Cost Analysis of R-CHOP Versus Dose-Adjusted R-EPOCH in Treatment of Diffuse Large B-Cell Lymphoma with High-Risk Features
 , Yenny Alejandra Moreno Vanegas2, 3,
, Yenny Alejandra Moreno Vanegas2, 3,  , Nancy Diehl4, Aaron C. Spaulding5,
, Nancy Diehl4, Aaron C. Spaulding5,  , Sue Visscher6, Han W. Tun2, Sikander Ailawadhi2,
, Sue Visscher6, Han W. Tun2, Sikander Ailawadhi2,  , Prakash Vishnu2, 7
, Prakash Vishnu2, 7Email: bhagirathbhai.r.dholaria@vumc.org
- DOI
- 10.2991/chi.d.200410.001How to use a DOI?
- Keywords
- Diffuse large B-cell lymphoma; chemotherapy; outcomes research; combined antineoplastic chemotherapy protocols
- Abstract
Dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA.R-EPOCH) is used for upfront treatment of high-risk diffuse large B cell lymphoma (DLBCL). In this study, we compared the outcomes in patients with high-risk DLBCL who received frontline rituximab, cycophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) or DA.R-EPOCH immunochemotherapy. Outcomes and treatment-related cost were analyzed. DLBCL with one of the following features were included in the study: MYC ± BCL2 or BCL6 rearrangement by FISH or MYC overexpression by immunohistochemistry, Ki67 index ≥ 80% or nongerminal center immunophenotype, tumor measuring ≥5 cm and NCCN- IPI score ≥4. A total of 80 patients were treated with R-CHOP (n = 52, 65%) or DA.R-EPOCH (n = 28, 35%), with a median follow-up of 11.2 months (range: 0.7–151.3 months). The hazard ratios (HRs) for progression-free survival and overall survival were 0.79 [95% confidence interval (CI) 0.28%–2.29%, p = 0.67] and 0.86 (95% CI 0.26%–2.78%, p = 0.80), respectively for DA.R-EPOCH compared to R-CHOP. The total mean cost was USD106,940 ± USD39,351 and USD58,509 ± 24,588 for DA.R-EPOCH and R-CHOP respectively (p < 0.001). In our analysis, DA.R-EPOCH resulted comparable clinical outcomes and increased treatment-related expenses compared to R-CHOP in high-risk DLBCL.
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma in adults, and accounts for approximately 30% of all B-cell malignancies worldwide [1,2]. It is recognized as an aggressive, clinically heterogeneous disease that can be categorized into various subtypes based on histology and gene expression profiles (GEP) [2]. There are both clinical and pathologic factors that have been associated with different prognoses in DLBCL when treated with the standard chemotherapy regimen [3]. The revised National Comprehensive Cancer Network-International Prognostic Index (NCCN-IPI) has been used to predict outcomes of DLBCL treated with rituximab-based chemoimmunotherapy [4]. The 5-year progression-free survival (PFS) and overall survival (OS) of DLBCL patients in the poor risk group (NCCN- IPI ≥ 6) was 30% and 33%, respectively [4]. Moreover, the cell of origin (COO) classification based on GEP studies has identified distinct molecular subtypes of DLBCL [5]. Germinal center B-cell-like (GCB) and activated B-cell-like (ABC) or non-GCB subtypes based on GEP have significantly different behaviors and response to therapy [6]. Patients with the GCB subtype have better outcomes than those with the ABC subtype when treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) [1,7,8]. Rearrangements of the MYC gene concurrently with BCL2 and/or BCL6 as detected by fluorescent in situ hybridization (FISH), are sometimes referred to as “double-hit lymphomas” (DHL) or “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements” as per revised 2016 World Health Organization classification [1,9]. Patients with DHL DLBCL treated with R-CHOP present have an 18% 5-year PFS and 27% 5-year OS [10]. DLBCL with overexpression of MYC and BCL2 protein as detected by immunohistochemistry (IHC) are referred to as “double-expressor lymphomas” (DELs) [11]. The 5-year OS and PFS of DLBCL with and without MYC/BCL2 co-expression when treated with R-CHOP were reported as 30% versus 75% (p < 0.001) and 27% versus 73% (p < 0.001), respectively [11]. NCCN- IPI score of ≥3, COO classification non-GCB and MYC ± BCL2/BCL6 rearrangements and overexpression have been added as high-risk features in the revised World Health Organization classification of lymphoid neoplasms [9]. Early identification of these features can help direct the selection of an appropriate chemotherapy regimen. Approximately 40% of patients with DLBCL who are treated with R-CHOP or R-CHOP–like chemotherapy will relapse or develop refractory disease [12]. Hence, higher intensity regimens have been studied to improve the outcomes