Interarm Differences in Brachial Blood Pressure and their Effect on the Derivation on Central Aortic Blood Pressure
- DOI
- 10.2991/artres.k.200201.002How to use a DOI?
- Keywords
- Interarm blood pressure differences; blood pressure monitoring; cardiovascular disease; aortic blood pressure
- Abstract
Background: Inter-arm differences in brachial systolic Blood Pressure (BP) are associated with increased cardiovascular risk. It is unclear whether anatomical factors contribute to brachial Interarm Blood Pressure (IABP) differences or whether brachial IABP differences translate to differences in derived central aortic BP. This study aimed to ascertain whether IABP differences in brachial BP correlate with anatomical factors (arm side, dominance, and geometry) and translate to differences in derived central BP.
Methods: Brachial BP and derived central BP were measured simultaneously in both arms in 77 community-dwelling adults (18–66 years, 38 male) using two SphygmoCor XCEL (AtCor Medical) BP devices. Measurements were taken 3–4 times in each participant, swapping devices between measurements. An optoelectronic volumeter (Perometer 350S) and hand-held dynamometer (Saehan) were used to measure arm volume and maximal hand-grip strength. Differences in brachial and derived central BP between arms were evaluated by paired t-tests. Regression analysis was used to examine predictors of IABP differences.
Results: Absolute IABP difference in brachial systolic BP was 4.2 ± 3.6 mmHg. Brachial systolic IABP differences were not different between arms (right/left, dominant/non-dominant, or large/small arm volume). Brachial systolic IABP differences were not correlated with differences in arm volume or grip strength. Male sex and diastolic BP were the only predictors. Brachial systolic IABP difference translated to a small (3.1 ± 2.4 mmHg) difference in derived central BP.
Conclusion: As there is only a single aortic BP, we consider the difference in derived central BP likely an artefact. The possibility that it results from BP variability warrants further investigation.
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Hypertension is a major health challenge affecting over 30% of adults worldwide [1] and is the major risk factor for cardiovascular and cerebrovascular disease [2]. Hence, accurate and comprehensive methods for diagnosing and monitoring hypertension are crucial to reduce this health burden. Clinical guidelines recommend that comprehensive screening for hypertension includes measuring arterial Blood Pressure (BP) in both arms at the initial visit [3,4]. This recommendation arises from epidemiological studies showing interarm differences in brachial systolic BP of ≥10 mmHg are associated with increased risk of vascular disease [5,6], and differences ≥15 mmHg are linked to widespread vascular disease and increased mortality [5–8]. Thus, identification of interarm BP (IABP) differences offers prognostic utility when screening for overall cardiovascular risk and may complement identification of a hypertensive phenotype.
Despite the relative simplicity of IABP measurement, several uncertainties around IABP differences prevail. First, although many studies have examined the presence of IABP differences, the prevalence of IABP differences, especially in normal community-dwelling adults, is unclear. In a systematic review by Clark et al. [9], the pooled analysis showed that approximately 20% of individuals had an IABP difference ≥10 mmHg and approximately 4% over 20 mmHg. However, these findings are likely to be an overestimate, given that most of the studies were conducted on diseased populations. Also, most studies measured IABP sequentially rather than simultaneously. Sequential measures yield artefactually higher IABP differences than simultaneous measures due to differences in timing, which reflect BP variability [10]. Second, it has been suggested that handedness and arm geometry may confound IABP differences. Potentially, increases in arm circumference secondary to handedness and grip strength may impact on the transmission of the cuff pressure to the artery, leading to an artefactually higher BP in the ‘larger’ arm.
It is also unknown how brachial IABP compares with central aortic IABP, as no studies have examined central BP derived from brachial BP measured simultaneously in each arm. Interest in this parameter stems from the fact that central BP is more predictive of vascular damage and cardiovascular outcomes then brachial BP, as it represents the direct load on the heart, brain and kidneys [11,12]. If the singular pressure value in the aorta gives rise to two different BP in each arm, this should be reflected in the pressure waveform. Working backwards, using these two waveforms in each arm to estimate central aortic BP should theoretically yield a single value of central aortic BP even where there are interarm differences in BP. However, whether or not brachial IABP differences, measured simultaneously with two identical BP monitors, translates to differences in derived central BP needs to be confirmed.
Thus, the main aim of this study was to determine if handedness and arm geometry influenced IABP differences in a cohort of community-dwelling adults. We hypothesized that simultaneously measured IABP differences would be higher in the dominant arm and the larger arm. The second aim was to compare the central BP derived from the brachial pressure waveform measured from each arm in community-dwelling adults. We hypothesised that IABP differences in measured brachial BP would not translate into differences in derived central BP, as there is only one single value of aortic pressure at any one time.
