The Prognostic Role of Aortic Stiffness in Patients Hospitalized for an Acute Heart Failure Syndrome
 , Mara Bougiakli1, Aris Bechlioulis1,
, Mara Bougiakli1, Aris Bechlioulis1,  , Anna Kotsia1, Lampros Lakkas1, Ioannis Girdis1,
, Anna Kotsia1, Lampros Lakkas1, Ioannis Girdis1,  , Konstantinos Pappas1, Georgios Chasiotis2,
, Konstantinos Pappas1, Georgios Chasiotis2,  , Eleni Bairaktari2,
, Eleni Bairaktari2,  , Andreas Kalogeropoulos3,
, Andreas Kalogeropoulos3,  , Lampros K. Michalis1,
, Lampros K. Michalis1,  , Katerina K. Naka1, *,
, Katerina K. Naka1, *, 
- DOI
- 10.2991/artres.k.200930.001How to use a DOI?
- Keywords
- Acute heart failure; prognosis; aortic stiffness; pulse wave velocity; pulsatile hemodynamics
- Abstract
Background: Although impaired arterial function has been associated with adverse prognosis in chronic Heart Failure (HF), its role in Acute HF Syndromes (AHFS) has been little studied. We prospectively investigated the prognostic role of arterial function on mortality and HF Hospitalizations (HHF) in patients with AHFS.
Design and Methods: A thorough assessment of arterial function was performed in patients hospitalized for AHFS 24–48 h before discharge and followed-up for 6 months for all-cause death and HHF. MAGGIC risk score was used to evaluate the additive predictive value of vascular biomarkers for clinical events.
Results: One-hundred patients were studied; aged 70 ± 11 years, 78% males, 61% had left ventricular ejection fraction ≤40% and 24% ≥50%. Mean aortic Pulse Wave Velocity (PWV) was 11.2 m/s, mean augmentation index 21% and median brachial flow-mediated dilation 3.14%. Higher PWV was associated with all-cause mortality (Hazard Ratio [HR] 1.32 per 1 m/s, p < 0.001) and the combined clinical event of mortality and HHF (HR 1.12 per 1 m/s, p = 0.012) even after adjustment for MAGGIC score. MAGGIC score predicted mortality (HR 3.40 per group increase, Area under Curve [AUC] = 0.741, p = 0.017) in our population; addition of PWV to MAGGIC score increased the predictive accuracy (AUC = 0.911, C-statistic p < 0.01 vs. MAGGIC score alone) for mortality.
Conclusion: In these AHFS patients, increased aortic stiffness was independently associated with mortality and further improved the predictive accuracy of an established risk model. Further research is needed to show whether a comprehensive assessment of AHFS patients focusing both on cardiac and vascular function, may improve management and ameliorate prognosis following an AHF hospitalization.
- HIGHLIGHTS
- •
The interaction between the heart and the arteries is a determinant of cardiovascular function.
- •
Increased aortic Pulse Wave Velocity (PWV) predicts mortality in acute heart failure.
- •
Aortic PWV increases predictive accuracy of MAGGIC score.
- •
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Heart Failure (HF) is a modern epidemic leading to increased mortality and impaired quality of life [1]. Acute Heart Failure Syndromes (AHFS), either in newly diagnosed HF or acutely decompensated chronic HF patients, are a common cause of hospitalization. Frequent Hospitalizations for HF (HHF) increase cost and portend a worse prognosis for patients as they indicate a deterioration of their health status [2]. There have been several efforts to identify contributors to rehospitalization after HHF in order to identify patients at increased risk and implement prevention strategies [3–5].
Arterial function, as assessed by measurement of endothelium-dependent vasodilation, aortic Pulse Wave Velocity (PWV), central aortic pressures and arterial elasticity indices, has been associated with atherosclerotic disease progression and cardiovascular events, including HHF, in various populations [6–9]. In patients with chronic HF, arterial function indices have been found to be impaired either as a result of advanced atherosclerosis or HF per se [10,11], leading to impaired ventricular-arterial coupling [12] that may be related to adverse prognosis [13–16].
Acute heart failure syndromes are associated with acute hemodynamic changes that may affect vascular function, while various vasoactive treatments administered during the acute phase could also affect arterial function. Few studies have addressed the role of on-admission [17,18] and pre-discharge [19] arterial function on risk stratification after an AHFS. Furthermore, the relative role of each marker (i.e. aortic stiffness, peripheral arterial elasticity, endothelial function etc.) in AHFS prognosis is not known.