of high-risk DLBCL patients with mixed results [13–16]. A regimen which includes rituximab, etoposide, cyclophosphamide, doxorubicin, vincristine and prednisone (R-EPOCH) is commonly used for the upfront treatment of high-risk DLBCL [12]. This has demonstrated improved survival over R-CHOP in DLBCL patients with high proliferative index (Ki-67) [17]. In a phase II CALGB study of DLBCL treated with dose-adjusted R-EPOCH (DA.R-EPOCH), the 4-year PFS was 81% for the entire study cohort but only 54% in the subgroup with high-risk IPI [18]. Few studies have compared the use of R-CHOP versus DA.R-EPOCH in DLBCL [17,19]. Initial data from a phase 3 trial (CALGB 50303) comparing R-CHOP and DA.R-EPOCH in untreated DLBCL did not show any difference in PFS or OS [16]. In this study, we evaluated the clinical outcomes and treatment-related expenses in patients with high-risk DLBCL who received DA.R-EPOCH or R-CHOP.
2. MATERIALS AND METHODS
2.1. Patient Selection
All patients who had histologically proven DLBCL per the WHO classification criteria, and presented with high-risk features were selected. The analyzed patients received treatment with R-CHOP or DA.R-EPOCH at our institute and had available clinical information and follow up of at least 6 months or until death. We collected basic demographic information, LDH, Ann-Arbor staging, NCCN-IPI score, tumor size, Ki-67 index, CD10, BCL2, BCL6, MUM1 and MYC expression measured by IHC, and MYC, BCL2 and BCL6 rearrangements detected by FISH. Treatment and follow up information were also collected. High-risk DLBCL was defined by the presence of any of the following features at diagnosis: MYC ± BCL2 or BCL6 rearrangement, MYC overexpression, Ki67 index ≥80% or nongerminal center immunophenotype by Hans algorithm [20], tumor measuring ≥5 cm and NCCN- IPI score ≥4.
2.2. Study Design and Objectives
This is a single institution, retrospective cohort study approved by the Institutional Review Board (IRB) of the Mayo Clinic, and was conducted in accordance with the declaration of Helsinki. Its primary objective was to perform a financial cost analysis of the R-CHOP and DA.R-EPOCH treatment in high-risk DLBCL patients. The secondary objectives were evaluation of treatment-related toxicity and survival outcomes in the two treatment arms.
2.3. MYC and BCL2 IHC
Epitope retrieval was performed with Cell Conditioning Solution (CC1) to process tissue samples with IHC reactions carried out on VENTANA BenchMark XT© automated slide stainers. The BCL2 antibody (124 clone) from DAKO was used. The Myc antibody (clone EP121) from Epitomics© was used. All IHC was performed as per the manufacturer's recommendations.
2.3.1. MYC, BCL2 and BCL6 FISH
FISH for the MYC, BCL2 and BCL6 genes was performed using Abbott molecular probes. Rearrangements involving MYC, BCL2 and BCL6, were detected using dual-color break-apart strategy probes. Translocations involving MYC were identified using dual-color, dual-fusion (D-FISH) strategy probes.
2.4. Chemotherapeutic Regimens, Toxicity and Side Effects
All 80 patients in our study received either R-CHOP or DA.R-EPOCH as fist-line treatment, based on provider and patient preference. All these patients were serially treated during the study period. We did not have any institutional protocol to assign either of these regimens in newly diagnosed patients with DLBCL. The R-CHOP regimen was given in an outpatient chemotherapy unit and included rituximab 375 mg/m2/day on day 0 or 1, cyclophosphamide 750 mg/m2 on day 1, doxorubicin 50 mg/m2 on day 1, vincristine 1.4 mg/m2 to a maximum of 2 mg on day 1, and prednisone 100 mg/m2 on days 1 through 5 every 21 days. For the DA.R-EPOCH regimen, rituximab was administered at the outpatient clinic on day 1, followed by inpatient continuous intravenous infusion of etoposide 50 mg/m2/d, vincristine 0.4 mg/m2/d, doxorubicin 10 mg/m2/d on days 1 to 4, cyclophosphamide 750 mg/m2/d on day 5 and oral prednisone 60 mg/m2/d on days 1 to 5. Pegfilgrastim 6 mg was administered subcutaneously on day 6 per protocol. The standard dose adjustment protocol for DA.R-EPOCH was followed based on interim absolute neutrophil count (ANC) and platelets [21]. Other dose adjustments were allowed depending on the patient's clinical status and side-effects from previous cycles. Most patients received between 2 and 8 cycles, with a median of 6 cycles for both regimens. Grades 3/4 adverse events were recorded as described in the Common Terminology Criteria for Adverse Events protocol version 4.0 (CTCAE v4.0) [22].
