Value of haemodynamic profiling to the response of antihypertensive therapy
Equal contributors.
- DOI
- 10.1016/j.artres.2014.07.004How to use a DOI?
- Keywords
- Antihypertensive agents; Hypertension; Vascular stiffness; Haemodynamics
- Abstract
Background: Essential hypertension is characterised by alterations in haemodynamics. Hence haemodynamic profiling could lead to improved blood pressure (BP) control in these patients. We tested if baseline haemodynamic indices predict the BP lowering effects of different classes of antihypertensive drugs in hypertensive patients.
Methods: In this double-blind placebo-controlled crossover study we randomised 53 hypertensive patients to receive doxazosin 4 mg, candesartan 16 mg, bisoprolol 5 mg, isosorbide mononitrate (ISMN) 50 mg, and placebo daily for 6 weeks. Brachial and central BP, augmentation index (AIx), aortic pulse wave velocity (aPWV), stroke volume (SV), cardiac output (CO), peripheral vascular resistance (PVR), and pulse pressure amplification (PPA) were measured at baseline and after each drug.
Results: Baseline AIx and PPA determined BP reduction with antihypertensive therapy, particularly with bisoprolol. In patients with low baseline AIx (1.7–28.9%) and high PPA (1.22–1.87), bisoprolol had a weak antihypertensive effect, while the opposite was observed in patients with high AIx (36.3–48.2%) and low PPA (1.05–1.11). With candesartan, BP reduction was the largest, regardless of baseline AIx or PPA levels.
Conclusions: Our study suggests that ARBs reduce BP the most irrespective of the underlying haemodynamic profile. Antihypertensive therapy guided by AIx and PPA may have some merit in the guidance of antihypertensive drug treatment, particularly if beta-blockers are considered for treatment. However, larger studies are needed to confirm these results.
Clinical Trials Registry number: EudraCT 2006-006981-40.
- Copyright
- © 2014 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Hypertension is a common disorder affecting about 1 billion people worldwide.1 Although a range of antihypertensive drugs are available, less than 30% of treated hypertensive patients are at or below blood pressure (BP) target levels.2 Although lack of compliance and presence of side effects may contribute to this, a significant number of patients remain above target despite adequate compliance with therapy. The physiological heterogeneity of hypertension may explain this phenomenon.3 Altered haemodynamics can play a central role in development and perpetuation of high BP.4 Hence a better understanding of the physiological basis for hypertension in these patients might lead to improved BP control, and a reduction in the number of antihypertensive drugs used.5 One could predict that drugs that reduce cardiac output (CO), (i.e beta-blockers),6 would be more efficacious in individuals with high CO. Similarly, vasodilators (i.e. alpha-blockers) could be more effective in individuals with increased peripheral vascular resistance (PVR).7 There is evidence that angiotensin II receptor blockers (ARBs) have BP-independent effects on arterial stiffness8 and could thus be efficacious in patients with high aortic pulse wave velocity (aPWV). Oral nitrate preparations have been shown to reduce augmentation index (AIx) significantly,9 indicating that they may be useful in patients with increased pulse wave reflection. The primary aim of the current study was to determine whether underlying haemodynamic abnormality in patients with hypertension determines their response to different antihypertensive agents. Secondly, we wished to obtain additional data on the physiological effects of the compared antihypertensive drugs.
Methods
Study subjects
Patients with essential hypertension were recruited from local hypertension outpatient clinics and from general practitioner clinics. Hypertension was defined as office seated brachial systolic BP > 140 and/or diastolic BP > 90 mmHg on 3 occasions. All studies were conducted in the Clinical Pharmacology Unit, University of Cambridge, Cambridge, United Kingdom, and in the Department of Cardiology, University of Tartu, Tartu, Estonia. We included newly diagnosed treatment-naïve hypertensive patients aged 18–80 years. We excluded patients with secondary hypertension, uncontrolled hypertension (brachial BP > 200/100 mmHg), and pregnant or nursing women and women of childbearing age not taking contraceptives. Additionally, patients with gout, asthma, heart failure, liver failure, renal failure, and terminal illnesses were excluded. The study protocol was approved by the relevant local research ethics committees, registered in the EU Clinical Trials Register (EudraCT No: 2006-006981-40), and the study was conducted according to the Declaration of Helsinki. All patients gave informed written consent.