2. MATERIALS AND METHODS
2.1. Participants
Seventy-seven community-dwelling adults volunteered for this study. Participants were recruited from the Faculty of Medicine and Health Sciences and the wider university community. Participants were only excluded if they were under 18 years of age or had previous arm pathology (previous upper limb surgery, injury or paralysis) or lymphoedema, which could confound [13] or contraindicate [14] BP measures, respectively. Females were not pregnant. This study was approved by the Macquarie University Human Research Ethics Committee and conformed to the standards set by the Declaration of Helsinki. All participants gave written informed consent.
2.2. Experimental Design
The experiment was conducted in a quiet, temperature-controlled (21°C) laboratory (Blood Pressure and Vascular Function Laboratory, Macquarie University). Participants attended on one occasion.
On arrival at the laboratory participants undertook anthropometric measures and a medical health screening questionnaire including smoking history, presence of Cardiovascular Disease (CVD), family history of CVD (including hypertension) when the family member under 65 years of age, medications and exercise. They then underwent non-invasive measurements of: (i) brachial and central BP; (ii) arm circumference and arm volume; and (iii) grip strength. Details are as follows:
2.2.1. Brachial and derived central BP
Brachial and central BP were measured bilaterally in the seated position using two identical automatic BP devices (SphygmoCor XCEL, AtCor Medical, Naperville, USA) with appropriately sized cuffs. Following a 5-min rest period BP was measured three to four times simultaneously on each arm, with 2 min rest between measurements. The arms were supported during measurements and SphygmoCor units were swapped between measurements to eliminate machine biases. The order of application of machine for the first arm measured was also randomised.
During operation the brachial cuff was initially inflated to a suprasystolic level and deflated as per the device control to measure brachial BP. It then inflated to a sub-diastolic pressure and a brachial cuff volumetric displacement waveform was acquired. The central (aortic) waveform was derived from the brachial waveform, using proprietary digital signal processing and a generalised transfer function built into the device [15]. Brachial Systolic and Diastolic BP (bSP and bDP, respectively) and the derived central Aortic Systolic and Diastolic BP (aSP and aDP, respectively) were recorded for each measurement. After measurements the position of the upper, lower border of the cuff, and midpoint were marked on the anterior and posterior aspect of the arm to standardise the arm circumference and volume measurements.
2.2.2. Arm circumference and volume
An optoelectronic volumeter (Perometer 350S, Pero-system GmbH, Wupertal, Germany) was used to measure arm circumference and volume. This comprised a moveable frame, embedded with rows of infrared light emitters at 90° and sensors on opposite sides. For measurement, participants were seated with their bare arm abducted to 90°, their elbow fully extended with their middle finger touching the end of the hand-rest, palm facing down. The participants’ arm was centred over the Perometer track and the assessor slid the frame slowly along the longitudinal axis of the limb from the ulnar styloid to the shoulder. As the frame was moved along the frame, the Perometer measured vertical and horizontal diameters at 5 mm intervals [16]. Measurements were taken twice on each arm at the level of the cuff, and the mean of the two measurements were used. Two segmental volumes were calculated at the level of the cuff position using the formulae for a truncated cone [for details see Eq. (1) in Dylke et al. [17]).
2.2.3. Grip strength
Maximal handgrip was measured in both arms using a hydraulic hand-held dynamometer (Saehan, Chungbuk, Republic of Korea). Measurements were taken with the participant standing with their elbow by their side and flexed at 90°. Handgrip strength was measured three times, with intervening rest. The best (peak) value of the 2–3 handgrips was taken to represent the maximal voluntary contraction and used as the measure of handgrip strength.
2.3. Statistical Analysis
Demographic, clinical and cardiovascular data for the entire cohort are presented as mean ± Standard Deviation (SD) or percentages. Data were analysed using the Statistical package for Social Sciences (IBM SPSS version 262, Survey, UK) or R (R Foundation for Statistical Computing, version 3.5.1, Vienna, Austria). Statistical significance was set at p < 0.05 for all comparisons.
Paired t-tests were used to compare differences in anthropometric, anatomical and cardiovascular measures. Paired t-tests were also used to compare BP between: (i) sides (right versus left arm); (ii) the dominant versus the non-dominant arm; and (iii) the arm with the larger versus smaller arm volume. Absolute IABP differences were also determined by subtracting the highest minus the lowest BP. Bi-directional stepwise linear regression was used to determine the associations between brachial systolic and diastolic IABP differences with male sex, age, heart rate, Body Mass Index (BMI), and Pulse Pressure (PP). Non-dominant arm bDP was also entered into the model for systolic IABP differences and vice-versa.