2.5. Response Assessment
Each patient had a positron emission tomography–computed tomography (PET-CT) scan at baseline and at 4–6 weeks after planned treatment completion. Interim imaging during treatment was performed at the provider's discretion. Surveillance imaging with contrast CT scan was performed every 4–6 month for the first two years after treatment completion. The response criteria were assessed based on the International Working Group Recommendations for Response Criteria for non-Hodgkin lymphoma using 5-point scoring (5PS) system for PET scan or CT scan with contrast [23,24]. Complete response (CR) required complete regression of all radiologic disease on CT scan, or no fluorodeoxyglucose (FDG) uptake, or score 1, 2 or 3 on 5PS. Partial response (PR) was defined as score 4 or 5 on 5PS with reduced FDG uptake by PET scan compared to baseline or a decrease of at least 50% in the sum of the products of the dimensions of measurable lesions by CT scan. Diffuse uptake compatible with reactive changes from chemotherapy was allowed. Progressive disease (PD) was defined as a score of 4 or 5 on 5PS with increase in FDG uptake compared to baseline, or a new FDG-avid lesion by PET scan, or increase in size of ≥50% of a single node, or new lesions on CT scan. Relapse was defined as new disease in CR patients or as PD in PR patients [23].
2.6. Statistical Analysis
The baseline patient characteristics were summarized using descriptive statistics including median, range for continuous variables, and proportions and frequencies for categorical variables. Categorical variables were compared between groups using the Fisher's exact test. The Wilcoxon rank-sum test was used to compare continuous variables. Survival analysis was performed using the Kaplan–Meier estimates. Differences were determined using a two-tailed log-rank test, and the significance level was set at p < 0.05. Ninety-five percent confidence intervals (CIs) were provided for survival probabilities and/or cumulative incidences. Cox proportional hazard regression was performed to assess the influence of clinical and treatment variables on OS and PFS.
2.7. Cost Analysis
Patients who did not receive their complete planned treatment at our facility were excluded from the cost analysis. Standardized costs were obtained from the Mayo Clinic Florida cost data warehouse based upon patient characteristics and treatments. Costs not associated with cancer treatments were excluded. Medicare reimbursement was assigned to all professional billed services, the appropriate Medicare Cost Report cost-to-charge ratios were multiplied by the charges for all hospital billed services and all resulting costs were adjusted to 2016 USD with the Gross Domestic Product (GDP) Implicit Price Deflator [25]. The resulting costs were then assigned to Berenson-Eggers Type of Service (BETOS) codes, in order to allow for comparisons between services, and Wilcoxon rank-sum tests were utilized to determine differences between DA.R-EPOCH or R-CHOP regimens [26]. The statistical analysis was performed using SAS (version 9.4; SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
3.1. Patient Characteristics
In this longitudinal cohort of 80 patients with high-risk DLBCL, 52 (65%) were treated with R-CHOP and 28 (35%) received DA.R-EPOCH. Table 1 shows their baseline demographics and clinical features. Most patients (71%) were ≥60 years of age. The DA.R-EPOCH cohort had a greater proportion of patients with higher Ann-Arbor stage, non-GCB immunophenotype and BCL2 expression. In total, 2 (2.5% tested) patients were found to have both MYC and BCL2 gene rearrangements and 3 (3.8% tested) had MYC protein overexpression with none being DEL. The median follow-up was 10.9 (0.7–151.3) and 13.3 (1–47.1) months for the R-CHOP and DA.R-EPOCH groups, respectively (p = 0.82).