Study protocol
This was a double-blind, randomised, placebo-controlled crossover study. After screening the eligibility of study subjects to the study, patients were called back for baseline measurements whereafter the subjects were allocated to receive study drugs in a random order. The study involved four different antihypertensive drugs and the drug dosages were gradually force titrated (please see Supplemental Table 1). We used candesartan 8 mg for 1 week and 16 mg thereafter; bisoprolol 2.5 mg for 1 week and 5 mg thereafter; isosorbide mononitrate MR (ISMN) 25 mg for 1 week and 50 mg thereafter; doxazosin SR 1 mg for week 1, 2 mg for week 2 and 4 mg thereafter; and placebo. All drugs were administered once a day. Each treatment phase lasted for 6 weeks after which, on day 42 of drug consumption, measurements were performed and the patient was switched to the next treatment phase. There were no wash-in or wash-out periods. Thus, each patient remained in the study for up to 30 weeks. All visits were performed between 08:00 and 11:00 h after an overnight fast and abstinence from any medication, tobacco, alcohol, tea, and coffee.
Haemodynamic measurements
The BP was measured from the dominant arm using a validated oscillometric technique (Omron HEM-705C, Tokyo, Japan). All measurements were taken in triplicate and the mean of the closest 2 readings was used in further analyses. Seated BP measurements were performed on the screening visit whereafter only supine BP measurements were performed.
Radial artery waveforms were recorded from the wrist of the dominant arm with a high-fidelity micromanometer (SPC-301; Millar instruments, Houston, TX, USA) and pulse wave analysis (Sphygmocor Px, Atcor Medical, Sydney, Australia) was used to generate a corresponding central waveform. From this, central aortic BP, AIx, mean arterial pressure (MAP), and heart rate (HR) were calculated as described previously.10 Briefly, AIx was defined as the difference between the second and first peaks of the central arterial waveform, expressed as a percentage of pulse pressure. Pulse pressure amplification (PPA) was calculated as the ratio of brachial pulse pressure to central pulse pressure. The aPWV was measured with the same device by sequentially recording ECG-gated carotid and femoral artery waveforms as described previously.10 Wave transit time was calculated by the system software using the R wave of a simultaneously recorded ECG as the reference frame. Path length for determination of aPWV was measured as the surface distance between the suprasternal notch and the femoral site minus the distance between the suprasternal notch and the carotid site using a tape measure. All measurements were made in duplicate and the mean values were used in analysis.
Stroke volume (SV) and CO were assessed noninvasively using the Innocor (Innovision, Odense, Denmark) inert gas re-breathing system. The PVR was calculated as MAP divided by CO.
Statistical analysis
Statistical analysis was performed using the SPSS software (version 18.0). Data are presented as mean ± SD. One-way repeated measures ANOVA with Bonferroni correction was used to analyse the effects of the drugs on haemodynamic parameters. The patients were divided by the tertiles of baseline haemodynamic parameters (aPWV, AIx, PVR, CO, SV, and PPA). Thereafter patients in the 1st and 3rd tertiles were compared regarding the change (from the values recorded after the placebo phase) in brachial systolic, diastolic, and central systolic BP for each drug using the independent samples t-test. The drug carry-over effect was assessed using treatment order as the independent variable. In all analyses, AIx was adjusted to HR and MAP; aPWV was adjusted to MAP. P < 0.01 was considered significant in Bonferroni corrected analysis. Otherwise, significance was defined as two-sided P < 0.05.
Results
Demographics and baseline characteristics
The descriptive characteristics of the study subjects are summarised in Table 1. Altogether 53 patients (41 patients studied at the University of Tartu, Estonia; 12 patients studied at the University of Cambridge, United Kingdom) completed the study. 56% of patients had systolic-diastolic hypertension, 40% of patients had isolated systolic hypertension, and 0.5% had isolated diastolic hypertension.
| Variable | Hypertensive patients (n = 53) |
|---|---|
| Age, y | 54.5 ± 12.1 |
| Sex, n | 25 M/28 F |
| Height, cm | 168.3 ± 8.5 |
| Weight, kg | 81.7 ± 13.1 |
| BMI, kg/m2 | 28.8 ± 4.1 |
| Brachial SBP, mmHg | 151.3 ± 18.6 |
| Brachial DBP, mmHg | 89.7 ± 9.7 |
| Brachial PP, mmHg | 61.6 ± 15 |
| Central SBP, mmHg | 142.8 ± 18.9 |
| Central DBP, mmHg | 90.8 ± 9.8 |
| Central PP, mmHg | 52 ± 14.7 |
| MAP, mmHg | 112.8 ± 12.4 |
| PPA | 1.21 ± 0.17 |
| AIx, % | 31.5 ± 6.2 |
| HR, bpm | 63.2 ± 9.4 |
| aPWV, m/s | 8.9 ± 3 |
| CO, l/min | 6.0 ± 1.7 |
| SV, ml | 81.2 ± 20.2 |
| PVR, dyne/s | 20.1 ± 5.2 |
| Glucose, mmol/l | 5.3 ± 0.6 |
| Total cholesterol, mmol/l | 5.6 ± 1 |
| HDL cholesterol, mmol/l | 1.4 ± 0.5 |
| LDL cholesterol, mmol/l | 3.8 ± 1 |
| Triglycerides, mmol/l | 1.5 ± 0.8 |
AIx, augmentation index; aPWV, aortic pulse wave velocity; BMI, body mass index; CO, cardiac output; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; PPA, pulse pressure amplification; PVR, peripheral vascular resistance; SBP, systolic blood pressure; SV, stroke volume.