The aim of the current study was to prospectively investigate the prognostic role of pre-discharge arterial function indices assessment for 6-month all-cause mortality and HHF in patients hospitalized for an AHFS.
2. MATERIALS AND METHODS
2.1. Study Population and Design
We prospectively studied consecutive patients who were hospitalized due to AHFS diagnosed based on the criteria established in current guidelines of the European society of cardiology [20]. All patients aged ≥18 years old were eligible for enrollment. Patients with an acute myocardial infarction that led to HF presentation or during the last 3 months, severe valvular disease, chronic atrial fibrillation, congenital heart disease or other severe chronic disease (e.g. cancer, chronic renal failure on hemodialysis etc.) were excluded. The study took place in the University Hospital of Ioannina between January 2012 and December 2013. During this period, we enrolled 100 patients that were eligible based on the above-mentioned inclusion and exclusion criteria. The study complied with the Declaration of Helsinki and all participants provided written informed consent. The study protocol was approved by the local Ethics Committee.
All patients were examined 24–48 h prior to discharge after having been stabilized and no longer requiring the administration of intravenous diuretics or vasoactive medications. A comprehensive medical record was recorded, and a detailed physical examination was performed. Blood samples were taken early in the morning after an overnight fasting for the determination of various metabolic parameters while a serum sample was kept frozen at −80°C for the determination of Brain Natriuretic Peptide (BNP). A detailed echocardiographic study was performed along with a thorough investigation of vascular function on the same day.
2.2. Definitions of Clinical Characteristics
The New York Heart Association (NYHA) scale I–IV was used to assess the functional status of all patients prior to discharge. A history of ischemic cardiomyopathy was defined as the documented presence of coronary artery disease that could explain the clinical presentation of HF in the patient. The presence of hypertension, hypercholesterolaemia, diabetes mellitus and smoking was recorded in all patients. Weight and height were measured, and body mass index was calculated in all study participants. Metabolic parameters in the blood were measured by standard methodology. Serum BNP levels were assessed using chemiluminescent microparticle immunoassay (ARCHITECT BNP iSystem, Abbott Laboratories, Diagnostics Division, Abbott Park, IL, USA) (assay sensitivity 10 pg/ml).
2.3. MAGGIC Score
The MAGGIC risk score for prediction of mortality in patients with HF [21] was constructed based on the Meta-Analysis Global Group in Chronic Heart Failure and has been shown to perform well in patients after discharge for an AHFS [22]. An integer risk score is calculated based on the assessment of 13 variables: age, Left Ventricular Ejection Fraction (LVEF), NYHA functional class, serum creatinine, diabetes, beta‐blocker use, systolic blood pressure, body mass index, time since diagnosis of HF, current smoker, chronic obstructive pulmonary disease, gender, and angiotensin‐converting enzyme inhibitor or angiotensin receptor blockers use, and the mortality risk was classified in six groups in the original publication [21]. Currently we use a 3-scale classification of the MAGGIC score i.e. A (groups 1 and 2, score ≤20), B (groups 3 and 4, score 21–28) and C (groups 5 and 6, score ≥29).
2.4. Vascular Measurements
Vascular studies were performed as previously described [23,24] in all participants and were performed by two skilled and experienced operators (SG, MB). All exams took place in the morning between 08.00 and 10.00 am.
2.4.1. Endothelial function
Endothelial function was assessed by measurement of endothelium dependent Flow-mediated Dilation (FMD) of the brachial artery, according to the published guidelines [25]. Optimal images of the brachial artery and Doppler indices were obtained using a Vivid I GE Healthcare (IL, US) ultrasound machine. All images were recorded at end-diastole coincident with the R-wave on the electrocardiogram and stored for offline analysis. The brachial artery was occluded for 5 min using a wrist cuff inflated to 300 mmHg causing an ischemic stimulus that resulted in brachial artery hyperemia. FMD was calculated as a percent increase in diameter during hyperemia compared with the brachial diameter at rest. The operators recorded the artery in a perpendicular plane so that the intima-media margins were visible both in the proximal and distal vessel wall if possible. Furthermore, the flow in the brachial artery immediately after the release of the cuff was recorded in order to make sure that an adequate increase in blood flow was achieved.