| R-CHOP (n = 52) | DA.R-EPOCH (n = 28) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Median age at diagnosis (range) | 64 (29, 88) | 68 (18, 87) | 0.33 |
| Male | 27 (51.9%) | 17 (60.7%) | 0.49 |
| Race, Caucasian | 47 (90.4%) | 24 (85.7%) | 0.71 |
| LDH | 0.81 | ||
| Normal <222 | 22 (48.9%) | 12 (44.4%) | |
| High ≥222 | 23 (51.1%) | 15 (55.6%) | |
| Missing data | 7 | 1 | |
| Extranodal involvement | 31 (59.6%) | 21 (75.0%) | 0.22 |
| Ann Arbor stage | 0.033 | ||
| 1–2 | 24 (46.2%) | 6 (21.4%) | |
| 3–4 | 28 (53.8%) | 22 (78.6%) | |
| ECOG performance status | 0.53 | ||
| ≤2 | 42 (80.8%) | 25 (89.3%) | |
| >2 | 10 (19.2%) | 3 (10.7%) | |
| Albumin | 0.056 | ||
| <3.7 | 8 (18.6%) | 11 (40.7%) | |
| ≥3.7 | 35 (81.4%) | 16 (59.3%) | |
| Missing data | 9 | 1 | |
| Transformed DLBCL | 8 (15.4%) | 5 (17.9%) | 0.76 |
| NCCN-IPI | 0.49 | ||
| 0–3 | 36 (87.8%) | 21 (80.8%) | |
| ≥4 | 5 (12.2%) | 5 (19.2%) | |
| Missing data | 11 | 2 | |
| High-Risk Features | |||
| Bone marrow involvement | 0.024 | ||
| No | 47 (95.9%) | 22 (78.6%) | |
| Yes | 2 (4.1%) | 6 (21.4%) | |
| Missing data | 3 | 0 | |
| Largest tumor size (cm) | 0.80 | ||
| <5 | 16 (34.0%) | 10 (38.5%) | |
| ≥5 | 31(66.0%) | 16 (61.5%) | |
| Missing data | 5 | 2 | |
| Ki-67 index | 0.040 | ||
| <80% | 13 (28.9%) | 2 (7.7%) | |
| ≥80% | 32 (71.1%) | 24 (92.3%) | |
| Missing data | 7 | 2 | |
| MYC positive (IHC) | 0.29 | ||
| No | 28 (90.3%) | 18 (100.0%) | |
| Yes | 3 (9.7%) | 0 (0.0%) | |
| Missing data | 21 | 10 | |
| MYC rearranged (FISH) | 0.082 | ||
| No | 26 (96.3%) | 18 (78.3%) | |
| Yes | 1 (3.7%) | 5 (21.7%) | |
| Missing data | 25 | 5 | |
| BCL2 positive (IHC) | 0.010 | ||
| No | 22 (53.7%) | 5 (20.0%) | |
| Yes | 19 (46.3%) | 20 (80.0%) | |
| Missing data | 11 | 3 | |
| BCL2/BCL6 rearranged (FISH) | 0.16 | ||
| No | 19 (82.6%) | 11 (61.1%) | |
| Yes | 4 (17.4%) | 7 (38.9%) | |
| Missing data | 29 | 10 | |
| Non-germinal center B-cell immunophenotype | 22 (43.1%) | 20 (71.4%) | 0.020 |
| Missing data | 1 | 0 | |
| Number of high-riska features present | 0.001 | ||
| 1–2 | 40 (76.9%) | 12 (42.9%) | |
| 3 | 12 (23.1%) | 11 (39.3%) | |
| 4–5 | 0 (0.0%) | 5 (17.9%) | |
| Median follow-up (months, range) | 13.3 (1–47.1) | 10.9 (0.7–151.3) | 0.82 |
DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab; cyclophosphamide; doxorubicin; vincristine and prednisone; DA.R-EPOCH, dose adjusted rituximab; etoposide; prednisone; vincristine, cyclophosphamide and doxorubicin; NCCN-ZIPI, national comprehensive cancer network international prognostic index; FISH, fluorescent in-situ hybridization; IHC, immunohistochemistry.
High-risk DLBCL was defined by the presence of any of the following features at diagnosis: MYC ± BCL2 or BCL6 rearrangement by FISH or MYC overexpression by IHC, Ki67 index ≥80% or non-germinal center immunophenotype by Hans algorithm, tumor measuring ≥5 cm and NCCN- IPI score ≥4. Bold values denote statistical significance at < 0.05.
