Central pressure should be used in clinical practice
- DOI
- 10.1016/j.artres.2014.11.001How to use a DOI?
- Keywords
- Haemodynamic; Aorta; Blood vessels; Brachial artery
- Abstract
The original purpose for recording brachial blood pressure (BP) more than 100 years ago was to estimate central (aortic) BP. While high brachial BP is an important cardiovascular risk factor, it is clear that major differences in central systolic BP (SBP; e.g. >30 mmHg) can occur among people with similar brachial SBP. It is also proven that central SBP responses to antihypertensive therapy can differ substantially from brachial SBP responses, such that true treatment effects cannot be gauged from conventional brachial BP. Importantly, assessment of central BP results in: 1) improved predictive accuracy of future cardiovascular events beyond brachial BP and other cardiovascular risk factors; 2) superior diagnostic accuracy over brachial BP and; 3) different patient management than usual care guided by brachial BP. Collectively, the above illustrates that central BP is a better cardiovascular risk biomarker than brachial BP. As with all medical advances there are areas of research need and international consensus is required on issues such as standardization of techniques. However, central BP can now be accurately estimated (with appropriate waveform calibration) using brachial cuff methods in an approach that is familiar to clinicians, acceptable to patients and amenable to widespread use. In other words, this modern BP technique can finally satisfy the original purpose for measuring central aortic BP as intended more than 100 years ago. Although the tipping point towards routine use is yet to be reached, the body of evidence continues to favour the view that central BP should be used in clinical practice.
- Copyright
- © 2014 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Since inception of the method to measure blood pressure (BP) by cuff at the arm in the 19th century, the purpose has been to gain an appreciation of central aortic pressure on the understanding that this would be the most clinically relevant measure of pressure exposure to the heart. The original 1896 reports from Riva-Rocci on the operation of the cuff sphygmomanometer described the technique as measuring the pressure (‘total charge’) “… either in the aorta itself” or “… a point fairly close to the aorta.”1 This technique was refined by Korotkoff in 1905 and the principles of measurement have since remained almost unchanged as used in clinical practice and research today. While it is accepted that high arm (brachial) BP is a powerful cardiovascular (CV) risk factor,2 there is incontrovertible evidence that aortic (central) systolic BP (SBP) can differ markedly (e.g. >30 mmHg) among people with the same or similar brachial SBP,3 and that antihypertensive drugs can differentially affect brachial compared with central SBP.4 These latter two facts alone should place a question mark over brachial BP holding sway as the reference standard in clinical practice. Indeed, ample added information has come to light in the 21st century to verify this claim.
Techniques to non-invasively estimate central BP have undergone major development in recent years, such that it is now possible to derive a good estimate of central BP using an automated device with similar appearance and operating characteristics to conventional brachial cuff methods. This measurement approach is highly familiar to doctors and theoretically should be appealing for widespread clinical use, or at least provides the opportunity for such. But despite this, clinical take up of central BP methods is virtually absent and, while acknowledging the pathophysiological, pharmacological and therapeutic interest of central BP, it is currently not recommended for routine clinical use in hypertension management guidelines.5 Notwithstanding several knowledge gaps and limitations in need of rectifying, there exists substantial evidence in favour of the case that central BP should be a useful tool for general use in clinical practice.
Evidence to support use of central BP in clinical practice
Brachial BP is a biological marker used to identify increased vulnerability to CV disease and is the most important modifiable CV risk factor worldwide.2 In order for central BP to be endorsed as a clinical assessment tool, several evidentiary criteria must be satisfied to ultimately prove greater clinical value over and above conventional brachial BP. In addition to accuracy, reproducibility and acceptance to patients, these criteria also include: diagnostic superiority; proof of elevated risk associated with central BP independent of other established CV risk factors and; evidence that knowledge of central BP changes patient management.6 To these ends, there is supporting data for central BP along multiple evidence streams that are summarised in Table 1. Data to support the first five summary points in Table 1 were recently reviewed in detail,7 but more corroborative evidence on the autonomous strength of central BP has since emerged and these studies are detailed below.
