CD3/CD19 Depletion for T-cell Reduction of Allogeneic Transplants: Mostly Efficient, but not Robust
 , Halvard Bonig1, 2, 3, *,
, Halvard Bonig1, 2, 3, *, 
- DOI
- 10.2991/chi.k.210725.001How to use a DOI?
- Keywords
- Immunomagnetic depletion; CliniMACS; haplo-identical transplantation; TCRαβ/CD19 depletion; graft-versus-host disease; graft failure
- Abstract
Aggressive T-cell depletion, in vitro or in vivo, is a prerequisite for survival of haplo-identical stem cell transplantation. The classical T-cell-depleted transplant, immunomagnetically enriched CD34+ cells, is very safe with respect to graft-versus-host reactivity, but associated with very high transplant-related and relapse mortality with an overall probability of survival of only 20%. Protocols for T- and B-cell depletion were therefore developed, reasoning that transplantation of the majority of Natural Killer (NK) cells and the substantial dose of residual T-cells might improve survival, which was, in principle, confirmed. Anecdotal reports of frequent failure to achieve adequate T-cell depletion prompted review of the aggregate data for transplant quality at our center. The first observation is the relative paucity of combined CD3/CD19 depletion processes as PTCy protocols have made inroads, 13 depletions in 8 years. Median T- and B-cell log-depletion were −3.89 and −1.92, respectively; instead of, CD34+ cell recovery was generally high (median 92%), as was NK-cell recovery (median 52%). However, the process failed to yield satisfactory T- and B-cell depletion in two out of 13 preparations, of which one product could be rescued by a second round of depletion, at the expense of CD34+ cell recovery. In our hands, the process is thus insufficiently robust for routine clinical use. Assuming similar observations in other centers, this may explain implementation of alternative protocols, such as TCRαβ/CD19 depletion or transplantation of unmanipulated grafts with subsequent in vivo depletion.
- Copyright
- © 2021 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Where well-matched donors are unavailable, transplant centers may resort to mismatched donors, most often haplo-identical family donors [1–3]. Such transplants will inevitably cause lethal acute graft-versus-host disease (GvHD) unless T-cells are aggressively depleted. The price for overzealous T-cell depletion, on the other hand, is graft failure, relapse due to lack of immunological control of residual blasts (graft-versus-leukemia, GvL), morbidity and mortality from opportunistic infections and dramatically delayed immune reconstitution [4–7]. The search for the ideal protocols providing GvHD control and useful immune function has been ongoing for approximately four decades, but achieving good separation of GvHD from GvL immune response is notoriously difficult. The initial haplo-identical transplant required mega-doses of CD34+ cells [8,9], immunomagnetically selected by, at the time, several competing technologies [10–12]. The disappointing clinical outcomes, with long-term survival rates of not much more than 20% [7], have largely curbed the enthusiasm for allogeneic “naked haplo” transplantation without immediate or delayed co-transplantation of an alloreactivity-attenuated T-cell product [7]. The use of CD34+ selected products is limited to correction of poor graft function following allogeneic hematopoietic stem cell transplantation [13–16]. In the autologous setting, CD34 selected transplants retain some relevance for tumor cell purging, such as in neuroblastoma [17,18]. The rationale for CD3/CD19 depletion of haplo-identical allogeneic grafts was the expectation that co-transplanted mature cells, especially Natural Killer (NK)-cells, might provide relevant benefit [19–21]. We will not dismiss the possibility that after the much less efficient T-cell depletion provided by CD3/CD19 depletion (more than one log compared to CD34 selection) therapeutically relevant T-cell doses might remain in the product. Despite strong evidence of (GvHD-) safety and quite satisfactory clinical outcomes with CD3/CD19 depleted transplants, typically supplemented with CD34-selected grafts to achieve the desired hematopoietic stem and progenitor cell (HSPC) megadoses without exceeding the targeted T-cell dose of 50,000/kg body weight [21,22], CD3/CD19-depleted HSPCs have not become a mass product. Several reasons have likely contributed, including the high cost of the transplant and the advent of alternative haplo-identical transplants, such as TCRαβ/CD19 depleted HSPCs [23,24] or unmanipulated bone marrow in combination with post-transplant intermediate-dose cyclophosphamide, which is safe and provides reasonable immune reconstitution [25–30]. We propose that inconsistent cell processing results during CD3/CD19 depletion, as demonstrated by the analyses reported here, may have contributed to its limited adoption. These inconsistencies arise despite use of quality-controlled devices, reagents and consumables, with multiply validated, Good Manufacturing Practice (GMP) compliant, rigid manufacturing protocols disseminated by the manufacturer of the materials, in the hands of a professional GMP-team consistently successfully manufacturing a host of other, much more complex products.