Patient demographics and disease characteristics of high-risk DLBCL
3.2. Treatment Responses and Chemotherapy Toxicity
The rates of treatment completion and CR, as well as the overall incidences of grade ≥3 neutropenia, neuropathy and unplanned hospitalizations were similar between the two treatment groups. Patients treated with DA.R-EPOCH required more red cell transfusions (p = 0.004) (Table 2).
| Treatment-related toxicities | R-CHOP (n = 52) | DA.R-EPOCH (n = 28) | p-Value |
|---|---|---|---|
| Grade 3/4 neutropenia | 29 (69.0%) | 22 (84.6%) | 0.25 |
| Grade 3/4 neuropathy | 3 (7.1%) | 1 (3.8%) | 1.00 |
| Unplanned hospitalization | 12 (28.6%) | 12 (46.2%) | 0.19 |
| Required red cell transfusion | 9 (20.9%) | 15 (55.6%) | 0.004 |
| Outcomes | |||
| Finished planned treatment | 47 (90.4%) | 23 (82.1%) | 0.31 |
| End of treatment response | 0.67 | ||
| Complete response | 44 (86.3%) | 20 (80.0%) | |
| Partial response | 3 (5.9%) | 2 (8.0%) | |
| Stable disease | 1 (2.0%) | 0 (0.0%) | |
| Progressive disease | 3 (5.9%) | 3 (12.0%) |
DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab; cyclophosphamide; doxorubicin; vincristine and prednisone; DA.R-EPOCH, dose adjusted rituximab; etoposide; prednisone; vincristine; cyclophosphamide and doxorubicin.
Treatment toxicities and outcomes of high-risk DLBCL patients treated with R-CHOP and DA.R-EPOCH
3.3. Survival Outcomes and Prognostic Factors
In a Cox-regression analysis, low baseline albumin, ECOG performance status ≥2, above-normal LDH and high NCCN- IPI were associated with poor OS and PFS in all the patients. There was no significant difference in the OS (p = 0.99) or PFS (p = 0.85) for patients between the two treatment cohorts. The 3-year PFS of patients treated with R-CHOP and DA.R-EPOCH was 71% (95% CI 50%–88.1%) and 77% (95% CI 52%–98%) and the 3-year OS was 66% (95% CI 46.8%–91.1%) and 80.1% (95% CI 56.1%–100%), respectively. The hazard ratio (HR) for PFS was 0.79 (95%CI 0.28%–2.29%, p = 0.67) and for OS 0.86 (95% CI 0.26%–2.78%, p = 0.80) (Figure 1). Low cumulative doses of vincristine, doxorubicin or cyclophosphamide were associated with poor OS and PFS (Table 3).
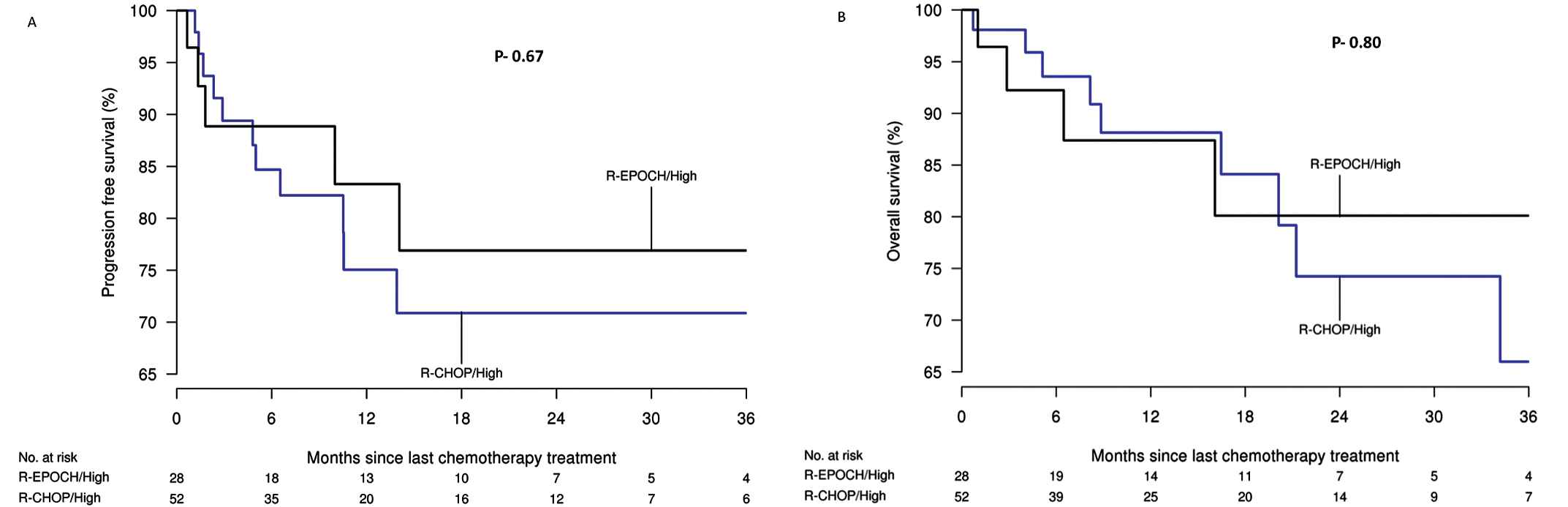
A) progression-free and B) overall survival of high-risk diffuse large B-cell lymphoma (DLBCL) treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) and dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA.R-EPOCH) (Kaplan–Meier analysis).
| Association with death |
Association with progression |
|||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Demographics | ||||
