 < 30). The difference between studied parameters was considered statistically significant (incidences, arithmetic means), when the calculated test value was equal or greater than the critical value obtained from chi-square tables (normal, t-Student) with appropriate number of degrees of freedom and error probability of less than 0.05.
Results
Table 1 presents general description of both analyzed groups and the distribution of risk factors. The A group was characterized by statistically significant higher mean diameter of AAA than group B (p < 0.001). The prevalence of carotid lesions, smoking and ischemic heart disease was significantly more frequent in patients from group B.
| Group characteristics | Total | A (AAA) | B (AAA + AS) | A vs B | ||||
|---|---|---|---|---|---|---|---|---|
| Statistical analysis | p value | |||||||
| Mean age | 67.28 ± 9.95 | 67.43 ± 10.2 | 67.13 ± 10.3 | 0.202 | 0.840 | |||
| Mean diameter of AAA | 42.47 ± 11.6 | 45.79 ± 14.27 | 39.14 ± 8.90 | 3.769 | 0.0002 | |||
| N | % | N | % | N | % | |||
| Sample size | 180 | 100 | 92 | 88 | ||||
| Sex | ||||||||
| Men | 157 | 87.2 | 78 | 84.8 | 79 | 89.8 | 1.005 | 0.316 |
| Women | 23 | 12.8 | 14 | 15.2 | 9 | 10.2 | ||
| Presence of carotid lesion | 114 | 63.3 | 46 | 50.0 | 68 | 77.2 | 14.418 | 0.0001 |
| Smoking | 113 | 62.8 | 40 | 43.5 | 73 | 83.0 | 21.8 | 0.00001 |
| Hypertension | 55 | 30.6 | 26 | 28.3 | 29 | 33.0 | 0.468 | 0.494 |
| Hyperlipidemia | 13 | 7.2 | 4 | 4.3 | 9 | 10.2 | 2.317 | 0.128 |
| Diabetes | 10 | 5.6 | 6 | 6.5 | 4 | 4.5 | 0.064 | 0.800 |
| Ischemic heart disease | 25 | 13.9 | 8 | 8.7 | 17 | 19.3 | 4.261 | 0.0390 |
| Stroke or TIA | 3 | 1.7 | 2 | 2.2 | 1 | 1.1 | 0.002 | 0.969 |
| Previous vascular surgery due to atherosclerosis | 2 | 1.1 | 4 | 4.3 | 6 | 6.8 | 0.158 | 0.691 |
A – isolated abdominal aorta aneurysm (AAA), B – the presence of atherosclerotic lesions in the abdominal aorta or arteries of the lower extremities concomitant to AAA (AAA + AS).
TIA – transient ischemic attack.
The numbers in bold indicate statistically significant differences.
Analyzed groups characteristics.
Figure 1 presents the distribution of atherosclerotic lesions in the carotid arteries among the patients of group A and B. The percentage rate of over 50% carotid artery stenoses (d and e) was 10.55%, i.e. 19 patients (d – 6.67%, i.e. 12 patients, and e – 3.89%, i.e. 7 patients). The carotid artery occlusion (f) was observed in 11 patients (6.11%) and its incidence was similar in both groups: i.e. 6 patients in group A (6.52%) and 5 patients in group B (5.68%).
