Mild Acute Inflammation does not Impair Maintenance of Blood Pressure during a Hypotensive Stimulus
 , Thessa I. M. Hilgenkamp2,
, Thessa I. M. Hilgenkamp2,  , Wesley K. Lefferts1,
, Wesley K. Lefferts1,  , Bo Fernhall1
, Bo Fernhall1- DOI
- 10.2991/artres.k.200513.001How to use a DOI?
- Keywords
- Acute inflammation; blood pressure; blood flow; LBNP
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Severe acute inflammation, such as sepsis, disrupts autonomic nervous system function and jeopardizes blood pressure regulation [1]. Acute hypotension under normal conditions stimulates baroreceptors to restore blood pressure by activating the sympathetic nervous system to increase heart rate and vasoconstrict peripheral vasculature. Severe acute experimental inflammation (endotoxin) blunts baroreflex-mediated increases in muscle sympathetic nerve activity during reductions in blood pressure and uncouples baroreflex control of heart rate [2]. Whether mild acute inflammation alters the ability to maintain blood pressure during a hypotensive challenge is less clear.
2. AIM
This study aimed to determine whether mild acute inflammation alters the ability to maintain blood pressure and peripherally vasoconstrict during lower body negative pressure in generally healthy young adults.
3. MATERIALS AND METHODS
3.1. Participants and Study Design
Fifteen healthy, young adults (18–35 years) completed the present protocol and were part of a previously published study [3]. The study was approved by the Institutional Review Board at the University of Illinois at Chicago. Participants provided written informed consent and reported to the lab having refrained from caffeine, alcohol, and exercise for ≥24 h and fasted for ≥10 h. Females were tested during their menstrual cycle or placebo pills if taking oral contraceptives.
Participants completed assessments before (baseline) and 24 h after the induction of acute inflammation via typhoid vaccination [4,5]. For each visit, a fasting blood sample was collected and then participants rested supine for 10 min in the Lower Body Negative Pressure (LBNP) chamber sealed at the waist before resting vascular and hemodynamic measures were collected. Following resting measures, LBNP was initiated at −20 mmHg. After 2 min of equilibration, vascular and hemodynamic measures were repeated.
3.2. Measures
Brachial blood pressure (systolic, diastolic, and mean pressure) and heart rate were measured in duplicate on the right arm using an automated blood pressure monitor (Mobil-o-graph 24 PWA, I.E.M., Stolberg, Germany). If blood pressure differed by >5 mmHg, a third measure was taken. Central blood pressure was estimated from pressure waveforms collected in duplicate via radial applanation tonometry (SphygmoCor Model EM3, AtCor Medical, Sydney, Australia) using a generalized transfer function and calibrated to brachial mean and diastolic blood pressure, as previously described [3].
Brachial blood flow was measured via ultrasonography (Hitachi-Aloka α7, Tokyo, Japan) and recorded for 1 min. The last 30 s was averaged and used for analyses. Offline analysis was completed for brachial artery diameter (Cardiovascular Suite, QUIPU, Pisa, Italy) and antegrade and retrograde flow to determine Mean Blood Velocity (MBV) (Brachial Analyzer, MIA, Coralville, IA, USA). Brachial blood flow (mL/min) was calculated as:
Brachial conductance (mL/min/mmHg) was calculated as:
Interleukin-6 (IL-6) and C-Reactive Protein (CRP) were analyzed in duplicate from venous blood samples to assess systemic inflammation using commercially available high-sensitivity enzyme-linked immunosorbent assay kits (HS600B, R&D Systems, Minneapolis, MN, USA; CrystalChem, Elk Grove Village, IL, USA).
3.3. Statistical Analysis
Results are expressed as mean ± standard deviation. Normality was assessed with the Kolmogorov–Smirnov test. All outcome variables were assessed with a two-way repeated measures ANOVA to compare the effects of LBNP before and during acute inflammation. When significant interaction effects were observed, post-hoc analyses were conducted with the Bonferroni correction for multiple comparisons. Data analysis was performed with SPSS software, version 24 (Chicago, IL, USA) and all p-values are two-sided with an a priori α-level of 0.05 deemed significant.
4. RESULTS
Nine females and six males were included in the study. Participants were 26 ± 4 years of age with a body mass index of 21.5 ± 2.0 kg/m2. At 24 h-post vaccination, acute inflammation was evident with increases in IL-6 (1.02 ± 0.53 to 2.05 ± 1.11 pg/mL, p = 0.003) and CRP (0.42 ± 0.93 to 1.33 ± 1.37 ng/mL, p = 0.009).