| Age at diagnosis (per each 5-year increase) | 1.08 (0.85, 1.37) | 0.53 | 1.10 (0.90, 1.34) | 0.37 |
| Gender—Male | 1.31 (0.43, 4.02) | 0.63 | 1.41 (0.51, 3.87) | 0.51 |
| Race—Caucasian | 1.27 (0.16, 9.75) | 0.82 | 1.81 (0.24, 13.70) | 0.57 |
| Diagnosis | ||||
| Extra-nodal involvement | 1.22 (0.38, 4.00) | 0.74 | ||
| NCCN-IPI | 1.99 (1.11, 3.59) | 0.022 | 0.49 (0.18, 1.31) | 0.15 |
| Ann Arbor stage (1–4) | 1.38 (0.82, 2.32) | 0.23 | 2.06 (1.10, 3.83) | 0.023 |
| LDH (loge) | 4.29 (1.72, 10.71) | 0.002 | 0.82 (0.30, 2.27) | 0.70 |
| ECOG (0–3) | 1.90 (1.06, 3.40) | 0.031 | 5.65 (2.22, 14.39) | 0.0003 |
| Albumin | 0.12 (0.04, 0.40) | 0.001 | 1.48 (0.81, 2.73) | 0.20 |
| High-Risk Features | ||||
| Bone marrow involvement | 1.68 (0.37, 7.59) | 0.50 | 0.13 (0.03, 0.50) | 0.003 |
| Largest tumor size (cm) | 0.52 | 0.40 | ||
| <5 cm | 1.00 | N/A | 1.00 | N/A |
| 5–10 cm | 1.85 (0.49, 6.93) | 0.33 | 2.23 (0.63, 7.96) | 0.22 |
| >10 cm | 0.83 (0.15, 4.53) | 0.83 | 1.12 (0.25, 5.01) | 0.88 |
| Ki-67 index | 0.99 (0.96, 1.02) | 0.39 | 0.99 (0.96, 1.02) | 0.51 |
| BCL2/BCL6 rearrangement by FISH | 1.79 (0.30, 10.72) | 0.52 | 5.41 (0.99, 29.66) | 0.052 |
| MYC IHC | N/A | 1.00 | N/A | 1.00 |
| MYC rearrangement by FISH | N/A | 1.00 | 3.19 (0.58, 17.51) | 0.18 |
| BCL2 IHC | 1.76 (0.46, 6.83) | 0.41 | 6.44 (0.80, 51.59) | 0.080 |
| GCB subtype | 0.77 (0.25, 2.36) | 0.65 | 0.50 (0.17, 1.43) | 0.19 |
| Cumulative Chemotherapy Dose | ||||
| Cumulative vincristine (mg/m2) dose | 0.61 (0.47, 0.80) | 0.0004 | 0.63 (0.43, 0.93) | 0.019 |
| Cumulative doxorubicine (mg/m2) dose (per each 50 unit increase) | 0.58 (0.39, 0.87) | 0.008 | 0.60 (0.38, 0.94) | 0.024 |
| Cumulative cyclophosphamide (mg/m2) dose (per each 500 unit increase) | 0.66 (0.51, 0.85) | 0.001 | 0.70 (0.51, 0.96) | 0.027 |
| Treatment Arm | ||||
| R-CHOP | 1.00 | N/A | 1.00 | N/A |
| DA.R-EPOCH | 0.86 (0.26, 2.78) | 0.80 | 0.79 (0.28, 2.29) | 0.67 |
| For associations with death and recurrence, HRs, 95% CIs, and p-values result from Cox proportional hazards regression models. | ||||
DLBCL, diffuse large B-cell lymphoma; NCCN-IPI, national comprehensive cancer network-international prognosis index; LDH, lactate dehydrogenase; ECOG, Eastern Co-operative Oncology Group; FISH, fluorescent in-situ hybridization; IHC, immunohistochemistry; GCB, germinal center B-cell; R-CHOP, rituximab; cyclophosphamide; doxorubicin; vincristine; prednisone; DA.R-EPOCH, dose-adjusted rituximab; etoposide; prednisone; vincristine; cyclophosphamide; doxorubicin; HR, hazard ratio; CI, confidence interval. Bold values denote statistical significance at < 0.05.