In a separate analysis, the prevalence of absolute systolic IABP differences above and below 10 mmHg was explored. The clinically significant IABP difference threshold of 10 mmHg is in accordance with the National Institute for Health and Clinical Excellence guidelines [18]. Differences in demographic and clinical characteristics between the two groups were evaluated by unpaired t-tests (numerical data) or Chi-square tests (categorical data).
3. RESULTS
As shown in Table 1, participants were 35 ± 15 years of age and had a BMI of 24 ± 5 kg/m2 (i.e. healthy weight). Half the participants were female. There were no differences in age or BMI between sexes (not shown). Most of the participants were right hand dominant and 87% identified as Caucasian. The self-reported medical history and lifestyle data from this general community-dwelling population revealed: (i) the majority of participants had never smoked; (ii) more males than females (six versus one) had known CVD including hypertension; (iii) almost half the participants had a family history of CVD; and (iv) approximately two-thirds of participants reported activity levels that would meet the current activity guidelines of ≥150 minutes of moderate intensity exercise per week, which included bilateral arm activities. All hypertensive participants were being managed with anti-hypertensive medications. No participants had diabetes, although one was pre-diabetic, and another had gestational diabetes.
| Participants (n = 77) | |
|---|---|
| Age (y) | 35 ± 15 (range 18–66) |
| Sex (male:female) | 38:39 |
| Height (m) | 1.72 ± 0.09 |
| Weight (kg) | 72 ± 15 |
| BMI (kg/m2) | 24 ± 5 |
| Heart rate (b/min) | 70 ± 12 |
| Right arm dominant arm [n (%)] | 68 (88) |
| Smokers | |
| Current [n (%)] | 3 (4) |
| Ex-smoker [n (%)] | 4 (5) |
| CVD [known (%)] | 7 (9) |
| Family history of CVD [n (%)] | 32 (42) |
| Cardiovascular medications [n (%)] | 6 (8) |
| Exercisea [n (%)] | 54 (70) |
Self-reported moderate intensity exercise ≥150 min week. Data are mean ± SD (or percentage).
BMI, body mass index; CVD, cardiovascular disease.
Demographic and clinical characteristics of all participants
Table 2 displays grip strength, arm volume and BP compared by side (right versus left arm), handedness (dominant versus non-dominant arm) and arm geometry (larger versus smaller arm volume). On average, participants had a stronger grip on their right, dominant and larger arm, than their left, non-dominant and smaller arm, respectively. Mean brachial and derived-central diastolic BP were the same in each arm, irrespective of side, dominance or arm volume. Arm, hand dominance and arm volume did not necessarily result in a higher bSP in the same individual. Only half of the participants had a higher mean bSP in their right arm (48%), their dominant arm (49%) or their larger arm (47%). In 9% of participants the bSP was no different between arms irrespective of side, dominance, or volume.
| Right arm | Left arm | Dominant hand | Non-dominant hand | Larger arm | Smaller arm | Absolute difference | |
|---|---|---|---|---|---|---|---|
| Handgrip strength (kg) | 39 ± 10 | 36 ± 10* | 39 ± 10 | 36 ± 10* | 38 ± 10 | 37 ± 11* | 3 ± 2 |
| Arm volume (ml) | 643 ± 168 | 634 ± 177 | 645 ± 169 | 633 ± 175* | 657 ± 177 | 620 ± 163* | 38 ± 6 |
| Brachial BP | |||||||
| bSP (mmHg) | 125 ± 12 | 125 ± 12 | 125 ± 13 | 124 ± 13 | 125 ± 13 | 125 ± 13 | 4.2 ± 3.6** |
| bDP (mmHg) | 77 ± 9 | 78 ± 9 | 78 ± 9 | 77 ± 10 | 77 ± 10 | 78 ± 9 | 2.4 ± 1.8** |
| Derived-central aortic BP | |||||||
| aSP (mmHg) | 111 ± 11 | 111 ± 10 | 111 ± 11 | 111 ± 12 | 111 ± 11 | 111 ± 11 | 3.1 ± 2.4** |
| aDP (mmHg) | 78 ± 9 | 79 ± 9 | 79 ± 10 | 78 ± 10 | 78 ± 10 | 79 ± 10 | 2.4 ± 1.8** |
p < 0.05, for comparisons between right versus left arm, dominant versus non-dominant arm, and larger versus smaller arm comparisons.
p < 0.001, compared with no difference.
Data are mean ± SD for 77 participants. BP, blood pressure; bSP, brachial systolic pressure; bDP, brachial diastolic pressure; aSP, derived-central systolic pressure; aDP, derived-central diastolic pressure.