2.4.2. Arterial stiffness
Aortic stiffness was assessed non-invasively by measurement of aortic PWV using applanation tonometry.
Carotid-femoral PWV was estimated using the Sphygmocor system (Version 7.01, AtCor Medical, Sydney, Australia). Pressure waveforms were recorded from the carotid and femoral arteries, mostly the right ones. The distance traveled by the pulse wave was measured over the body surface as the distance between the suprasternal notch and femoral artery subtracting the distance from suprasternal notch to the carotid artery. The wave transit time between the two sites was calculated using as reference frame the R-wave on the simultaneously recorded electrocardiogram. PWV was calculated as the ratio distance/transit time in m/s. PWV >10 m/s is considered abnormal [26].
2.4.3. Pulsatile hemodynamics
Augmentation Index (Alx), Central Pressures and peripheral arterial compliance were assessed non-invasively using applanation tonometry.
Augmentation index was extracted by the pressure waveform acquired from the right radial artery using the Sphygmocor system (Version 7.01, AtCor Medical). After 15–20 waveforms were acquired, the software generated the corresponding central aortic pressure waveform using a generalized transfer function. Alx was calculated as the difference between the second and first systolic peaks of the central pulse waveform. Alx was expressed as a percentage of the central pulse pressure and was finally corrected for heart rate (Alx@75). Pulse pressure was defined as systolic minus diastolic pressure. All measurement that had a quality index provided by Sphygmocor >80, were stored for further analysis while other measurements were rejected.
The Large Artery Elasticity Index (LAEI) and Small Artery Elasticity Index (SAEI) artery elasticity index were calculated using the HDI/Pulsewave CR-2000 Cardiovascular Profiling System (Hypertension Diagnostic, Eagan, MN, USA) as previously described [27]. A suitably sized blood pressure cuff was placed around the patient’s left arm for simultaneous blood pressure recordings. A rigid, plastic wrist stabilizer was placed on the right wrist in order to achieve stability during the recording of pulse waveform. This methodology uses a modified Windkessel model to derive arterial compliance of proximal (LAEI) and distal (SAEI) arteries, by analyzing the diastolic portion of the pulse pressure contour. Abnormal values of both indices according to age and gender have been previously published [27]. Measurements with a Relative Strength Index >15% (provided by the HDI/Pulsewave CR-2000 Cardiovascular Profiling System) were stored for further analysis while other measurements were rejected.
Reproducibility analysis of vascular studies has been previously published [23]. In studies performed on two separate days (7–10 days apart) in 10 subjects by a single operator, the within-subject coefficient of variation of FMD, PWV, LAEI and SAEI were 6.9%, 5.6%, 6.8% and 9.1% respectively.
2.5. Echocardiography Measurements
A detailed two-dimensional and Doppler transthoracic echocardiogram was performed by an experienced operator, using a Vivid I, GE Healthcare (IL, US) machine. Classical echocardiographic indices of Left Ventricular (LV) systolic and diastolic function were recorded. LVEF was measured using the biplane Simpson’s method. Based on this, patients were classified as Heart Failure with Reduced Ejection Fraction (HFrEF, i.e. LVEF ≤ 40%), Heart Failure with mid-range Ejection Fraction (HFmrEF, LVEF > 40% and <50%) or Heart Failure with preserved Ejection Fraction (HFpEF, i.e. LVEF ≥ 50%). Left atrial volume index was used as a measure of left atrial size. Tissue Doppler imaging was used to assess the early diastolic velocity of the septal and lateral mitral annulus (Eʹ average) in order to further calculate the E/Eʹ ratio as a measure of LV filling pressures and diastolic function.
2.6. Follow-up and Outcomes
All participants were followed-up at 6 months to record any clinical events; a telephone interview of the patients who could not attend the 6-month follow-up (or their relatives) was performed. Pre-specified clinical events (death and new HF hospitalizations) were confirmed through medical records or interviews with physicians.
2.7. Statistical Analysis
Continuous data are presented as median (interquartile range) for not normally distributed variables and mean ± SD for normally distributed variables. Cox regression analysis was used to identify associations between clinical outcomes at 6-month follow-up and vascular parameters, BNP and MAGGIC score. Multivariate Cox regression analysis was used to adjust the association of PWV with clinical events for MAGGIC score. The area under the curve of regression models for the prediction of mortality including MAGGIC score alone and MAGGIC score and PWV were calculated, and their predictive accuracy was compared using the methodology described by Hanley and McNeil (C-statistic) [28]. p-Values were always two-sided and a value of p < 0.05 was considered significant. The SPSS statistical software package (IBM SPSS Statistics, Version 23, NY, US) was used.