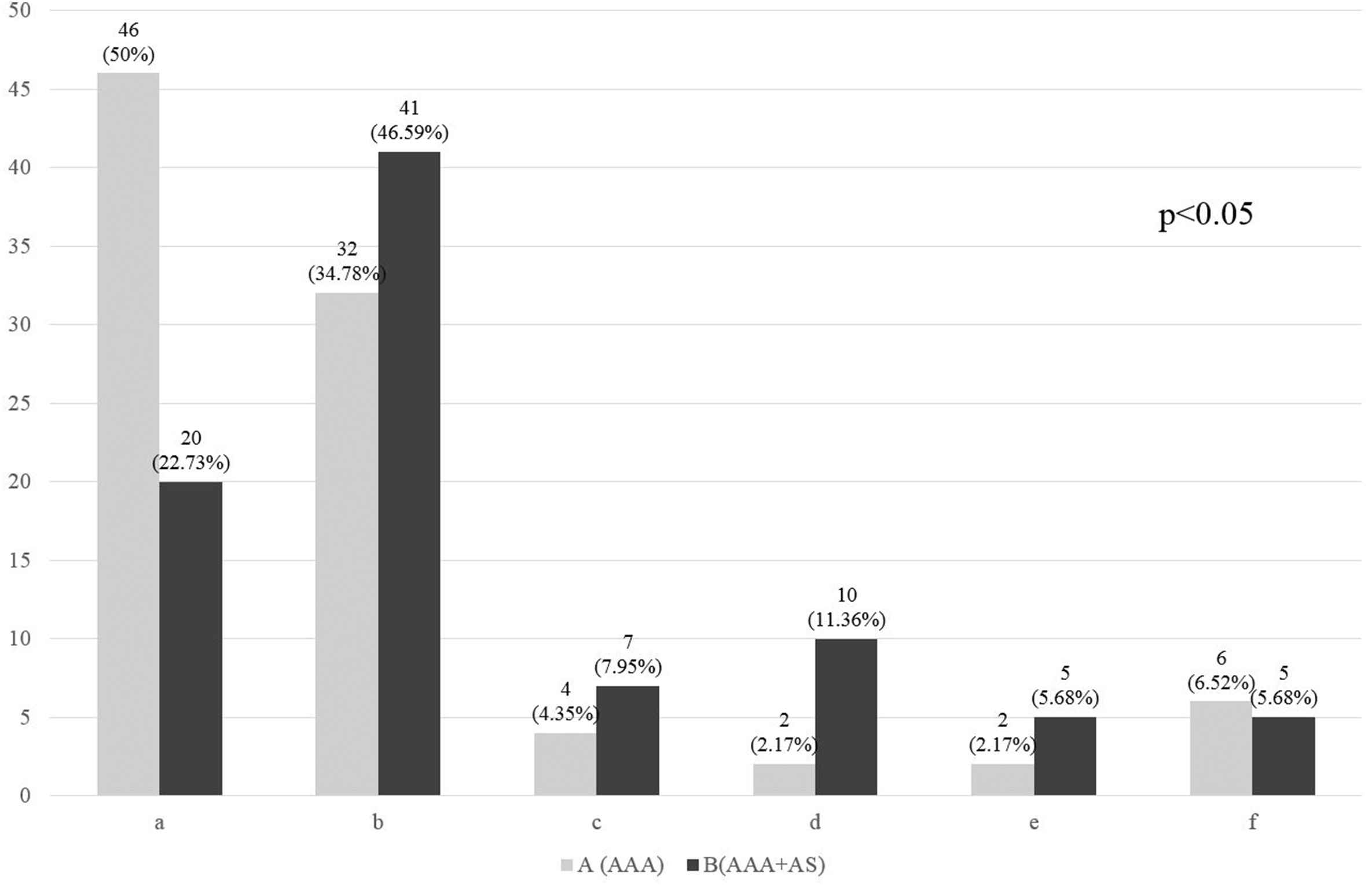
Distribution of atherosclerotic lesions in carotid arteries in particular groups, including stenosis grade. A – isolated abdominal aorta aneurysm (AAA), B – the presence of atherosclerotic lesions in the abdominal aorta or arteries of the lower extremities concomitant to AAA (AAA + AS). a – no atherosclerotic lesions (0%), b – low-grade stenosis (1–29%), c – medium-grade stenosis hemodynamically insignificant (30–49%), d – medium-grade stenosis hemodynamically significant (50–69%), e − high-grade stenosis (70–99%),f – artery occlusion (100%). The difference between the groups, as a whole, is statistically significant.
The difference in the distribution of carotid atherosclerotic lesions was statistically significant (p < 0.05) between groups A and B as a whole.
Statistically significant differences (p < 0.05) between groups A and B were observed for carotid stenoses 50–69% (d), cumulated non-occlusive stenoses over 50% (d + e) and such stenoses combined with occlusion group (d + e + f).
No statistically significant association was observed between the aneurysm diameter and carotid artery stenosis both for isolated aneurysm, and for AAA concomitant with atherosclerosis of aorta and/or arteries of the lower extremities (Fig. 2).