Baseline characteristics.
Changes in haemodynamic indices with treatment
The haemodynamic indices after placebo and all active drugs are shown in Table 2. All drugs significantly reduced brachial and central BP and MAP. However, candesartan reduced brachial and central systolic BP, and pulse pressure the most. All drugs except bisoprolol increased PPA (P < 0.01). The aPWV corrected to MAP did not change with any drug. The AIx corrected to MAP and HR was reduced by all drugs except bisoprolol, with ISMN having the relatively largest effect (Δ for ISMN: −5.4%) (P < 0.01). The CO increased with doxazosin (Δ: +0.4 l/min) and was not affected by the other drugs (P < 0.01). Bisoprolol (Δ: +14.4 ml) and doxazosin (Δ: +7.3 ml) significantly increased SV, while ISMN (Δ: −5.2 ml) reduced it (P < 0.01). Doxazosin, candesartan, and bisoprolol significantly decreased PVR, with doxazosin having the largest effect (Δ for doxazosin: −3.7 dyne/s) (P < 0.01). Treatment order as the independent variable did not affect the results of the analysis.
| Placebo | Doxazosin | Candesartan | Bisoprolol | ISMN | P value | |
|---|---|---|---|---|---|---|
| Brachial SBP, mmHg | 148.7 ± 16 | 131.6 ± 15.8a | 127.3 ± 13.4a | 132.5 ± 15.2a | 135.6 ± 16.8ab | <0.001 |
| Brachial DBP, mmHg | 87 ± 8.5 | 78.8 ± 9.6a | 77.5 ± 7.8a | 78.3 ± 8.6a | 80.5 ± 9a | <0.001 |
| Brachial PP, mmHg | 61.7 ± 13 | 52.8 ± 12.8a | 49.8 ± 11.2a | 54.2 ± 12.8ab | 55.1 ± 13.6a | <0.01 |
| Central SBP, mmHg | 139.6 ± 16.3 | 120.9 ± 15.1a | 117.3 ± 14.7a | 125.8 ± 14.3ab | 124.3 ± 17.5a | <0.001 |
| Central DBP, mmHg | 88.1 ± 8.5 | 79.7 ± 9.4a | 79 ± 8.3a | 79.3 ± 8.7a | 81.4 ± 9.2a | <0.001 |
| Central PP, mmHg | 51.6 ± 12.8 | 41.2 ± 11.1ad | 38.3 ± 12.2ad | 46.5 ± 12.1a | 42.8 ± 14.2a | <0.001 |
| MAP, mmHg | 109.4 ± 10.8 | 97.2 ± 11a | 95 ± 9.7a | 98.6 ± 10a | 99.1 ± 11.4a | <0.001 |
| PPA | 1.2 ± 0.2 | 1.3 ± 0.3ad | 1.4 ± 0.4ad | 1.2 ± 0.2 | 1.3 ± 0.2ad | <0.001 |
| aPWV, m/s¶ | 8.2 ± 1.6 | 8.5 ± 1.4 | 8.6 ± 1.7 | 8.3 ± 1.6 | 8.5 ± 1.6 | 0.8 |
| AIx, %§ | 30.6 ± 11.5 | 28 ± 10.5a | 27.7 ± 12a | 28.1 ± 9.3 | 25.2 ± 10.6a | <0.001 |
| HR, bpm | 63.1 ± 9.4 | 61.8 ± 9d | 63.3 ± 8.6d | 54.6 ± 8.3a | 64.2 ± 8.6d | <0.001 |
| CO, l/min | 5.9 ± 1.8 | 6.3 ± 1.8acd | 6.1 ± 1.6 | 5.9 ± 1.5 | 5.8 ± 1.8 | <0.001 |
| SV, ml | 81.7 ± 23.3 | 89 ± 25.2ac | 86.3 ± 22.9 | 96.1 ± 22abc | 76.5 ± 23.1b | <0.001 |
| PVR, dyne/s | 20.1 ± 5.9 | 16.4 ± 4.2acd | 16.6 ± 4.8ac | 17.7 ± 4.1a | 18.5 ± 5.1 | <0.001 |
AIx, augmentation index; aPWV, aortic pulse wave velocity; CO, cardiac output; DBP, diastolic blood pressure; HR, heart rate; ISMN, isosorbide mononitrate; MAP, mean arterial pressure; PP, pulse pressure; PPA, pulse pressure amplification; PVR, peripheral vascular resistance; SBP, systolic blood pressure; SV, stroke volume. Values in the final column represent the results of one-way repeated measures ANOVA.