Mean baseline grip strength, arm volume and cardiovascular parameters in all participants
Across all participants the absolute IABP differences, irrespective of direction (i.e. highest – lowest arm pressure), for bSP (4.2 ± 3.6 mmHg) and bDP (2.4 ± 1.8 mmHg) were significantly different from zero (p < 0.001). These absolute interarm differences translated to small but significant differences in aSP (3.1 ± 2.4 mmHg) and aDP (2.4 ± 1.8 mmHg) (p < 0.001, Table 2).
Participants were stratified into those with an absolute bSP difference above 10 mmHg and those below 10 mmHg (Table 3). Nine participants (i.e. 12% of the cohort) had an interarm bSP difference ≥10 mmHg, of which one had an interarm bSP difference of 15 mmHg. There were no significant differences in age, sex (p = 0.087), BMI, or BP between the two groups. The percentage of individuals who: (i) had an elevated BMI (≥25 kg/m2); (ii) a current or previous smoking habit; (iii) used cardiovascular medications; or (iv) reported exercising above the standard activity guidelines [19], were no different between groups.
| Absolute aSP IABP differences (mmHg) | ||
|---|---|---|
| <10 mmHg | ≥10 mmHg | |
| Number [n (%)] | 68 (88) | 9 (12) |
| Age | 36 ± 15 (range 19–66) | 29 ± 13 (range 18–63) |
| Male [n (%)] | 31 (46) | 7 (78) |
| BMI (kg/m2) | 24 ± 5 | 25 ± 4 |
| BMI over 25 kg/m2 [n (%)] | 28 (41) | 3 (33) |
| Current or ex-smokers [n (%)] | 7 (10) | 0 (0) |
| Cardiovascular medications [n (%)] | 6 (9) | 0 (0) |
| Exercisea [n (%)] | 45 (66) | 7 (78) |
| BP (non-dominant arm) | ||
| bSP | 125 ± 13 | 123 ± 14 |
| bDP | 78 ± 9 | 72 ± 8 |
| aSP | 111 ± 12 | 108 ± 14 |
| aDP | 79 ± 10 | 73 ± 8 |
Self-reported moderate intensity exercise ≥150 min week. In this table BP was reported for the non-dominant arm to conform with that measured in clinical practice. There were no significant between-group differences for any of the demographic and clinical characteristics.
Data are mean ± SD (or percentage). IABP, interarm blood pressure; BMI, body mass index; CVD, cardiovascular disease; bSP, brachial systolic pressure; bDP, brachial diastolic pressure; aSP, derived-central systolic pressure; aDP, derived-central diastolic pressure.
Comparison of demographic and clinical characteristics between participants with absolute bSP IABP differences above and below 10 mmHg