3. RESULTS
The baseline characteristics of the study population (n = 100) are presented in Table 1. The mean age was 70 years, 78% were male, 37% had diabetes, 54% had impaired renal function [Estimated Glomerular Filtration Rate (eGFR) <60 ml/min/1.73 m2), and 53% had ischemic cardiomyopathy. Most patients (61%) had LVEF ≤ 40%, while 24% had LVEF ≥ 50%. Median length of stay was 8 days. Pre-discharge metabolic and other biochemical parameters of the population are shown in Table 1. Prior to discharge, most patients (78%) were on NYHA class I and II. Median BNP value was 436 ng/ml. Standard pre-discharge echocardiographic parameters are shown in Table 2. Analysis of vascular biomarkers prior to discharge showed a PWV >10 m/s, an abnormal LAEI and an abnormal SAEI in 8% and 68% of the patients respectively. Mean AIx was 21% and median FMD was 3.14% (Table 2).
| Age, years | 70 ± 11 |
| Male gender, n | 78 |
| History of dyslipidemia, n | 61 |
| History of hypertension, n | 85 |
| History of diabetes, n | 37 |
| Current smoking, n | 28 |
| Chronic kidney disease, n* | 54 |
| Anemia, n** | 37 |
| Chronic pulmonary disease, n | 16 |
| Ischemic cardiomyopathy, n | 53 |
| Paroxysmal atrial fibrillation, n | 23 |
| HFpEF (LVEF ≥ 50%), n | 24 |
| HFrEF (LVEF ≤ 40%), n | 61 |
| Previous decompensations within 12 months, n | 21 |
| Medications on admission | |
| Beta blockers | 60 |
| ACE-I/ARB | 57 |
| Aldosterone antagonists | 34 |
| Furosemide | 53 |
| Digoxin | 4 |
| Amiodarone | 11 |
| Statin | 46 |
| Body mass index, kg/m2 | 27.5 ± 4.4 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 58.7 ± 17.9 |
| Hemoglobin, g/dl | 13.3 ± 1.9 |
| Fasting glucose, mg/dl | 137 (97, 191) |
| Urea, mg/dl | 57 (42, 78) |
| Sodium, mmol/l | 138 ± 3 |
| Potassium, mmol/l | 4.3 ± 0.6 |
| Uric acid, mg/dl | 7.5 ± 2.3 |
| Total cholesterol, mg/dl | 166 ± 38 |
| High density lipoprotein cholesterol, mg/dl | 40 ± 10 |
| Low density lipoprotein cholesterol, mg/dl | 99 ± 31 |
| Triglycerides, mg/dl | 120 (90, 140) |
| Albumin, mg/dl | 3.9 (3.6, 4.2) |
| Brain natriuretic peptide, ng/ml | 436 (110, 832) |
Chronic kidney disease defined as eGRF < 60 ml/min/1.73 m2.
Anemia defined as hemoglobin <12 gr/dl for women and 13 gr/dl for men.
Continuous variables are presented as mean ± SD.
ACE-I, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker.
Descriptive characteristics, metabolic and biochemical parameters of the study population (n = 100)
| Systolic blood pressure, mmHg | 123 ± 20 |
| Diastolic blood pressure, mmHg | 72 ± 10 |
| Heart rate, beats per minute | 69 (60, 79) |
| Pulse wave velocity, m/s | 11.2 ± 3.4 |
| Pulse wave velocity >10 m/s, n | 57 |
| Augmentation index, % | 21 (17, 29) |
| Aortic pulse pressure, mmHg | 37 (29, 51) |
| Flow-mediated dilation, % | 3.14 (2.37, 3.70) |
| Large artery elasticity index, ml/mmHg * 10 | 11.2 (8.0, 16.2) |
| Abnormal large artery elasticity index, n | 8 |
| Small artery elasticity index, ml/mmHg * 100 | 3.4 (2.5, 4.8) |
| Abnormal small artery elasticity index, n | 68 |
| Left ventricular end-diastolic diameter, mm | 57 ± 10 |
| Left ventricular mass index, gr/m2 | 137 ± 40 |
| Increased left ventricular mass, n* | 71 |
| Left atrial volume index, ml/m2 | 44.8 (34.0, 54.0) |
| E/Eʹ | 12.6 (9.0, 18.6) |
| E/Eʹ > 14 | 40 |
| Left ventricle ejection fraction, % | 35 (27, 45) |
| Pulmonary artery systolic pressure, mmHg | 35 (20, 42) |
| Pulmonary artery systolic pressure >35 mmHg | 43 |
| Inferior vena cava diameter, mm | 15 (11, 20) |
Increased left ventricular mass defined as left ventricular mass index >95 and 115 g/m2 for women and men respectively.