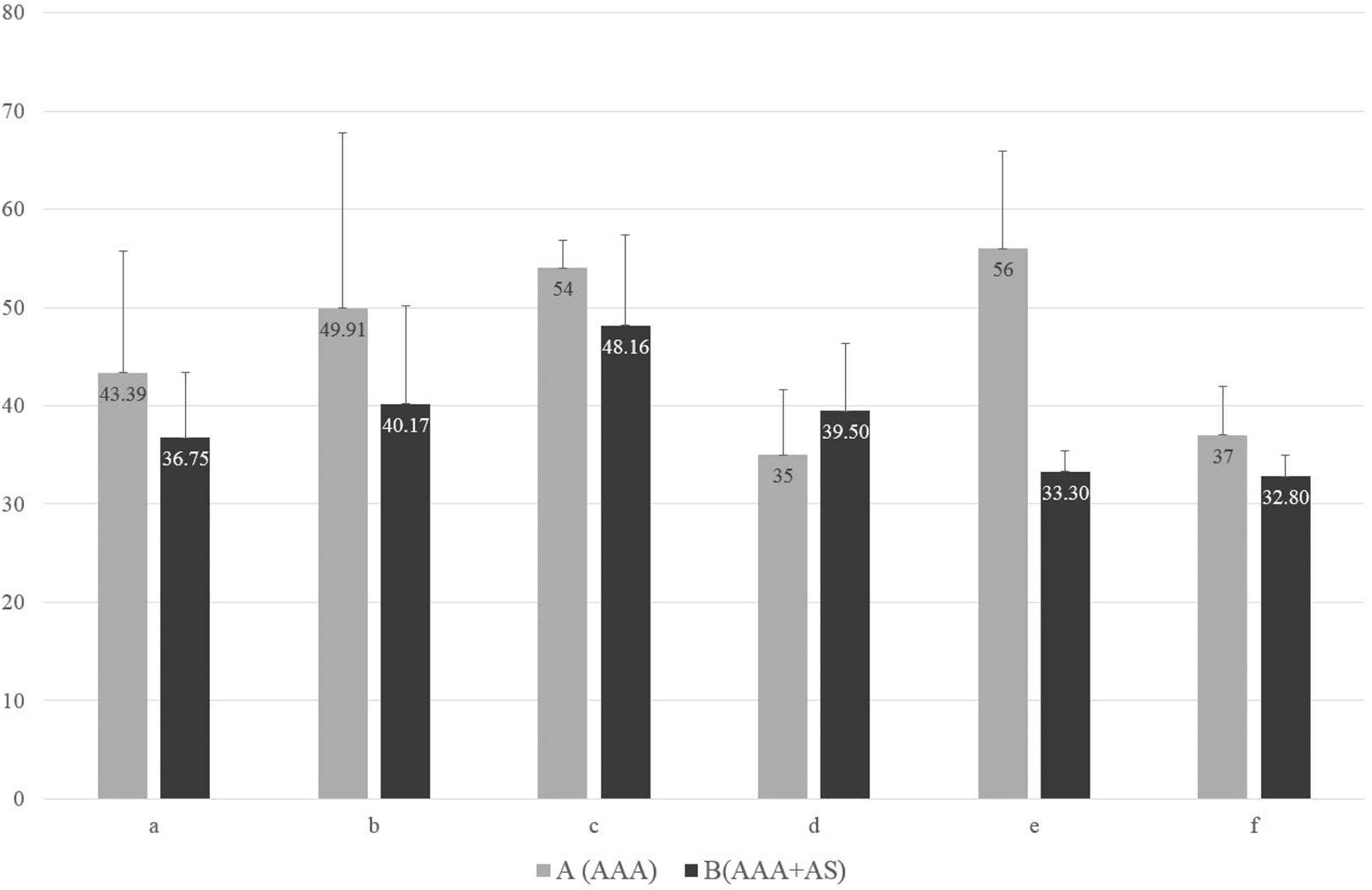
Aneurysm diameter (mm) and carotid lesions in groups. O – total, A – isolated abdominal aorta aneurysm (AAA), B – the presence of atherosclerotic lesions in the abdominal aorta or arteries of the lower extremities concomitant to AAA (AAA + AS). a – no atherosclerotic lesions (0%), b – low-grade stenosis (1–29%), c – medium-grade stenosis hemodynamically insignificant (30–49%), d – medium-grade stenosis hemodynamically significant (50–69%), e − high-grade stenosis (70–99%), f – artery occlusion (100%). The difference between the groups, as a whole, is not statistically significant.