Cox-regression analysis of patient characteristics with death after last chemotherapy date and disease progression
3.4. Cost Analysis
After excluding the patients who did not receive their full treatment at our facility, we analyzed 66 (DA.R-EPOCH = 41, R-CHOP = 25) for their treatment-related cost. The total costs associated with the use of DA.R-EPOCH were greater than those in the R-CHOP group, with the mean cost USD 106,940 ± USD39,351 and USD58,509 ± 24,588, respectively (P < 0.001). DA.R-EPCOH was associated with higher expenses related to laboratory testing, hospital services and evaluation and management, compared to R-CHOP (P < 0.001). All other costs related to chemotherapy, imaging (BETOS category 3) and other (BETOS category 6, 7 which included vaccines, follow-up visits, certain injections and drugs) were similar between the two groups (Table 4).
| R-CHOP (n = 41) | DA.R-EPOCH (n = 25) | p-Value | |
|---|---|---|---|
| Total Cost Inflated to 2016 USD | <0.0001 | ||
| Mean (SD) | 57,115 (25,904) | 106,939 (39,351) | |
| Range | (98–134,728) | (2,812–169,534) | |
| Chemotherapy | |||
| Mean (SD) | 38,459 (20,600) | 90,506 (34,416) | <0.0001 |
| Range | (0–99,251) | (2,812–140,326) | |
| Nonchemotherapy | |||
| Procedures | 0.0736 | ||
| Mean (SD) | 2,041 (1,407) | 1,435 (1,194) | |
| Range | (0–7,839) | (0–4,027) | |
| Imaging | 0.0002 | ||
| Mean (SD) | 1,812 (886) | 877 (853) | |
| Range | (0–4,255) | (0–2,475) | |
| Tests | 0.4718 | ||
| Mean (SD) | 1,249 (966) | 1,297 (1,510) | |
| Range | (98–4,328) | (0–7,042) | |
| Hospital Services | 0.3359 | ||
| Mean (SD) | 2,712 (5,395) | 5,337 (9,192) | |
| Range | (0–25,242) | (0–32,920) | |
| Blood Transfusion | 0.0057 | ||
| Mean (SD) | 308 (755) | 1,041 (1252) | |
| Range | (0–3,509) | (0–4,166) | |
| Eval and Mgmt | 0.3958 | ||
| Mean (SD) | 831 (756) | 949 (1,026) | |
| Range | (0–3,871) | (0–4,010) | |
| Other | 0.0099 | ||
| Mean (SD) | 9,702 (6,696) | 5,497 (4,591) | |
| Range | (0–22,937) | (0–13,897) |
Treatment-related expenses for high-risk DLBCL treated with R-CHOP versus DA.R-EPOCH (inflated to 2016 USD)
4. DISCUSSION
DA.R-EPOCH has been proposed as a higher intensity regimen over standard R-CHOP for high-risk DLBCL. In our study, DA.R-EPOCH was associated with increased treatment-related costs, without improving survival outcomes compared to R-CHOP. There was no significant difference in the side effects such as neutropenia, peripheral neuropathy or unplanned hospitalizations between the two treatment regimens. However, patients treated with DA.R-EPOCH required more red cell transfusion support for symptomatic anemia compared to those who received R-CHOP. Few studies have compared the efficacy of R-CHOP versus DA.R-EPOCH in specific DLBCL subgroups. A report by Huang et al. of DLBCL with Ki-67 index ≥80%, showed that the 3-year PFS with R-EPOCH and R-CHOP was 86.6% and 59.7% (P = 0.024), and the 3-year OS was 89.9% and 70.2% (P = 0.041), respectively [17]. A meta-analysis by Howlett et al. comparing intermediate dose R-EPOCH and standard dose R-CHOP in the first-line setting in patients with DHL showed no significant difference in terms of OS between the two regimens, but the former led to a reduction in the risk of progression in those patients [27]. Similarly, a phase III study (CALGB 50303) did not to show a difference in event-free survival (EFS) and OS between the DA.R-EPOCH and R-CHOP as a first-line treatment of DLBCL patients [16].