Continuous variables are presented as mean ± SD or median (interquartile range).
E/Eʹ, early diastolic mitral inflow velocity to mitral annular early diastolic velocity ratio.
Vascular and echocardiographic parameters of the study population prior to discharge (n = 100)
All patients were followed-up according to study protocol. At 6-month follow-up, 32% of the study population presented a pre-specified clinical event; 9% of the patients died, while 23% had a HHF. MAGGIC risk score (as a 3-scale variable) was assessed in all patients and deaths were recorded in 0%, 11% and 20% in MAGGIC groups A–C respectively. MAGGIC score was associated with mortality at 6 months [HR 3.40 per group increase (95% CI 1.25, 9.26), p = 0.017], but not with HHF [HR 1.30 per group increase (95% CI 0.82, 2.07), p = 0.266] or the combined event of mortality and HHF [HR 0.91 per group increase (95% CI 0.52, 1.60), p = 0.749]. BNP levels were not related to any of the studied clinical events. The associations of vascular parameters with clinical events at follow-up in univariate analysis are shown in Table 3. PWV was associated with mortality (HR 1.32 per 1 m/s increase, p < 0.001) and the combined event of mortality and HF re-hospitalizations (HR 1.12 per 1 m/s increase, p = 0.014) at 6 months. After adjustment for MAGGIC score, PWV remained an independent predictor of mortality [HR 1.38 per 1 m/s increase (95% CI 1.19, 1.63), p < 0.001] and the combined event of mortality and HHF [HR 1.12 per 1 m/s increase (95% CI 1.03, 1.22), p = 0.012]. None of the other studied vascular markers was related to any of the clinical events at follow-up (Table 3). In Receiver Operating Characteristic (ROC) curve analysis, MAGGIC risk score predicted mortality with an AUC = 0.741, p = 0.017). The addition of PWV to MAGGIC score resulted in AUC = 0.911, p < 0.001 (p < 0.01 vs. MAGGIC score alone) (Figure 1).
| Mortality and hospitalization for heart failure at 6 months | |
| Systolic BP per 10 mmHg increase | HR 1.15 (95% CI 0.97, 1.37), p = 0.097 |
| Diastolic BP per 10 mmHg increase | HR 1.38 (95% CI 0.98, 1.96), p = 0.066 |
| PWV per 1 m/s | HR 1.12 (95% CI 1.02, 1.22), p = 0.014 |
| AIx per 5% increase | HR 1.04 (95% CI 0.87, 1.25), p = 0.653 |
| cPP per 5 mmHg | HR 1.10 (95% CI 0.99, 1.22), p = 0.072 |
| FMD per 1% increase | HR 1.03 (95% CI 0.77, 1.38), p = 0.849 |