indicates data corrected for MAP and HR;
indicates data corrected for HR.
P < 0.01 vs placebo.
P < 0.01 vs candesartan.
P < 0.01 vs ISMN.
P < 0.01 vs bisoprolol.
Crossover comparison of the haemodynamic variables following treatment with each of the four antihypertensive drugs.
Haemodynamic indices and reduction in blood pressure
Comparison of the 1st and 3rd tertiles of the baseline haemodynamic parameters revealed baseline AIx and PPA as determinants of BP reduction. The patients in the lowest tertile of baseline AIx (AIx value: 1.7–28.9%), comprising mainly women, were significantly older, shorter, and more hypertensive than the patients in the highest tertile (AIx value: 36.3–48.2%) (Table 3). There was a significant difference in BP change between the tertiles of AIx for all drugs except ISMN (Fig. 1). With candesartan, reduction in brachial and central systolic BP was the largest, regardless of the baseline AIx tertile. Bisoprolol was relatively weak at reducing brachial and central BP in the 1st tertile of AIx. However, in the 3rd tertile the BP lowering effect of bisoprolol (Δ brachial systolic BP: −23.4 mmHg) was roughly comparable to that of doxazosin (Δ brachial systolic BP: −22.3 mmHg).
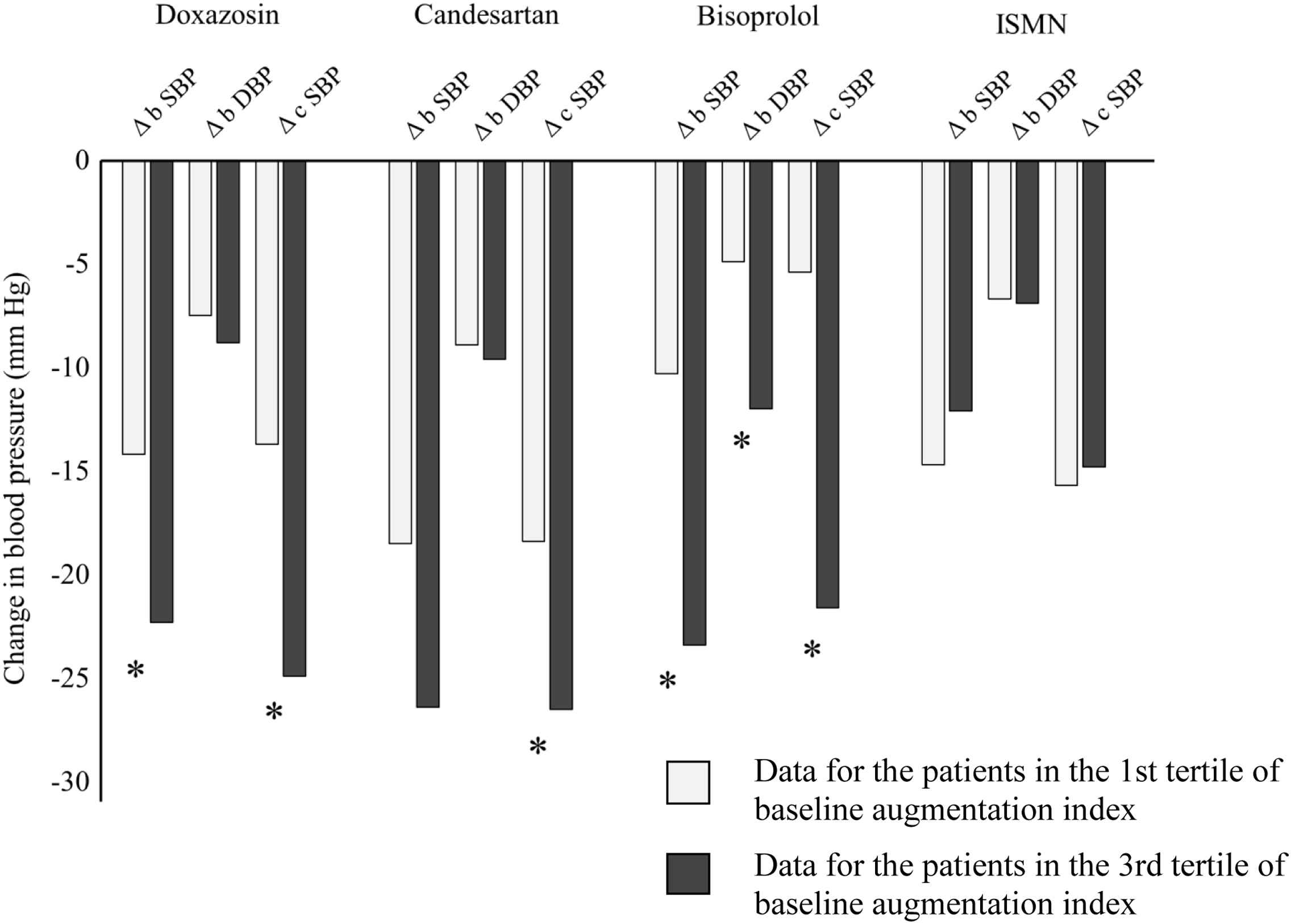
Comparison of blood pressure change in patients divided by tertiles of baseline augmentation index. * indicates P < 0.05. b DBP, brachial diastolic blood pressure; b SBP, brachial systolic blood pressure; c SBP, central systolic blood pressure; ISMN, isosorbide mononitrate.
| Characteristic | Tertiles of augmentation index (%) | ||