In our study, a higher proportion of patients in the DA.R-EPOCH group had higher Ann Arbor stage, positive BLC2 IHC and ABC immunophenotype compared to the R-CHOP group. While these factors may have influenced the physicians' decision for using DA.R-EPOCH instead of R-CHOP, it remains unknown if these patients would have fared poorly if they all had received R-CHOP or better if they all received the DA.R-EPOCH regimen. Nevertheless, these factors were not associated with higher risk of death or recurrence when analyzed independently. A Cox-regression analysis indicated that NCCN- IPI, LDH, ECOG status and low albumin were associated with worse survival and a higher risk of recurrence. In this analysis, the treatment regimen was not a significant factor influencing PFS and OS after adjusting for other clinical variables.
A recent single-center study has shown increased direct treatment-related cost associated with DA.R-EPOCH compared to R-CHOP, but full details are not published [19]. The cost analysis of our cohort demonstrated that the DA.R-EPOCH regimen is associated with higher overall actuarial costs compared to R-CHOP. The difference in costs was primarily related to hospitalization and laboratory test-related charges. At other centers where DA.R-EPOCH is routinely administered in outpatient settings, this difference may not exist; however, requirement of an infusion pump and related administration costs associated with DA.R-EPOCH makes it more resource intensive compared to R-CHOP, even in outpatient settings. DA.R-EPOCH also requires weekly complete blood counts per protocol and routine administration of pegfilgrastim, which all add to the expenses associated with this protocol.
There are several limitations to our study. It is a single-center retrospective post-hoc analysis with a relatively small sample size and follow-up. Despite being statistically comparable, baseline high-risk factors were imbalanced between the treatment groups, with DA.R-EPOCH used in the patients with higher proportion of risk factors. There were several patients for whom the data on IHC and FISH results for MYC, BCL2 and BCL6 were not available. Also, the cost analysis was largely influenced by location of treatment administration which limits its utility.
5. CONCLUSION
Our study showed that patients with high-risk DLBCL treated with either R-CHOP or DA.R-EPOCH had equivalent PFS and OS. Red cell transfusion requirement and treatment-related costs were higher in DA.R-EPOCH treated patients. Higher intensity chemotherapy needs prospective validation in the patients with DLBCL with poor risk features, and R-CHOP remains the standard of care for such patients.
CONFLICTS OF INTEREST
The authors delcare they have no conflicts of interest the final article.
AUTHORS' CONTRIBUTION
BD, PV, YM contributed to the conception and design of the study; BD, PV, YM contributed to the writing of the manuscript; ND, AS, SV analyzed the data and provided statistical support; all authors critically reviewed the manuscript and approved the final version.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Bhagirathbhai Dholaria AU - Yenny Alejandra Moreno Vanegas AU - Nancy Diehl AU - Aaron C. Spaulding AU - Sue Visscher AU - Han W. Tun AU - Sikander Ailawadhi AU - Prakash Vishnu PY - 2020 DA - 2020/04/23 TI - Cost Analysis of R-CHOP Versus Dose-Adjusted R-EPOCH in Treatment of Diffuse Large B-Cell Lymphoma with High-Risk Features JO - Clinical Hematology International SP - 117 EP - 124 VL - 2 IS - 3 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.200410.001 DO - 10.2991/chi.d.200410.001 ID - Dholaria2020 ER -