Regression analysis was used to examine association between absolute brachial IABP differences and grip strength and arm geometry. There were no associations between differences in interarm bSP, or bDP with interarm differences in grip strength or arm volume (Figures 1 and 2, respectively). Stepwise linear regression exploring wider associations between the absolute systolic IABP with sex, anthropometric (age and BMI) and cardiovascular parameters (HR, bSP, bDP and brachial PP) showed male sex and bDP were the only predictors of absolute systolic IABP difference, and PP was the only predictor of the absolute diastolic IABP difference (Table 4).
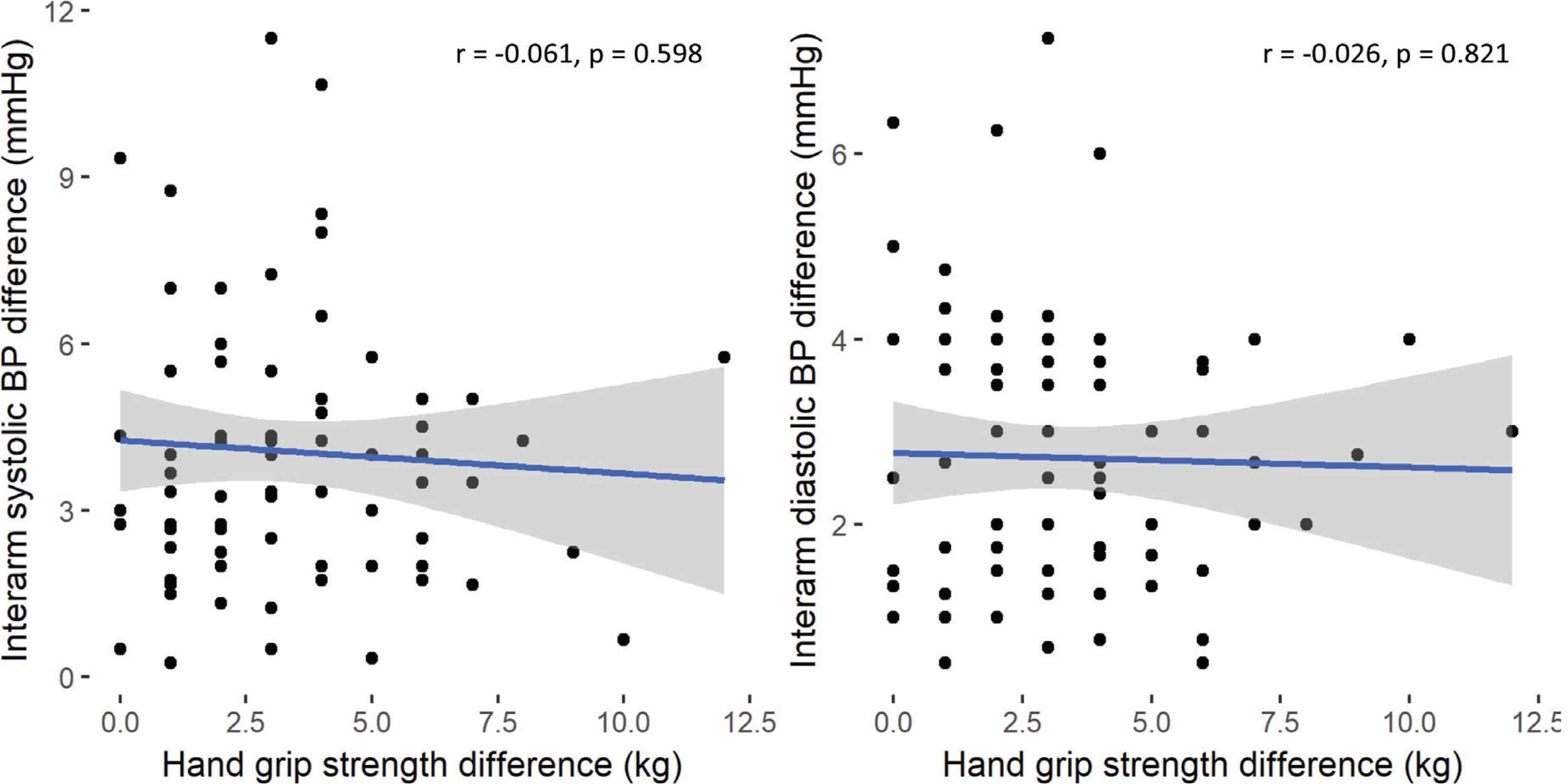
Correlation analysis of hand grip strength difference with interarm systolic (left panel) and diastolic (right panel), blood pressure (BP) differences, respectively
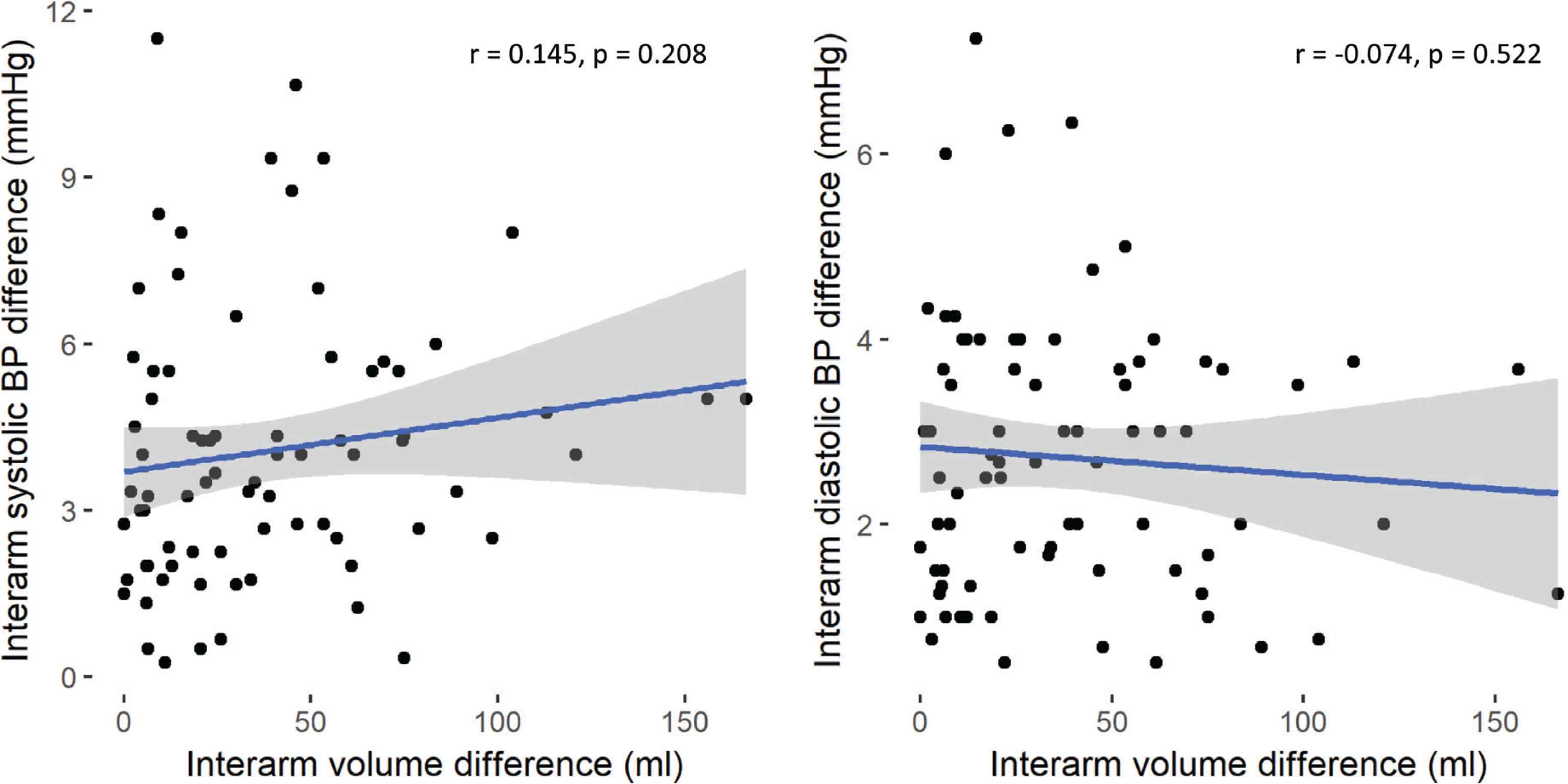
Correlation analysis of arm volume difference with interarm systolic (left panel) and diastolic (right panel, blood pressure (BP) differences, respectively
| Standardised β-coefficient | p-value | |
|---|---|---|
| Brachial systolic IABP | ||
| Age | −0.16 | 0.159 |
| Male sex | 0.29 | 0.012 |
| bDP | 0.19 | 0.031 |
| Brachial diastolic IABP | ||
| Pulse pressure | 0.35 | 0.002 |
IABP, interarm blood pressure; bDP, brachial diastolic pressure.
Standardised beta coefficients exploring predictors of the brachial IABP differences
4. DISCUSSION
This study used simultaneous BP in combination with anatomical measurements to address uncertainty around the impact of arm volume and handedness on IABP differences, and the impact of IABP differences on derived-central BP in a general community-dwelling population. Despite significant differences in grip strength and arm volume in the dominant and/or larger arm, we found no interarm differences in systolic or diastolic BP. Thus, in contrast to our hypothesis, IABP differences were not influenced by arm dominance, or arm geometry. Indeed, male sex and higher diastolic BP were the only clinical predictors of absolute systolic IABP differences; age, BMI or heart rate were not predictive.