| LAEI per 5 ml/mmHg * 10 increase | HR 0.97 (95% CI 0.76, 1.24), p = 0.818 |
| SAEI per 2 ml/mmHg * 100 increase | HR 0.92 (95% CI 0.67, 1.25), p = 0.575 |
| Mortality at 6 months | |
| Systolic BP per 10 mmHg increase | HR 1.07 (95% CI 0.78, 1.46), p = 0.683 |
| Diastolic BP per 10 mmHg increase | HR 1.10 (95% CI 0.58, 2.08), p = 0.781 |
| PWV per 1 m/s | HR 1.32 (95% CI 1.15, 1.53), p < 0.001 |
| AIx per 5% increase | HR 0.85 (95% CI 0.58, 1.23), p = 0.384 |
| cPP per 5 mmHg | HR 1.01 (95% CI 0.83, 1.23), p = 0.944 |
| FMD per 1% increase | HR 0.56 (95% CI 0.27, 1.18), p = 0.127 |
| LAEI per 5 ml/mmHg * 10 increase | HR 0.81 (95% CI 0.47, 1.41), p = 0.461 |
| SAEI per 2 ml/mmHg * 100 increase | HR 0.79 (95% CI 0.39, 1.62), p = 0.522 |
| Hospitalization for heart failure at 6 months | |
| Systolic BP per 10 mmHg increase | HR 1.17 (95% CI 0.96, 1.43), p = 0.115 |
| Diastolic BP per 10 mmHg increase | HR 1.44 (95% CI 0.95, 2.16), p = 0.083 |
| PWV per 1 m/s | HR 1.05 (95% CI 0.94, 1.18), p = 0.483 |
| AIx per 5% increase | HR 1.04 (95% CI 0.84, 1.29), p = 0.704 |
| cPP per 5 mmHg | HR 1.11 (95% CI 0.98, 1.25), p = 0.095 |
| FMD per 1% increase | HR 1.09 (95% CI 0.79, 1.52), p = 0.599 |
| LAEI per 5 ml/mmHg * 10 increase | HR 1.02 (95% CI 0.77, 1.35), p = 0.892 |
| SAEI per 2 ml/mmHg * 100 increase | HR 0.87 (95% CI 0.59, 1.28), p = 0.467 |
BP, blood pressure; CI, confidence interval; cPP, central aortic pulse pressure; HR, hazard ratio; Ln, natural logarithm.
Univariate associations of vascular parameters with clinical events at follow-up
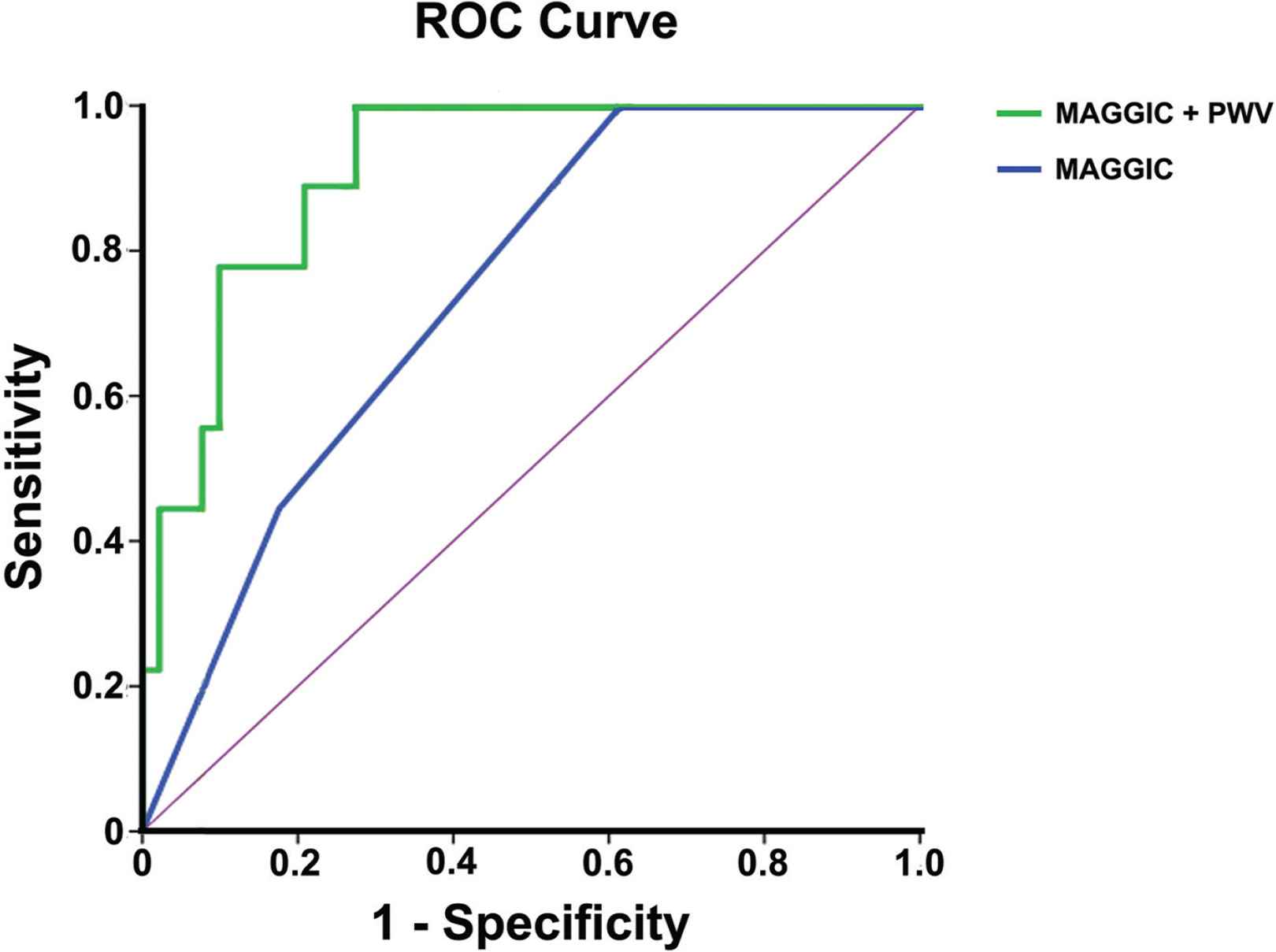
Receiver operating curves for the two models, i.e. MAGGIC score only and MAGGIC score plus pulse wave velocity, for the prediction of mortality at 6 months.
4. DISCUSSION
In the present study, arterial function indices were assessed in patients hospitalized with AHFS prior to discharge. Aortic PWV, an index of aortic stiffness, was independently associated with the occurrence of death and HHF at 6-months.