|---|---|---|---|
| First (1.7–28.9) | Third (36.3–48.2) | P value | |
| (n = 17) | (n = 18) | ||
| Age, y | 49.1 ± 14.1 | 58.1 ± 9.6 | <0.05 |
| Males, % | 15 (88.2) | 4 (22) | <0.001 |
| Weight, kg | 86.9 ± 13.5 | 78.2 ± 6.9 | <0.05 |
| Height, cm | 176.5 ± 6.7 | 163.9 ± 6.1 | <0.001 |
| BMI, kg/m2 | 27.9 ± 3.6 | 29.2 ± 2.9 | 0.3 |
| Aortic PWV, m/s | 8.1 ± 1.4 | 8.7 ± 1.6 | 0.3 |
| Systolic BP, mmHg | 146.9 ± 17 | 162.4 ± 20.9 | <0.05 |
| Diastolic BP, mmHg | 86.9 ± 8.3 | 95.7 ± 10.0 | <0.01 |
| Heart rate, bpm | 62.1 ± 9.7 | 64.0 ± 10.8 | 0.6 |
| MAP, mmHg | 107.5 ± 10.3 | 122.9 ± 12.8 | <0.001 |
DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
Subject characteristics at baseline according to tertiles of baseline augmentation index.
The patients in the highest tertile of PPA (baseline PPA value: 1.22–1.87) comprising mainly men, were significantly younger, taller, less hypertensive and had lower AIx and HR than the patients in the lowest tertile of PPA (baseline PPA value: 1.05–1.11) (Table 4). There was a significant difference in BP change between the tertiles of PPA for all drugs except candesartan (Fig. 2). However, candesartan reduced brachial and central BP significantly more than the other drugs, irrespective of the tertile of baseline PPA. With bisoprolol, the reduction in brachial and central systolic BP was more significant in patients with low baseline PPA (Δ brachial systolic BP: −23.1 mmHg and Δ central systolic BP: −21.1 mmHg) compared to patients with high baseline PPA (Δ brachial systolic BP: −11.1 mmHg and Δ central systolic BP: −7.7 mmHg) (P < 0.05).