| Evidence summary | Evidence strength | Clinical advantage beyond brachial BP |
|---|---|---|
| 1. Major differences in central BP occur among people with similar brachial BP | +++ | More accurate assessment of risk related to BP |
| 2. The response to antihypertensive drugs differs between central and brachial BP | +++ | More accurate assessment of BP response to treatment |
| 3. Central BP indices independently relate to end organ disease | ++ | Better discriminator of cardiovascular risk |
| 4. Changes in end organ disease after therapy independently relate to central BP | ++ | |
| 5. Central BP indices independently relate to cardiovascular events and mortality | ++ | |
| 6. Central BP improves the predictive accuracy of future cardiovascular events beyond brachial BP and other cardiovascular risk factors | + | |
| 7. Central BP has superior diagnostic accuracy over brachial BP | + | Higher probability of clinicians making relevant management decisions |
| 8. Care guided by central BP results in different management decisions than usual care | + | Improved patient care |
Summary of current evidence in support of clinical use of central blood pressure (BP; updated from).7
Improved prognostic capacity
A critical step in determining the practical worth of a new biological marker is to assess whether it improves the predictive accuracy for clinical events beyond the conventional marker after adjusting for known risk factors in an optimised statistical model.6 This is best evaluated with the incremental change in the concordance index (c statistic) for central BP versus brachial BP in predicting outcomes.8 Cheng and colleagues9 recently validated central BP thresholds for diagnosing hypertension based on prediction of CV and stroke mortality. Optimal central BP and ‘central hypertension’ thresholds were estimated at <110/80 mmHg and ≥130/90 mmHg respectively in a derivation cohort (n = 1272) and then tested in a separate (validation) cohort (n = 2501). Stronger associations of CV mortality with both central pulse pressure and systolic BP (SBP) compared with brachial cuff BPs were observed. Moreover, central BP had an additional contribution to the prediction of future CV and stroke mortality beyond brachial BP and independent from traditional CV risk factors of sex, age, body mass index, smoking and serum lipids (demonstrated by improved incremental c statistic).9
Although the study of Cheng et al.10 had some limitations and raises questions yet to be answered (e.g. racial generalizability, calibration methods),11 this important work is the first to produce outcome-based diagnostic thresholds for central BP. The increased discriminatory power of central BP proves the concept that it should be a better clinical biomarker of CV disease risk than brachial BP. Similar findings were reported in two other recent investigations that explored the independent utility of central BP indices (aortic excess pressure and a marker termed ‘reflection magnitude’) for predicting incident CV events in the general population as well as higher risk patients treated for hypertension.12,13 In the latter population there was an improvement of the c statistic with the inclusion of aortic excess pressure into predictor models for CV events already containing conventional Framingham risk factors (including brachial BP).13 This novel marker, representing excess left ventricular (LV) work, has been validated in humans,14 and can be produced from pressure waveforms (oscillometric cuff or tonometry) without using a generalised transfer function, and is a promising area for future study.
Superior diagnostic capacity
If clinicians are to be guided by central BP in the management of patients with hypertension, there should be satisfaction with the diagnostic performance of central BP monitoring devices. The first such assessment was performed recently.10 In a robust design, 138 untreated subjects had simultaneous recording of invasive central BP (as the comparator gold standard) in addition to non-invasive brachial BP and central BP using an oscillometric monitor validated for measuring brachial BP15 and central BP (using multivariate modelling of arterial waveform features).16 As per convention, the threshold to denote hypertension based on brachial BP was 140/90 mmHg,5 and was 130/90 mmHg for central BP in accordance with the abovementioned study.9 On all performance measures, the non-invasive central BP method was diagnostically superior to brachial BP for delineating hypertension (e.g. sensitivity 93% vs. 49%; specificity 95% vs. 94%; positive predictive value 96% vs. 90%; negative predictive value 93% vs. 63%; accuracy 94% vs. 70%) irrespective of sex, age or presence of diabetes or coronary artery disease. Twenty four percent of misclassified diagnosis based on brachial BP was correctly reclassified with central BP. Thus, accuracy of hypertension diagnosis should be markedly improved with central BP, at least using the device examined in this study, but could also raise question as to whether the diagnostic capability of all central BP devices should be scrutinised to this level? This is not a requirement for brachial BP.
Changed patient management
On the premise that high brachial BP is associated with poor clinical outcomes and reducing high brachial BP with medication improves clinical outcomes,17,18 a ‘more is better’ approach to treating hypertension has been advocated (more medication to lower BP more). This apparent axiomatic fact has been challenged on the basis of emergent reports that aggressive BP lowering may not be beneficial or even have adverse effects in some patient populations (especially individuals with higher CV risk).19 While acknowledging this area is yet to be fully understood,20 the possibility of harm should alert to the need for examining other ways of guiding hypertension management beyond focus on brachial cuff BP, for which the responses to antihypertensives are different to central BP.21 Indeed, it is possible that brachial SBP does not change, or only undergoes a small drop, in response to vasoactive medications despite major (or relatively greater) decreases in central SBP.4,21 This differential central-to-brachial BP change with treatment could lead to drug overtitration (and undesirable outcomes such as increased falls risk in older people22) when decisions are based on brachial BP. The availability of non-invasive central BP monitoring offers a potential alternative to help refine BP management decisions.