Further examination of brachial IABP differences, irrespective of the arm (i.e. highest minus lowest arm BP) showed a mean absolute brachial IABP difference of approximately 4 mmHg, which translated to a small (3 mmHg) interarm difference in derived central BP. The small numerical differences in derived central BP between the two sides is artefact, given that there can only ever be one value for aortic pressure at any given time. Collectively, our findings suggest that IABP differences are generally small in the community-dwelling adults, and aside from male sex, general demographic, anatomical, and clinical measures are not predictive of IABP differences, even in those who have an absolute IABP difference ≥10 mmHg.
4.1. Methodological Considerations
Blood pressure recording can be inherently unreliable if not undertaken in a standardised manner. Indeed, much of the difficulty in interpreting IABP differences within the literature has arisen because of the lack of conformity in the BP measurements. In this study, we took a stringent approach to measuring BP and adhering to clinical BP measurement guidelines. All BP measurements were made using two identically automated BP measuring devices (Sphygmocor XCEL) in a quiet, temperature-controlled environment. All measurements were taken simultaneously as sequential measurements are known to overestimate IABP differences [20]. Measurements were repeated three to four times after 5 min rest, and devices were switched between measurements, to reduce machine bias. Thus, we feel confident that our measurement technique adhered to current recommendations for measuring BP and evaluating IABP differences.
The accuracy of the BP monitoring device is also critical to BP measurement [21] and accordingly the interpretation of IABP differences. In this study we used the Sphygmocor XCEL device, which is a well validated device for measuring brachial BP and acquiring a non-invasive estimate of central aortic BP [15]. To the best of our knowledge, this is the first study to compare IABP differences in the brachial artery with those derived near-simultaneously in the aorta. This is important given the increasing interest in central aortic parameters for assessing cardiovascular risk [11,22] and the potential to incorporate non-invasive estimation of central aortic pressure, alongside brachial BP measurement in the clinical setting [15].