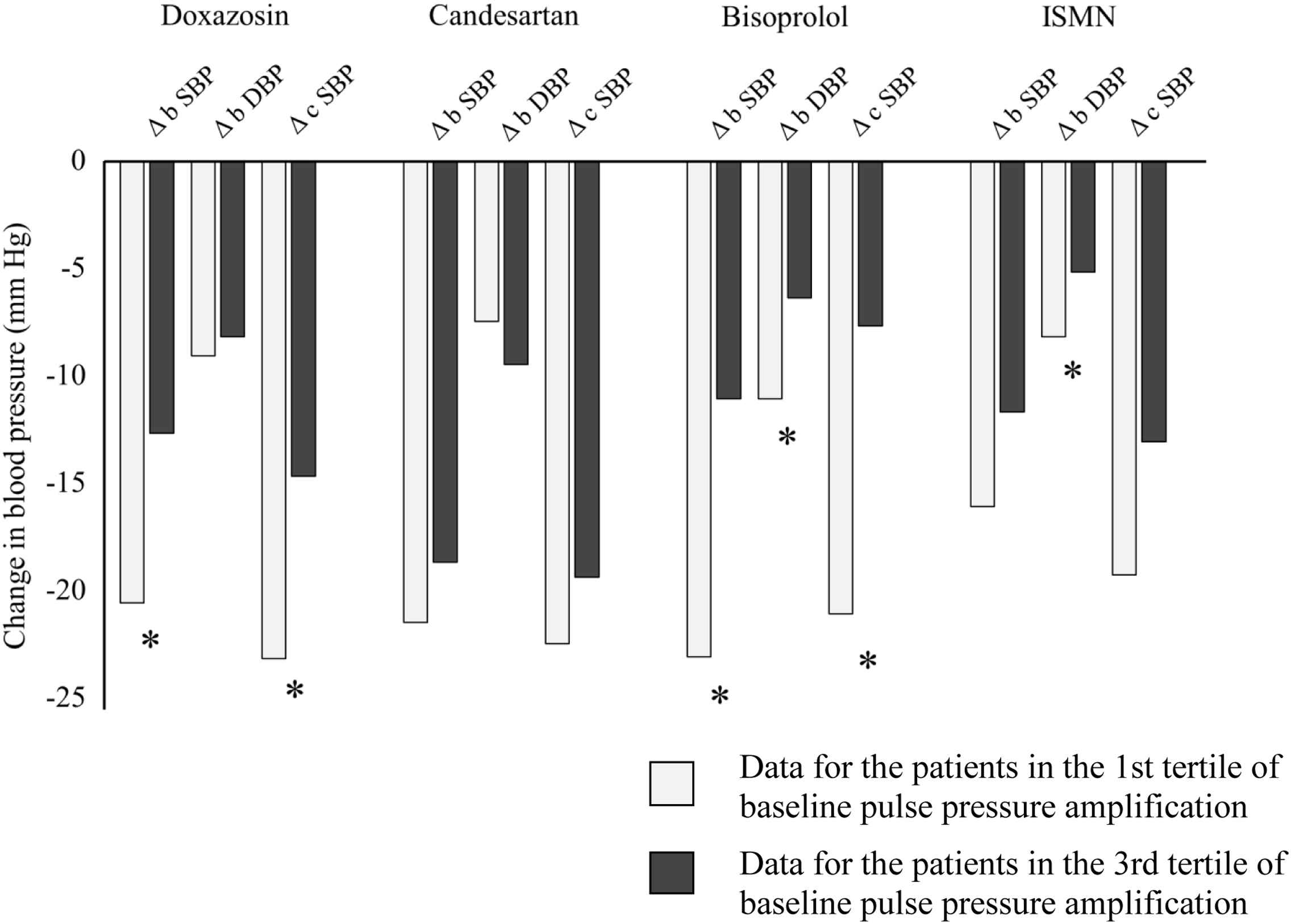
Comparison of blood pressure change in patients divided by tertiles of baseline pulse pressure amplification. * indicates P < 0.05. b DBP, brachial diastolic blood pressure; b SBP, brachial systolic blood pressure; c SBP, central systolic blood pressure; ISMN, isosorbide mononitrate.
| Characteristic | Tertiles of pulse pressure amplification | ||
|---|---|---|---|
| First (1.05–1.11) | Third (1.22–1.87) | P value | |
| (n = 17) | (n = 18) | ||
| Age, y | 57.4 ± 9.3 | 47.5 ± 12.7 | 0.01 |
| Males, % | 4 (24) | 15 (83) | 0.001 |
| Weight, kg | 78.9 ± 11.3 | 86.1 ± 13.6 | 0.1 |
| Height, cm | 162.7 ± 6.4 | 174.0 ± 8.1 | <0.001 |
| BMI, kg/m2 | 29.8 ± 3.9 | 28.4 ± 3.6 | 0.3 |
| AIx, % | 38.5 ± 3.9 | 25.4 ± 13.1 | 0.001 |
| Aortic PWV, m/s | 8.9 ± 1.7 | 8.1 ± 1.4 | 0.2 |
| Systolic BP, mmHg | 159.9 ± 20.6 | 145.9 ± 14.5 | <0.05 |
| Diastolic BP, mmHg | 91.8 ± 10.8 | 87.8 ± 7.3 | 0.2 |
| Heart rate, bpm | 57.4 ± 5.7 | 70.2 ± 10.4 | <0.001 |
| MAP, mmHg | 118.7 ± 12.8 | 108.1 ± 7.9 | 0.007 |
DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
Subject characteristics at baseline according to tertiles of baseline pulse pressure amplification.