The BPGUIDE study was the first study that sought to determine the value of central BP-guided care compared with best-practice in a randomised, open-label, blinded endpoint trial of 286 moderate-risk patients (aged 64 ± 8 years) with treated hypertension followed over 1 year.23 The primary endpoints were antihypertensive medication quantity, LV mass index (3-dimensional echocardiography) and quality of life, with the hypotheses that central BP-guided care would result in less medication use (due to knowledge of central BP) and better quality of life (due to better medication choices), but no change in LV mass (due to appropriate BP control). The central BP group had a highly significant reduction in medication (Fig. 1) across all drug classes, but appropriate BP control was still achieved (gauged by 24 h ambulatory BP and 7-day home BP) and there was no significant change in LV mass between groups. Contrary to mistaken assertions around study design24 and the impact of central SBP underestimation,25 drugs were only downtitrated and maintained at a lower level if non-office BP values were confirmed to be normal, thereby showing clear discriminatory value of central BP separate from brachial BP methods. Quality of life improved in both groups and there was also no change in aortic stiffness (an important surrogate endpoint26 in addition to cardiac hypertrophy). Although the long-term clinical sequelae of this drug-minimising approach to patient care are unknown, the maintenance of appropriate non-office BP, together with central SBP kept below the hypertension threshold (130 mmHg), should weight the balance towards no harmful effects.
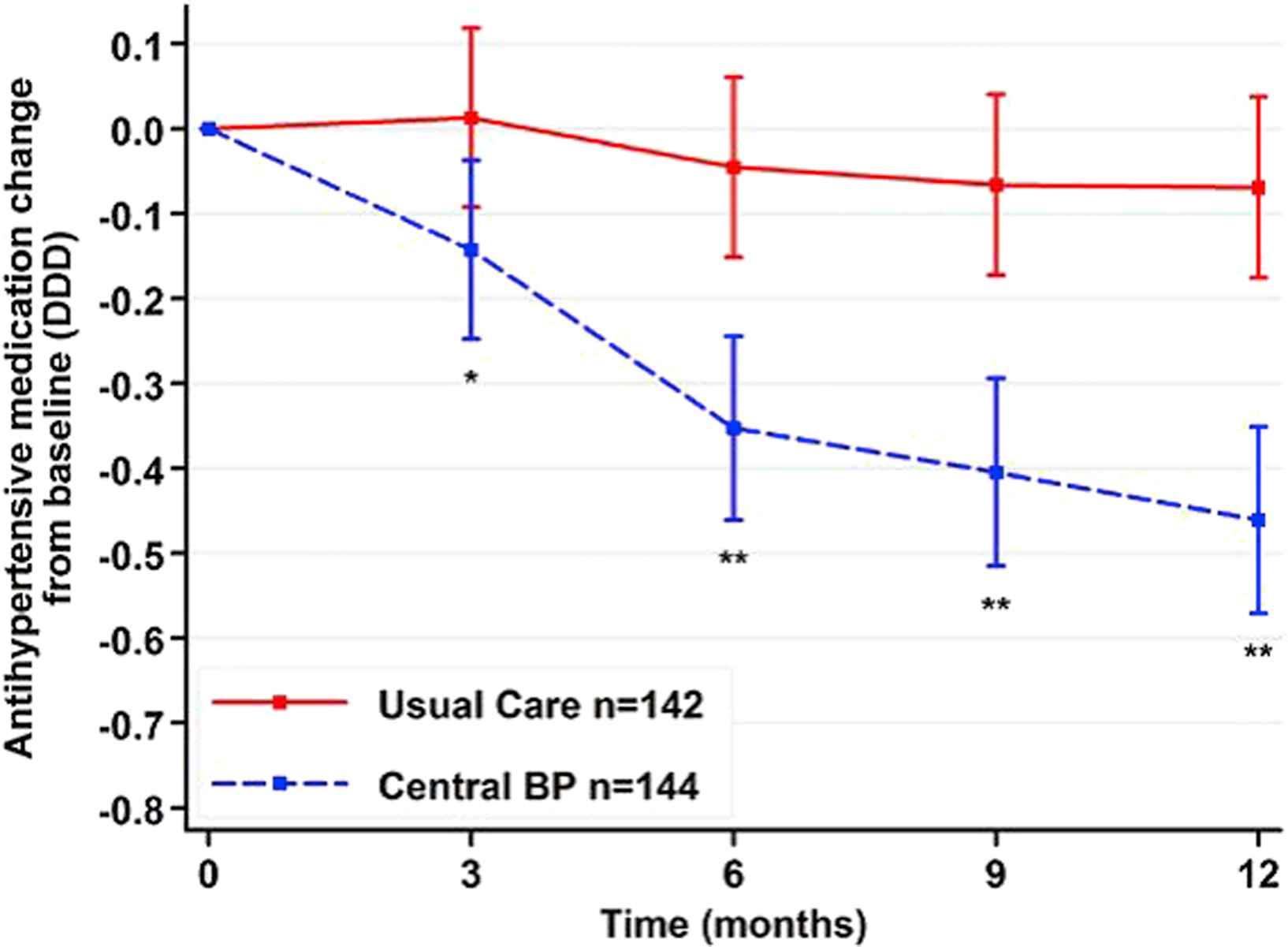
The change in daily defined dose of antihypertensive medications between groups randomized to central blood pressure-guided care or best-practice usual care. Data adjusted for age, sex and body mass index. Error bars indicate 95% confidence interval and *P = 0.008, **P < 0.001. BP, blood pressure (from Sharman et al.28 with permission from Wolters Kluwer Health).
The only other randomised, prospective (6 months) study to test the strategy of clinical decision making based on central BP indices was in 50 patients with chronic heart failure NYHA class ≥ II.27 Treatment withdrawal in this higher risk population may have been ethically questionable and all patients started the trial on maximal guideline-directed medical therapy. The goal of active treatment was to reduce augmentation index to 0% with antihypertensive medications (provided central SBP was in an acceptable range; deemed > 85–100 mmHg), thereby ‘pressure unloading’ the left ventricle and enabling improved cardiac ejection and exercise tolerance. As hypothesised, there was a significant improvement in exercise capacity with intervention compared with controls, and augmentation index decreased in both study arms. As could be expected, more drugs were initiated and maintained throughout the study in intervention patients, but this was regarded as optimising therapy in this population. This proof of concept study,27 together with that of BPGUIDE,28 emphasise the additional utility and potential flexibility of central BP in aiding clinical decisions among diverse patient populations requiring different care strategies pertaining to BP control. This area remains open for further enquiry, but there are several evidence gaps and technological issues that need to be clarified.
Future studies, problems and solutions
More data and standardisation of techniques
Although enthusiasm for the goal of widespread clinical use of central BP is defensible, the body of confirmatory data may simply need to be larger before a tipping point will be reached toward general acceptance of central BP. It may be supposed that health economic benefits could be achieved for example through more accurate hypertension diagnosis,10 or medication cost reduction,28 or potential quality adjusted life years gained from improved functional capacity,27 but such analyses require more data. Another research need is testing the validity of proposed central BP thresholds9,29 via randomised clinical trials with CV endpoints. There is a pressing need for standardisation of techniques to assess performance of central BP technology, where currently there exists a diverse approach to testing the degree of validity (accuracy), reproducibility (precision) and diagnostic performance (if at all). The identification of probable variability in estimating central SBP among different devices or techniques25 provides fodder to challenge credibility30 and has implications for generalizability of central BP results, even though this same deficiency of variability applies to brachial BP monitors due to individual proprietary algorithms or deflation rates.24,31 As with all medical tests, neither brachial nor central BP techniques are perfect, and taking the field forward requires solutions-focussed effort. International consensus is needed for guidance, particularly in light of the quantity of central BP devices available and potentially emerging in the future, but also to clarify standpoint on other key issues such as accuracy of estimated central BP due to waveform calibration.