As intended, we recruited a wide age-range of adults from the local community. We did not restrict entry into the study, on the basis of any known cardiovascular, metabolic or renal disease, as we wished to recruit a ‘typical’ community-dwelling cohort (with an equal balance of males and females), relevant to those in the primary care setting. We believe our cohort satisfies this criterion. Although a few of our participants had known CVD, the majority were free from vascular disease including hypertension. The average BMI of 25 kg/m2 indicated that most participants fell with the normal healthy weight range. The average resting bSP for most individuals (92%) lay within the normal range (bSP < 130 mmHg) [3]. Most of our participants (70%) undertook 150 min or more of exercise per week, which is above average for people between 18 and 64 years of age. Accordingly, we extend our definition of the cohort to that of a healthy adult population.
4.2. Comparison with Previous Literature
Interest in brachial IABP differences have spanned over 50 years; however, the magnitude of IABP disparities and the frequency of brachial systolic IABP differences ≥10 mmHg is highly variable, due, at least in part, to differences in study populations and methods of BP measurement. Therefore, the following comparisons with previous literature are largely restricted to studies involving healthy adults, in the community setting, and those who have measured IABP from simultaneous, repeated BP recordings.
The magnitude of our IABP differences in bSP (4.2 ± 3.6 mmHg) and bDP (2.4 ± 1.8 mmHg) are in accord with healthy controls in other studies [23,24]. For instance, Clark et al. [24] reported similar systolic and diastolic IABP differences (3.8 ± 3 mmHg and 2.5 + 2.8 mmHg, respectively) in 285 control participants (mean age 56 ± 13 years, 44% males). On the other hand, the prevalence of an absolute IABP ≥10 mmHg, of 12% in our study is higher than others. In 2016, Clark et al. [25], performed a comprehensive systematic review with meta-analyses to examine the prevalence of systolic IABP differences in the primary care setting. The pooled prevalence of IABP differences in control participants (specifically those without hypertension or diabetes) was 3.6%. However, it needs to be recognised there was considerable heterogeneity in their control cohort. For example, even within those who used simultaneous methods, there were interstudy variations in: (i) the ethnic mix of cohort (IABP differences in Asian populations were lower than those in Western/Caucasian populations [25]); (ii) how many times sequential measures were undertaken; and (iii) age. The implication being that no one’s study is directly comparable to ours and while we do not believe one single factor accounted for the higher prevalence of IABP differences ≥10 mmHg in our study, subtle variation in the myriad of interacting differences could be responsible.
4.3. Possible Mechanisms for Brachial IABP Differences
The mechanisms underpinning IABP differences are not well understood. Our study was designed to comprehensively explore the impact of anatomical variations on IABP differences as follows.
First, we considered the possibility that bSP may be higher in the right arm than the left arm. This has been a matter of contention as some studies [23,26,27] have confirmed this to be the case, whereas others [28,29] have reported the bSP is not consistently higher in the right arm. Advocates for the theory that right bSP is higher, propose that this disparity arises from different vascular anatomy between the right and left sides, and their potential to incur localised right-sided pathology. For example, previous studies [26,30] suggest the right brachial artery may be exposed to greater pressure from the heart than the left, and is therefore more vulnerable to atherosclerotic changes. In our study, we found no consistent differences between sides, and inconsistency between which arm had the highest and lowest BP between measures within a given participant. Thus, there is no evidence that asymmetrical right-sided occlusion impacted on our small absolute interarm differences, in our cohort.
Second, we examined whether hand dominance influenced the BP in the right and left arms. Proponents of this theory suggest that the hand dominance influences the muscle mass in the arm, such that bSP in the dominant is artefactually higher due to greater external compression required by the cuff to transmit the pressure to on the brachial vessel. As expected, our participants had a stronger hand grip and larger arm volume in their dominant arm. Nevertheless, these functional and anatomical differences did not result in any differences in bSP or bDP. Hence arm dominance did not appear to impact on brachial BP. We also considered the possibility that this comparison could be confounded by the assumption that arm volume is higher in the dominant arm. However, because there were no differences between the BP in the larger and smaller arm, this was not the case. Indeed, the lack of correlation between differences in grip strength and arm volume, and absolute IABP differences, indicates any potential impact of handedness or arm volume on arterial compression, did not influence absolute IABP differences. Collectively, these findings leave the unanswered question of what factor/s did cause absolute IABP differences.
Detailed statistical analysis revealed the small but significant systolic IABP differences were associated with sex and diastolic BP. Specifically, absolute IABP differences were greater in males than females, and in participants with a lower bDP. Moreover, diastolic IABP differences were related to PP. This study was not designed to examine the mechanism/s underpinning these observations. Hence, future mechanistic studies investigating the influence of sex and bDP on absolute IABP differences are warranted.