2. MATERIALS AND METHODS
Mobilized apheresis products from haplo-identical family donors were collected by the apheresis services supporting the respective transplant centers, subjected to quality control in agreement with locally specified quality attributes and released by a Qualified Person. The products were hand-carried by designated couriers at 4–8°C in temperature-controlled containers. Products were processed within 24 h of the end of the apheresis. Product quality varied widely, reflecting typical donor variability but, more importantly, the 10-fold difference in recipient weight (median, 45 kg, interquartile range 27–54 kg, range 8.4–83 kg) and accordingly, difference in target doses for CD34+ and T-cells. In most cases, apheresis products were asymmetrically divided, for one part to be subjected to CD3/CD19 depletion, and the remainder to CD34 selection. Collection of sufficient cells provided, the fraction slated for CD3/CD19 depletion reflected the maximum cell dose for total cells, T- or B-cells allowed by the manufacturer (Miltenyi Biotech (MACS), Bergisch Gladbach, Germany) for reagent kits and tubing set, whichever cell population turned out to be limiting. The remainder was subjected to CD34 selection and is not considered here. CD3/CD19 depletion followed company-provided protocols as previously described, without any modifications, using commercially available reagents throughout. In brief, platelets were removed from the apheresis product by soft-spin, the white blood cells (WBCs) were re-suspended in MACS buffer, albumin-supplemented saline. Antibody-bead-complex was added as manufacture-recommended. A depletion tubing set 161-01 (Miltenyi Biotech) was used with the cognate Depletion program on CliniMACS (Miltenyi Biotech). Depicted in Table 1 are the attributes of the fraction subjected to CD3/CD19 depletion. The process was initiated on the morning after the apheresis, approximately 18 h after its end; in one case, the products from two successive aphereses were combined and subjected to CD3/CD19 depletion, at which time the first apheresis product was 24-h old. Quality controls were performed on incoming apheresis products and target cell bags. The volume was assessed by gross product weight corrected for tare and assuming 1 g = 1 mL. Concentrations of WBC and five-way differential were measured with Sysmex XT1800i (Norderstedt, Germany), although eosinophils and basophils were neglected for the purpose of these analyses. CD34+ cells (HSPCs) in the apheresis product and end product, as well as T-cells in the apheresis product were enumerated by single-platform flow cytometry, using Becton-Dickinson (BD) Canto II (BD, Heidelberg, Germany) with Diva software. CD20+ and CD56+ cells before and after depletion as well as T-cells after depletion were enumerated using an in-house generated multi-parametric flow cytometry panel containing anti-CD45, anti-CD34, anti-CD3, anti-CD20, anti-CD56 and anti-CD14, as well as 7-aminoactinomycin (7AAD) as a viability dye (all antibodies and 7AAD:BD). The latter panel is optimized and formally validated for enumeration of very low frequencies of T- and B-cells, whereby T- and B-cells are quantified by normalizing their frequency against International Society for Hematology and Graft Engineering (ISHAGE, now ISCT)-gated CD34+ cells according to the multi-color panel and the concurrently analyzed CD34+ concentration from the single platform assay (Stem Cell Enumeration (SCE), BD) [31]. Sterility was assessed using the European Pharmacopoeia (EP)-conforming rapid test “BacT/Alert” (BioMérieux, Marcy-l’Étoile, France) as described [32]; all products were negative for microbial growth.