Comparison of the 1st and 3rd tertiles of baseline SV, CO, aPWV, or PVR revealed no significant differences in the BP change with any drug.
Because there was a significant difference in BP values in the patients in the 1st and 3rd tertile of AIx and PPA, we additionally investigated the baseline characteristics of the patients in the 1st and 3rd tertiles of SV, PVR, aPWV, and CO. There was a significant difference in BP values between the patients in the 1st and 3rd tertile of PVR (Supplemental Table 2), however no differences existed between the tertiles of SV, aPWV, or CO.
Discussion
We studied whether the haemodynamic profile of patients with essential hypertension determines the efficacy of an alpha-blocker, a beta-blocker, an ARB, or a nitrate. To our knowledge, this is the first study to systematically investigate a complex of haemodynamic parameters (i.e. SV, CO, aPWV, AIx, PPA, and PVR) which could influence the efficacy of antihypertensive drugs. We found that baseline AIx and PPA determined the response to antihypertensive therapy by the extent of brachial and/or central BP reduction. Haemodynamic profiling by baseline AIx determined BP reduction with doxazosin, bisoprolol, and candesartan. Haemodynamic profiling by PPA determined BP reduction with doxazosin, bisoprolol, and ISMN. The largest effect of haemodynamic profiling by AIx and PPA which determined BP reduction was demonstrated with bisoprolol. The characterisation of the tertiles of these haemodynamic indices revealed in turn that BP could be a confounding factor for these results. Baseline aPWV, SV, CO, and PVR did not determine the response to the drugs used in our study. Candesartan had the most impressive brachial and central BP reducing properties, regardless of the baseline haemodynamic profile.
Augmentation of the pulse wave is dependent on the speed of pulse wave travel, the amplitude of the reflected wave, the reflectance sites and the duration and pattern of ventricular ejection, including HR and ventricular contractility.11 In contrast to other drugs, only bisoprolol did not change AIx in our study. It is well known that beta-blockers have a negative or neutral effect on AIx. As expected, only bisoprolol reduced HR significantly, which may explain our result. Due to HR reduction, beta-blockers favour the arrival of the reflected wave in the relatively earlier phase in the systole, instead of the diastole.12,19 Nitrates can be regarded as the most potent drug to reduce pulse wave reflection in ISH patients.9 In our study, although ISMN reduced AIx the most, the change in AIx was smaller than expected from previous studies.9 The modest effect of ISMN on AIx may explain the relatively small reduction in BP with this drug. Among the patients in the 3rd tertile of AIx, the alpha-blocker, the beta-blocker, and the ARB effectively reduced BP. Among these drugs, alpha-blockers and ARBs are known to cause vasodilation in the peripheral arteries.7,12 However, the results concerning beta-blockers are intriguing. Our results suggest that in patients with low AIx, beta-blockers are comparatively less effective at reducing BP, compared to the patients in the 3rd tertile. These results can be explained by the baseline characteristics of these patients. Patients with higher AIx had substantially higher baseline BP. There is evidence that higher baseline BP is associated with a larger extent of decrease in BP.14
Brachial BP differs from central BP owing to the phenomenon of PPA.15 There is evidence that central BP predicts cardiovascular risk better than brachial BP does.16 Moreover, different antihypertensive drugs reduce central BP differentially,15,17 which can be a better determinant of subclinical organ damage and clinical outcome.18,19 In our study candesartan reduced both brachial and central BP the most. Hence, direct comparison of on-treatment central BP may underestimate the differential effect of study drugs on central BP. The PPA could be a better measure of inter-drug comparison in our study because it takes into account both brachial and central BP. All drugs except bisoprolol increased PPA, which is in accordance with previous evidence.12 Baseline PPA predicted the BP lowering effects of doxazosin, bisoprolol, and ISMN. However, the results regarding PPA as an underlying haemodynamic alteration cannot be considered independently. This is because it is determined by pulse wave reflections within the arterial tree and by arterial stiffness.20
There is long-term evidence from the REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double-Blind) study where atenolol and perindopril + indapamide reduced aPWV to a similar degree.20 The post-hoc analysis of the REASON study showed that aPWV predicts BP reduction in hypertension.21 However, in our study aPWV corrected for MAP was not changed by any drug and baseline aPWV did not predict the reduction of BP by the studied drugs. It is possible that in our study the period for detection of complete BP reduction and “destiffening” (6 weeks) was inadequate.22
In our study CO did not predict BP reduction with antihypertensive treatment, which would have been expected with bisoprolol. The failure to show CO as a predictor of BP reduction with the beta-blocker in our study may be due to the neutral effect of bisoprolol on CO. The mean baseline CO in our study patients was 6.0 l/min, which is lower than it was in a similar group of patients (6.4 l/min)23 and even in young normotensive subjects (6.9 l/min).3 It is possible that the compensatory mechanism, i.e. increase in stroke volume (Δ for bisoprolol: +14.4 ml) mitigated the decrease of CO with bisoprolol. The PVR is positively related to MAP and inversely related to CO. While doxazosin reduced MAP similarly to the other drugs it significantly increased CO, which might have contributed to its larger effect on PVR in our study. However, haemodynamic profiling with the use of PVR failed to predict BP reduction with doxazosin.