4.4. The Impact of Interarm Differences in Brachial Systolic BP on Derived Central BP
An unexpected, but important, finding was that absolute brachial IABP differences translated to small differences in derived central BP. Given that there can only be one aortic pressure we consider these differences to be an artefact of timing. In other words, despite measuring BP simultaneously in each arm, the implied differences in derived central BP reflect beat-to-beat differences in the arrival of the brachial pressure waves, which are used to calibrate the derived-central waveform in each arm. These beat-to-beat differences are likely to reflect BP variability.
Because the generalised transfer function to derive central BP was developed using invasive studies likely to be conducted in only one arm (either left or right, it is unknown which), we considered the possibility that the algorithm was not applicable to the arm for which invasive measurements were not taken in for development of the transfer function. However, given the transfer function is generalisable between males and females, and people of different heights [31], it is unlikely that vascular anatomical differences between left and right arms are of a magnitude that would substantially alter the parameters of the transfer function. Indeed, if that were the case, we would expect that there would be systematic differences (biases) in the left and right centrally derived BP, which we did not observe. Hence, the influence of BP variability remains the most likely reason the derived central BP appeared to be different between arms.
On the basis of the above, and assuming the generalised transfer function is truly generalisable, it seems plausible that the observed brachial IABP differences are also an artefact of small beat-to-beat changes in BP. These suggestions prompt further investigation of the influence of BP variability on IABP differences. Indeed, given that: (i) previous studies have shown that the magnitude of the IABP differences are greater in people with known CVD [32] and diabetes [24]; and (ii) increased BP variability is greater in people with hypertension [33], we suggest the magnitude of systolic IABP differences may be correlated with the magnitude of beat-to-beat BP variability.
4.5. Limitations
This study has several limitations. First, targeting community dwelling adults with higher cardiovascular risk would have improved statistical power when comparing the demographic and clinical characteristics of participants with IABP differences above (high interarm bSP difference group) and below 10 mmHg (low interarm bSP difference group). Indeed, the fact there were no between-group differences in sex despite males representing 78% of the high interarm difference group compared with 46% in the low interarm difference group, is most likely due to the small number of people in the high interarm difference group. That said, it is worth re-iterating that exploring the prevalence and reasons for bSP interarm differences based on a 10-mmHg threshold was not the main purpose of this study. Also, our non-selective approach to participant recruitment was made with the intention to explore IABP differences in a ‘typical’ community population. That the majority of our participants appeared to be healthier than normal, is likely a reflection of recruitment from a largely university-based population. Nevertheless, future studies exploring IABP differences, against a clinically relevant IABP threshold in community-dwelling adults should pre-select for higher cardiovascular risk.
Second, there were slight differences in the number of BP recordings we averaged to obtain the participants BP. Specifically, in 58% of the cohort four readings were averaged and in 42% three were averaged. Previous studies [34,35] have shown that IABP differences decline with the number of recordings. However, the main difference lies within the first and second readings, and there is little change thereafter [34]. Hence, we do not believe averaging three or four pairs of recordings confounded our results.
5. CONCLUSION
Current BP guidelines advocate that IABP differences are measured in the initial clinic visit, as a tool to stratify for cardiovascular risk [3]. However, uncertainty has arisen as to whether differences in arm, hand dominance, arm geometry influence IABP differences, particularly in the community-dwelling adults, which has implications on the clinical relevance of interarm disparities. Our detailed analysis confirms that the aforementioned factors do not contribute to IABP differences. Contrary to our hypothesis, it also showed that IABP differences in brachial systolic pressure translate to small differences in the derived central BP. We suggest the interarm differences in derived-central BP are an artefact due to pulse-to-pulse BP variability. This warrants further investigation exploring IABP differences and BP variability, simultaneously.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
MB, KP and AA were responsible for the conception and design of the research. MC and DT performed the experiments. MB, KP, IT, MC and DT analysed the data and interpreted the results of the experiments. MB, KP and IT prepared figures; KP and MB drafted the manuscript. KP, IT, MC, DT, AA and MB edited and revised the manuscript. All authors approved the final version.
ACKNOWLEDGMENTS
We wish to acknowledge Louise Koelmeyer (Department of Clinical Medicine, Macquarie University) the loan of the optoelectronic volumeter.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Karen C. Peebles AU - Isabella Tan AU - Mitchell T.D. Cook AU - Davis A. Theobald AU - Alberto P. Avolio AU - Mark Butlin PY - 2020 DA - 2020/02/11 TI - Interarm Differences in Brachial Blood Pressure and their Effect on the Derivation on Central Aortic Blood Pressure JO - Artery Research SP - 89 EP - 96 VL - 26 IS - 2 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.200201.002 DO - 10.2991/artres.k.200201.002 ID - Peebles2020 ER -