No changes were observed in brachial or central blood pressure during acute inflammation or during LBNP (Table 1, p > 0.05). An interaction was observed for heart rate (p < 0.01) in which heart rate was elevated at 24 h-post compared to baseline, and heart rate increased during LBNP at baseline but not during LBNP at 24 h-post. No effect of acute inflammation was observed for brachial blood flow or conductance (Figure 1, p > 0.05), however, brachial blood flow (Baseline rest: 45.9 ± 13.7, Baseline LBNP: 38.7 ± 12.2, 24 h-post rest: 47.2 ± 15.8, 24 h-post LBNP: 41.8 ± 14.9 mL/min) and conductance (Baseline rest: 51.1 ± 14.6, Baseline LBNP: 42.5 ± 12.5, 24 h-post rest: 52.2 ± 16.0, 24 h-post LBNP: 46.5 ± 15.4 mL/min/100 mmHg) were reduced during LBNP (p < 0.05).
| Baseline | 24 h-Post | p-value (η2) | |||||
|---|---|---|---|---|---|---|---|
| Rest | LBNP | Rest | LBNP | Inflammation | LBNP | Interaction | |
| Systolic blood pressure (mmHg) | 114 ± 12 | 114 ± 11 | 114 ± 9 | 113 ± 10 | 0.56 (0.03) | 0.52 (0.03) | 0.23 (0.10) |
| Diastolic blood pressure (mmHg) | 70 ± 8 | 71 ± 8 | 70 ± 6 | 70 ± 6 | 0.72 (0.01) | 0.17 (0.13) | 0.62 (0.02) |
| Mean arterial pressure (mmHg) | 90 ± 9 | 91 ± 9 | 90 ± 7 | 90 ± 7 | 0.56 (0.03) | 0.73 (0.01) | 0.40 (0.05) |
| Central systolic blood pressure (mmHg) | 101 ± 10 | 102 ± 9 | 102 ± 8 | 101 ± 9 | 0.94 (0.00) | 0.95 (0.00) | 0.23 (0.10) |
| Central diastolic blood pressure (mmHg) | 70 ± 8 | 72 ± 8 | 70 ± 7 | 71 ± 6 | 0.53 (0.3) | 0.052 (0.24) | 0.29 (0.08) |
| Heart rate (bpm) | 53 ± 11 | 58 ± 11* | 57 ± 11* | 58 ± 11* | 0.06 (0.23) | <0.01 (0.56) | <0.01 (0.45) |
| Diameter (mm) | 3.75 ± 0.80 | 3.75 ± 0.83 | 3.82 ± 0.80 | 3.74 ± 0.78 | 0.46 (0.04) | 0.45 (0.04) | 0.15 (0.14) |
| Velocity (cm/s) | 7.4 ± 3.1 | 6.2 ± 2.8 | 7.3 ± 3.3 | 6.6 ± 2.5 | 0.79 (0.01) | 0.02 (0.36) | 0.55 (0.03) |
Different from rest at baseline, p < 0.05. Data are represented by mean ± standard deviation.
Hemodynamic response to −20 mmHg Lower Body Negative Pressure (LBNP) at baseline and during acute inflammation (24 h-post vaccination).
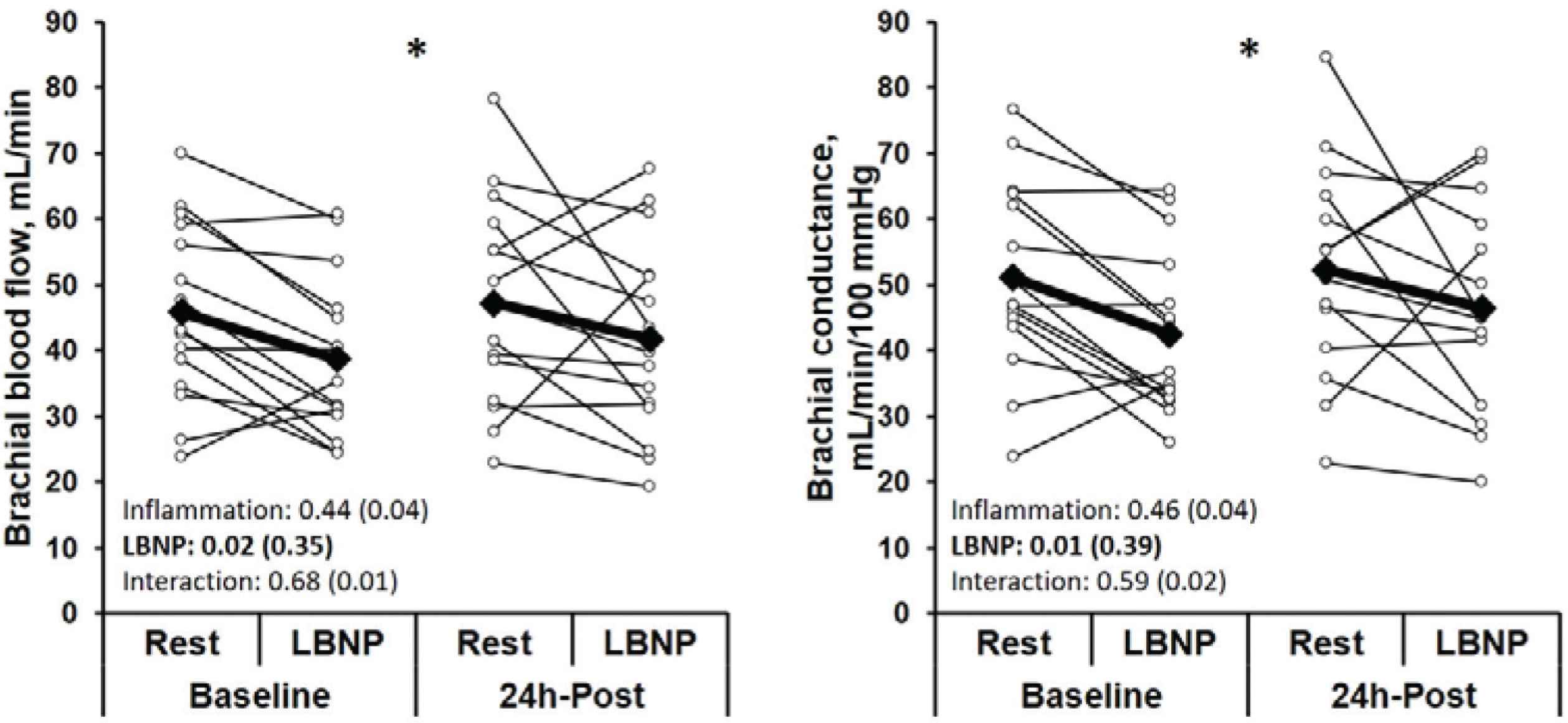
Brachial blood flow and conductance in response to −20 mmHg Lower Body Negative Pressure (LBNP) at baseline and during acute inflammation (24 h-post vaccination). Values on graph represent p-value (η2). *Effect of LBNP, p < 0.05.
5. CONCLUSION
This study sought to determine the effect of mild acute inflammation on the ability to maintain blood pressure during a hypotensive challenge in young healthy adults. Our results suggest young adults were able to peripherally vasoconstrict during the hypotensive challenge to maintain blood pressure (central and peripheral) despite inflammation altering the heart rate response to LBNP. As such, our data may suggest that mild acute inflammation alters baroreflex control of heart rate during a hypotensive challenge.