Our population consisted of HF patients with both reduced and preserved LVEF in a relatively stable condition pre-discharge, mostly in functional class NYHA I and II with residual congestion (as indicated by increased BNP and echocardiographic findings of increased E/Eʹ ratio >14 and systolic pulmonary artery pressure >35 mmHg in >40% of the patients). Patients presented increased aortic stiffness (i.e. PWV > 10 m/s), abnormal small artery distensibility and severely impaired endothelial function (i.e. median brachial FMD ca. 3.0%) in the majority of our population. Impairment of arterial function indices in HF patients has been attributed to increased age, high prevalence of cardiovascular risk factors (i.e. hypertension, diabetes) and coronary artery disease [11,29], characteristics also found in our population. Aortic stiffness may accelerate HF decompensation in vulnerable patients by increasing systolic load and worsening ventricular-vascular coupling. Excessive neurohormonal activation and inflammation as part of the HF syndrome itself, as occurring in AHFS, may further aggravate arterial function [30–32], while following the acute phase and transitioning to chronic HF, arterial stiffness may decrease [31]. Volume retention causes perturbations of pulsatile haemodynamics, manifested by an abrupt increase in arterial stiffness and blood pressure that acutely impair LV diastolic dysfunction leading to an AHFS [33,34]. This may be the rationale for vasodilator therapy in AHFS [35].
Currently, we demonstrated that in AHFS patients, increased aortic PWV pre-discharge was independently associated with increased risk for mortality or the combined clinical endpoint of mortality and HHF. Furthermore, adding PWV to MAGGIC score, a risk score for mortality in chronic HF patients [21] which has been also validated in patients after AHFS [22], increases considerably the prognostic accuracy of the combined model for mortality at 6 months. However, our results must be interpreted with caution because the absolute number of events was small. In addition, this is the first study to show a prognostic role of MAGGIC risk score for mortality in short-term (i.e. 6-months follow-up); previous studies have validated the prognostic role of the risk score for 1–3 years follow-up [21,22]. Pre-discharge aortic PWV has been previously shown to be an independent predictor of mortality following a HHF [19,36]. On-admission PWV (in patients hospitalized with AHFS) has been also associated with post-discharge adverse outcomes, but this association was lost after adjustment for age, eGFR, hemoglobin, and natriuretic peptides [18].