Discussion
Until recently atherosclerosis was considered the primary cause for the formation of abdominal aorta aneurysms.1 Commonly observed atherosclerotic plaques in the intima of aneurysmatic aorta and processes associated with their formation and evolution, such as rupture and dissection secondary to the hemorrhagic conversion may influence the natural course of aneurysm, resulting in the reduction of wall strength and raise in the risk of aneurysm rupture. One of the main phenomenon taking place during blood vessel aging is the degradation of elastic fibers, which intensifies in such conditions as atherosclerosis, arterial hypertension and diabetes.3,4 Some studies indicated the high elastase activity in human aorta extracts, which increased exponentially with age and correlated with the severity of atherosclerotic lesions. The impairment of elastin/laminin receptor function and its permanent stimulation (e.g. during aging) was proven responsible for the overproduction of elastases and elastin degradation.4 The positive correlation between the concentration of elastin degradation products and aneurysm growth rate was found.5,6 The atherosclerosis-like process that was previously considered responsible for pathological aorta dilatation was not confirmed in recent studies. Although numerous studies concerning the processes of vascular damage revealed many common features between atherosclerosis and aortic aneurysm, the detailed analyses proved that they are generally different pathological phenomena, showing their morphological and immunological dissimilarities.7,8 The proliferation of arterial intima is the dominating element in the pathology of atherosclerosis, whereas the weakening of media and adventitia contribute to the reduction in elasticity and tensile strength of the aorta. It results in the reduction of wall thickness, increase in wall tension, dilation of aortic lumen, which may eventually lead to aortic rupture. The discovery of these phenomena and processes, which contribute to the pathological aortic dilation led to common recognition of multifactorial aneurysm etiology.
One of the main issues concerning aneurysm etiology is pathological processes taking place in its wall and concomitance of various systemic diseases. The etiological factors that accompany aortic aneurysms include: degenerative diseases, congenital developmental defects, smoking, infections, vasculities and trauma. The significant factors determining the formation of aortic aneurysm include: inflammation, proteolytic connective tissue degradation and biomechanical factors, that alter the quality and structure of aortic wall and consequently contribute to the reduction of aortic resistance to forces associated with pulsatile blood flow.11 It is favored by factors associated with wall structure itself, characteristic of blood flow and reduction of perfusion in abdominal aortic wall supplied by supplying vessels.12–14
Commonly recognized pathological phenomenon is the presence of atherosclerotic lesions in patients with abdominal aorta aneurysm in the aorta itself, and the arteries of the lower extremities, carotid arteries and coronary arteries as well.15–23 Frequent incidence of these two pathologies was confirmed both in clinical observation, and autopsy as well, especially among older men.15,24,25 Their correlation is being discussed for years and several theories were developed, aiming at explaining the mutual influence of these pathological phenomena.26
The first theory of atherosclerosis-based aneurysm etiology led to the development of the term “atherosclerotic aneurysm” that is present in medicine for over 100 years and is still being used by some researchers.26–28 It assumes the presence of causal relationship between atherosclerotic degeneration of aortic wall and the formation of aneurysmatic dilation of its lumen. It is based on the known and well-described in the literature phenomenon of compensatory dilation of arterial lumen resulting from its wall remodeling in response to shear stress, should the lesion leading primary to stenosis of the vessel lumen, such as atherosclerosis, occur.29 The degradation of intracellular matrix proteins and proteolytic elastin damage may result from chronic inflammatory process secondary to atherosclerosis and concomitant arterial thrombosis.22,29 As far as the remodeling may explain the marked reduction of media thickness, it has no significant influence on adventitia inflammation observed in aneurysmatic aorta. Should aneurysm have causative atherosclerotic background, the atherosclerotic lesions should be present in aortic or arterial wall of all patients. It is contradicted by published papers, which authors indicate various incidence of both pathologies in patients, yet they never reach 100% in AAA-patients.30
The second theory presumes the independent occurrence of both aortic pathologies, yet common contribution of some genetic and environmental risk factors, whereas the type of the lesion being developed is associated with different pathophysiological mechanism.21 It is supported by the observation of many similarities in pathogenesis and pathophysiology of both diseases.31–34 The opponents of this theory indicate obvious differences among risk factors characteristic in patients with atherosclerosis developing in carotid, coronary and lower extremities arteries and patients with aortic aneurysm.35–38 The significant difference in the incidence of diabetes was found in both groups, which is negative or neutral risk factor for AAA, but positive for atherosclerosis.22 Other authors indicate different genetic factors that may be responsible for each of the mentioned vascular pathologies.39 They supported their hypothesis by identifying the gene that is responsible for hereditary aortic aneurysm.10
The third possibility to explain the association between atherosclerosis and aortic aneurysm is their mutual stimulatory interaction, regardless of the fact which pathology occurs first.26 Remodeling occurrence in patients with the primary atherosclerosis of aortic wall and being a result of flow disturbances in the atherosclerotic abdominal aorta, interaction with oxidized LDL and the release of proinflammatory cytokines from mural thrombi leads to the secondary proteolysis and degradation of intracellular matrix and inflammation that may lead to wall weakening and aneurysmatic dilation of aortic lumen.