We observed an increase in heart rate during acute inflammation and no change in heart rate during mild acute inflammation in response to a hypotensive challenge. This is in line with Sayk et al. [2] who observed an increase in resting heart rate during endotoxin, a more severe acute inflammation. Additionally, they examined the baroreflex using drug infusions during which heart rate was unresponsive during endotoxin and remained fixed at an elevated level [2]. In our sample, the uncoupling appeared to be highly driven by the female participants, thus, a future study powered to look at the sex differences in response to acute inflammation may be warranted. Overall, even during mild acute inflammation, it appears heart rate may also be uncoupled from baroreflex regulation.
During our mild inflammation, we observed no change in peripheral or central blood pressure or blood flow in response to a hypotensive challenge. Previous studies using endotoxin suggest more severe inflammation reduces resting muscle sympathetic nerve activity [2], blunts the baroreflex response to vasodilation [2], and decreases vascular responsiveness to norepinephrine [6]. However, our data suggest preservation of the ability to successfully increase muscle sympathetic nerve activity and peripherally vasoconstrict during mild acute inflammation as both blood flow and conductance were reduced during LBNP similarly at baseline and 24 h-post vaccination.
Overall, our findings suggest even mild acute inflammation potentially uncouples heart rate from baroreflex control despite the maintenance of blood pressure during a transient hypotensive challenge. A stronger inflammatory stimulus may be required to disrupt muscle sympathetic nerve activity and render blood pressure regulation vulnerable to the impaired baroreflex control of heart rate. As such, mild acute inflammation that accompanies regular vaccination may present a blood pressure regulation challenge among individuals with impaired blood pressure regulation.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
Conceptualization: ECS, BF; Data curation: ECS; Formal analysis: ECS; Investigation: ECS, TIMH, WKL; Methodology: ECS, BF; Supervision: BF; Writing – original draft: ECS; Writing – review & editing: ECS, TIMH, WKL, BF.
FUNDING
EC Schroeder is currently supported by an
WK Lefferts is currently supported by the
TIM Hilgenkamp is currently supported by the
B Fernhall is currently supported by the
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Elizabeth C. Schroeder AU - Thessa I. M. Hilgenkamp AU - Wesley K. Lefferts AU - Bo Fernhall PY - 2020 DA - 2020/05/25 TI - Mild Acute Inflammation does not Impair Maintenance of Blood Pressure during a Hypotensive Stimulus JO - Artery Research SP - 180 EP - 182 VL - 26 IS - 3 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.200513.001 DO - 10.2991/artres.k.200513.001 ID - Schroeder2020 ER -