Calibration of arterial pressure waveforms to derive central BP
A recent meta-analysis showed that true central SBP was substantially underestimated by radial tonometry when waveforms were calibrated with brachial SBP and DBP.32 Inaccurate cuff BP is one source of error in estimated central BP,33 but amplification of SBP from brachial to radial arteries is also a likely contributor to central SBP underestimation by the radial tonometry method.34 Another problem of calibrating radial arterial waveforms using brachial SBP is systematic underestimation of true brachial SBP (e.g. ≈10–15 mmHg, whether by auscultation or oscillometric methods)35–37 which directly causes underestimation of the true central SBP.38,39 However, when peripheral arterial waveforms are calibrated using oscillometric mean arterial pressure (MAP; which is close to aortic MAP)40 and DBP, the derived central SBP values become closer to true (invasive) central SBP.38 An advantage of this approach is that the variability of derived central SBP will be lower than that derived using brachial SBP calibration because the pressure range for MAP is smaller than for brachial SBP. This should lead to better fitted statistical models for the relation between central BP and clinical outcomes. Furthermore, the MAP/DBP calibration approach creates a relative disconnection of derived central SBP from the inaccurate brachial SBP recording, which are otherwise highly correlated if waveforms are calibrated by brachial SBP and DBP (e.g. r > 0.9).24
The remaining difficulty of MAP/DBP calibrated waveforms is conceptual only, that is in reconciling that central SBP values may appear to be higher than brachial SBP values in some people, which would be non-physiological. This appearance is only an artefact of the non-invasive brachial SBP being lower than the true brachial SBP, together with the synthesised central SBP being closer to the true (higher) central SBP.38,41 In the end it may be simpler to focus acceptance on only using BP methods that are validated to measure a representation of central aortic BP, as originally intended in the 19th century,1 rather than dissecting central from peripheral BP, which continues to fuel uncertainty in the field. This concept would require international cooperation to harmonise and validation of central BP-focussed thresholds, but may be preferred to lessen confusion and because central-to-peripheral BP amplification delivers no added (or little) prognostic power separate from central BP indices.12 In any case, the issue of appropriate calibration is likely to have profound implications on the way that central BP should be measured into the future.
The first data to impress the consequence of calibration was recently published in a cohort of 229 patients with hypertension, and showed that central SBP derived from oscillometric MAP and DBP calibrated brachial pressure waveforms (using Mobil-O-Graph, IEM over 24 h) was the strongest independent correlate of LV mass index (with higher discriminatory power to predict LV hypertrophy) compared with 24 h brachial SBP or central SBP calibrated with brachial SBP and DBP.41 The univariable pearson r correlations with LV mass index were 0.511, 0.399 and 0.332 respectively, with the slope of the relationship with MAP/DBP calibrated waveforms being significantly greater than for the other calibration methods. Whether this may be a device-specific phenomenon related to the algorithm to determine MAP is unknown and will need to be tested using other techniques. As always these findings generate new questions to resolve, but could represent a major turning point in terms of designing trials to gain greater clarity between the clinical worth of central compared with brachial BP. The expectation is that oscillometric MAP and DBP calibrated brachial waveforms will be the most robust method to accurately estimate central SBP and yield the strongest relationships with clinical outcomes.
Conclusions
More than one century ago the original invention to non-invasively measure brachial BP had the main goal of obtaining a clinically meaningful value that represented the pressure loading within the aorta (now commonly referred to as central BP). This important advancement opened the way for widespread use and elaborated understanding on the relation between BP and CV risk. Yet in recent decades, unassailable evidence has shown that the brachial BP method does not necessarily produce BP values that are representative of the true underlying central BP. Development of non-invasive methods to more accurately estimate central BP (and other indices from waveform analysis) has led to a consistent and growing volume of data indicating that central BP is a stronger CV risk determinant than brachial BP, and there is nothing to suggest a risk for harm (only advantage) by using central BP in clinical practice. The identification of some methodological and technical issues could jeopardise progression of the discipline and underscores the imperative for international collaboration to provide guidance. Altogether, it is clear that central BP techniques will help refine clinical decision making for doctors and enhance patient care above and beyond conventional brachial BP.
Conflict of interest
Dr Sharman has received research equipment or funding for research projects from AtCor Medical, IEM and Pulsecor. He has not received consultancies from companies that manufacture central BP devices and has no financial interest in central BP methods.
Grants
Dr Sharman is supported by a NHMRC Australian Clinical Research Fellowship (reference 409940).
Acknowledgement
The author is very grateful to Dr Siegfried Wassertheurer for providing helpful intellectual feedback on a draft of this manuscript.
References
Cite this article
TY - JOUR AU - James E. Sharman PY - 2014 DA - 2014/12/04 TI - Central pressure should be used in clinical practice JO - Artery Research SP - 1 EP - 7 VL - 9 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2014.11.001 DO - 10.1016/j.artres.2014.11.001 ID - Sharman2014 ER -