The presence of thrombi in the aneurysmal sac that contains high concentration of proteolytic enzymes and plasminogen activators contributes to the injury of the aortic wall, which leads to the aneurysm growth and rupture.40–42 On the other hand however, their presence hampers oxygen perfusion to the aortic wall, which increases vessel stiffness, reduces its compliance and ability to adapt to blood pressure changes, which in turn contributes to the formation of atherosclerotic plaques should certain environmental factors interact.43,44
The Norwegian TROMSO Study that included 6,500 adults from general population attempted to determine the relationship between the atherosclerosis of carotid, femoral and coronary arteries and the aortic aneurysm diameter.30 The results excluded the existence of such relationship, whereas the authors suggested that there are no data that could confirm the causative relationship between atherosclerosis and AAA. They suggested that atherosclerotic lesions develop in the aortic wall parallel to its aneurysmal dilation or are secondary to AAA. The SMART study, determining the thickness of intima-media complex in the common carotid artery in various patient groups revealed significant differences between patients with peripheral atherosclerosis and patients with AAA. Therefore the authors concluded that the development of aneurysmal dilation of abdominal aorta, may not simply be explained by atherosclerotic pathology of vessel wall and is determined by other pathophysiologic mechanism.45 There is insufficient evidence to confirm that AAAs are the result of atherosclerosis.46
A positive correlation was found between both the incidence and the grade of carotid artery stenosis and the presence of chronic lesions in aorta and/or arteries of the lower extremities, although it differed in both groups. The percentage of patients with atherosclerotic lesions in carotid arteries was relatively lower (50.0) in patients with isolated abdominal aorta aneurysm and the incidence of over-50% stenoses did not exceed 5%. The significantly higher rate of carotid lesions (77.27%) and higher grade of carotid artery stenosis (17.04% stenoses over 50%) was observed in patients with AAA concomitant to atherosclerotic lesions in peripheral arteries.
The routine evaluation of extracranial segments of carotid arteries during the duplex Doppler examination of aorta and the arteries of the lower extremities in patients with chronic peripheral vascular diseases, regardless of diagnostic or therapeutic indications, may pose a valuable screening tool. The presented comparisons between study groups may allow to identify patients, in which the screening for carotid disease may be most justified and show greatest cost-effectiveness ratio. The statistical analysis revealed significant differences (p < 0.001) in the distribution of carotid artery lesions between the patients with isolated AAA and patients with AAA concomitant to atherosclerotic lesions in aorta and/or arteries of the lower extremities.
The above-mentioned observations may confirm the theory of differing etiology of atherosclerosis and abdominal aorta aneurysm, and their independent incidence. The lack of statistical significance in the difference between the diameter of abdominal aorta aneurysm and the carotid artery stenosis that was observed both for isolated aneurysm, as well as for aneurysm concomitant to atherosclerosis of aorta and/or arteries of the lower extremities seems to additionally confirm such conclusion.
Conclusions
The presented results confirm the common occurrence of atherosclerotic lesions in aorta and/or arteries of the lower extremities and carotid arteries in patients with abdominal aorta aneurysm, yet their etiology is different. Such patients represent a heterogeneous group in respect to the presence of carotid arteries stenoses.
Conflict of interest
None.
References
Cite this article
TY - JOUR AU - Piotr Kaźmierski AU - Michał Pająk AU - Katarzyna Bogusiak PY - 2016 DA - 2016/09/19 TI - Concomitance of atherosclerotic lesions in arteries of the lower extremities and carotid arteries in patients with abdominal aorta aneurysm JO - Artery Research SP - 11 EP - 17 VL - 16 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2016.08.003 DO - 10.1016/j.artres.2016.08.003 ID - Kaźmierski2016 ER -