Perturbations of the central aortic pulsatile hemodynamics have been suggested to be involved in the development of AHF [37], while post-discharge events commonly occur in patients with incomplete recovery of the pulsatile hemodynamics [19]. Increased aortic PP showed only a trend, although non-significant, to higher incidence of HHF at 6 months in our study. Increased aortic PP, assessed either on admission [18] or pre-discharge [19] has been independently related to worse clinical outcomes in mixed populations with AHFS (both HFrEF and HFpEF). Of note, in AHFS patients with HFrEF only, lower aortic PP has been independently associated with higher all-cause mortality [17,36]. The role of aortic PP in patients with HFrEF is more complex as the lower systolic boost of the forward wave because of severe LV dysfunction and low stroke volume, rather than increased peripheral vascular resistance and stiffness, mainly determine aortic PP [38]. In the current study of a mixed AHFS population, aortic PP has not shown a clear predictive value probably because the conceptually anticipated effects of a lower PP in HFrEF is cancelled out but the effects of a higher PP in HFpEF. Nevertheless, larger future studies are needed to clarify these hypotheses.
Furthermore, endothelial function in our population was found to be severely impaired as assessed by the low brachial artery FMD (i.e. median value ca. 3.0%); previous studies in chronic HF patients have demonstrated higher FMD values (i.e. mean values >4.0–5.0%) [14,39], probably suggesting that FMD may be further attenuated in the acute setting of HF decompensation. Indeed, impairment in NO-dependent and independent pathways of endothelial function have been implicated in AHFS [40]. In chronic HF patients, endothelial dysfunction has been shown to be common [14,39] and has been associated with poor outcomes irrespective of HF etiology [14,41,42] providing incremental prognostic information in addition to BNP [42]. Nevertheless, FMD was not found to be associated with clinical outcomes probably because endothelial function was severely impaired invariably in our HF patients.
In contrast to central aortic stiffness, markers of peripheral arterial compliance have not shown a prognostic significance in our study. SAEI and LAEI indices have not been previously studied in the setting of HF. The greater prognostic importance of central aortic versus peripheral arterial elasticity indices in AHFS, implied in our study, needs to be further validated.
Interestingly, the natriuretic peptide BNP at discharge did not correlate with adverse clinical events at follow-up in our study. The exact time of measurement of natriuretic peptides (admission vs. discharge) and their change during hospitalization has been extensively studied with contradicting results in relation to prognosis [43].
4.1. Limitations
This was a single center study and the results may be affected by local clinical practice in the management of AHF patients. The sample size is relatively small and although incidence of clinical events was relatively high in our population (9% and 23% for mortality and HHF respectively at 6 months), the prognostic role of various vascular indices should be evaluated in larger studies, as the absolute number of events was small. The small sample size precluded the analysis of the predictive value of studied parameters based on Cox-regression models and hence ROC analysis was performed. The results cannot be generalized in HF patients with atrial fibrillation since these patients were excluded from the current study; vascular studies’ protocols have not been validated in the presence of atrial fibrillation.
5. CONCLUSION
The interaction between the heart and the systemic arterial vasculature is a key determinant of cardiovascular performance and assessment of arterial function, especially central aortic stiffness, may be a valuable tool in the prediction of adverse events following an AHF hospitalization. Increased aortic stiffness (i.e. increased PWV) may predict mortality and HF re-hospitalizations in AHF patients. Measurement of aortic PWV may further increase the prognostic ability of well-known risk scores for mortality in AHF patients. Further research is needed to investigate whether a comprehensive evaluation of AHF patients, assessing both cardiac and vascular function, allowing individualized targeted management, may ameliorate prognosis following an AHF hospitalization.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SG and KKN contributed in study conception, manuscript drafting, data-analysis and data collection. MB contributed in study conception, data-analysis and data collection. AB contributed in study conception, manuscript drafting, data-analysis. AK, LL, IG, KP, GC and EB contributed in critical appraisal and data collection. AK contributed in critical appraisal and manuscript editing. LKM contributed in study conception, critical appraisal, manuscript editing.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Sophia Giannitsi AU - Mara Bougiakli AU - Aris Bechlioulis AU - Anna Kotsia AU - Lampros Lakkas AU - Ioannis Girdis AU - Konstantinos Pappas AU - Georgios Chasiotis AU - Eleni Bairaktari AU - Andreas Kalogeropoulos AU - Lampros K. Michalis AU - Katerina K. Naka PY - 2020 DA - 2020/10/06 TI - The Prognostic Role of Aortic Stiffness in Patients Hospitalized for an Acute Heart Failure Syndrome JO - Artery Research SP - 7 EP - 13 VL - 27 IS - 1 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.200930.001 DO - 10.2991/artres.k.200930.001 ID - Giannitsi2020 ER -