Smith et al. have shown that antihypertensive treatment guided by impedance cardiography (measurement of cardiac index and systemic vascular resistance index) during antihypertensive treatment improves BP control.5 As in the above study the patients had been treated previously and they had also received combination therapy where the effects of individual drug classes could not be identified. However, this result indicates that additional haemodynamic indices can have importance in predicting the efficacy of antihypertensive treatment. In addition, it would have been interesting to analyse the data separately in patients with resistant hypertension. However, our study was not powered for such analysis and this remains to be investigated in the future.
There are several limitations in our study. Firstly, we had a relatively small sample size. Secondly, there was no wash-in or wash-out period. However, treatment order was used as an independent variable in the analysis which lessens the importance of this limitation. Thirdly, we studied acute effects of drugs lasting for 6 weeks for each phase, rather than chronic use which might have different effect.21 In addition, there was a difference in baseline BP between the tertiles of AIx and PPA. However, there was also a difference in baseline BP between the tertiles of PVR which did not predict BP reduction with treatment. All patients in the study were treatment-naïve, hence we cannot interpret the results of the study to all hypertensive patients.
To our knowledge, this is the first study to systematically investigate whether haemodynamic alterations in patients with hypertension determines their response to different antihypertensive agents. Among the drug classes used in our study, the ARB reduced BP the most regardless of the haemodynamic profile. Our study suggests that haemodynamic profiling with the use of AIx or PPA could be beneficial, especially if beta-blockers are being considered for therapy in essential hypertension. However, larger studies are needed to confirm these results.
Funding and support
I.B.W. and C.M.M. receive support from the British Heart Foundation. The authors acknowledge the support from the National Institute of Health Research, the Cambridge Biomedical Research Centre and the West Anglia Comprehensive Local Research Network, as well as from the Estonian Scientific Foundation grant no. 8273 and target financing nos. 0180105s08 and SF0180001s07, and institutional research funding no. IUT2-7.
Disclosure
All authors are employed by the University of Cambridge, Addenbrooke’s Hospital, Cambridge, United Kingdom (K.M.-P., C.M.M., I.B.W.), Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom (J.G., J.C.), University of Tartu, Tartu, Estonia (M.S., P.K., M.Za., J.K., M.Zi., J.E.) or Tartu University Hospital, Tartu, Estonia (P.K., J.K., J.E.).
Conflict of interest
None.
Acknowledgements
We are grateful to the Evelyn Trust. We thank the referring clinicians and research nurses for their contribution, particularly Michaela Watts and Eva-Brit Mölder.
Appendix A
Supplementary data
Supplementary data related to this article can be found at
References
Cite this article
TY - JOUR AU - M. Serg AU - J. Graggaber AU - P. Kampus AU - M. Zagura AU - J. Kals AU - K. Mäki-Petäjä AU - J. Cheriyan AU - M. Zilmer AU - J. Eha AU - C.M. McEniery AU - I.B. Wilkinson PY - 2014 DA - 2014/09/22 TI - Value of haemodynamic profiling to the response of antihypertensive therapy JO - Artery Research SP - 189 EP - 196 VL - 8 IS - 4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2014.07.004 DO - 10.1016/j.artres.2014.07.004 ID - Serg2014 ER -