| Apheresis product | Depleted product | |
|---|---|---|
| Median (IQR/range) | Median (IQR/range) | |
| Volume [mL] | 258 (193–310/130–380) | 206 (155–224/99–292) |
| WBC [×109] | 61 (57–74/30–105) | 35 (30–44/18–77) |
| CD34+ frequency [% CD45+] | 0.78 (0.65–0.92/0.13–2.3) | 1.0 (0.86–0.1.50/0.15–4.13) |
| CD34+ total dose [×106] | 529 (246–577/131–809) | 408 (200–534/119–594) |
| CD34+ dose [×106/kg] | 20.06 (8.71–15.14/3.43–66.47) | 9.42 (6.90–10.34/3.15–60.70) |
| CD3+ frequency [% CD45+] | 24 (21–28/12–36) | 0.006 (0.005–0.016/0.002–0.079) |
| CD3+ total dose [×106] | 15,473 (14,629–16,586/10,354–29,934) | 2.2 (1.8–3.1/0.9–13.1) |
| CD3+ dose [×106/kg] | 443 (316–549/232–1755) | 0.053 (0.049–0.093/0.034–0.386) |
| Monocytes [% CD45+] | 22 (18–29/15–32) | 30 (27–35/22–51) |
| Granulocytes [% CD45+] | 31 (27–34/16–38) | 7.6 (4.7–14.3/2.2–25.9) |
| CD56/16+ frequency [% CD45+] | 4.6 (3.8–6.2/2.4–22.8) | 4.6 (3.8–8.9/1.6–25.5) |
| CD56/16+total dose [×109] | 3.2 (2.5–4.0/1.4–9.5) | 2.5 (1.1–3.2/0.4–6.0) |
| CD56/16+ dose [×106/kg] | 71 (60–199/25–574) | 39 (21–96/13–385) |
| CD20+ frequency [% CD45+] | 4.4 (3.5–5.3/1.7–8.1) | 0.1 (0.01–0.17/0.01–0.27) |
| CD20+ total dose [×106] | 2604 (2305–3358/1322–4595) | 32.4 (8.2–59.6/1.6–135.2) |
| CD20+ dose [×106/kg] | 68 (64–79/21–547) | 0.889 (0.388–1.673/0.059–3.210) |
Product properties: median, interquartile range (IQR) and range for product-defining values before and after manipulation are presented for all consecutive CD3/CD19-depletion performed between 2012 and 2020
Data were extracted in a pseudonymized fashion from the batch records. Analyses represent our regulatory and JACIE-mandated efforts at periodic product quality review. The Goethe University Medical School ethics committee has confirmed that such exercise does not require specific donor consent. Log-depletion was calculated as log10 (total cell number after depletion/total cell number before depletion). Descriptive statistics were calculated and graphs drawn in Excel 2010 (Microsoft, Redmond, WA).
3. RESULTS AND DISCUSSION
A total of 13 products from 14 aphereses were processed over the course of 8 years. All preparations initially proceeded without planned or unplanned deviations. In two cases, a far less than the expected −3.5 to −4.0 log T-cell depletion was achieved. In one of these, the product was subjected to a second round of antibody incubation and column separation. The successive depletion cycles succeeded in reducing T-cell dose to a more typical −3.47 log-depletion, to 18,000 T-cells/106 CD34+ cells. A root cause analysis failed to identify reasons for the process failure; all reagents were, of appropriate quality, cell numbers in the apheresis product were well within the manufacturer-suggested range for the antibody dose and column capacity; a handling error was not apparent. The typical, and after the second depletion cycle markedly above-average, depletion of B-cells from the product support the impression that handling and device function during both runs were flawless. Unidentified product or donor properties seem to be responsible. Inconsistent antibody quality would be an alternative explanation. In their reply to our customer complaint, the manufacturer reported that no other complaints had been received for the batch, rendering deficient CD3 reagent an unlikely explanation. The re-depletion was recorded as a deviation; the product was released as within specification. For the second inefficiently T-cell depleted product, a second depletion was not attempted, since the bulk of the desired CD34+ cell dose, 3.5 × 106/kg, could be administered as a CD34-selected graft, topped up with part of the CD3/CD19-depleted graft containing only 1.5 × 106/kg CD34+ cells, for a total dose of T-cells from both products combined of the targeted 50,000/kg (and of 5 × 106/kg of HSPCs). The practice of combining CD34-selected and CD3/CD19-depleted product to achieve the desired HSPC doses without exceeding the targeted T-cell dose was previously reported, as was the occasional need to perform a second round of column purification [22].
On average, approximately 80% of the CD34+ cells and 55% of the NK-cells were recovered, similar for CD34+ cells and slightly inferior for NK-cells than in a previously reported series [22]. The one outlier for CD34+ cells, with a recovery of only 52%, is the product which, because of insufficient T-cell depletion, had been subjected to two depletion cycles. Excessive cell loss would indeed be expected, given the many centrifugation cycles, two CliniMACS runs, temporally extended processing and repeated quality control sampling which all removed some cells. The next worst recovery for CD34+ was 69%, a figure well within the range of what is also achieved with CD34 selection, i.e. recovery for 12/13 depletions was perfectly adequate. Average log depletion of T-cells approached −4, and of B-cells −2. T-cell depletion was thus in a similar range, possibly marginally more efficient, than in the aforementioned series [22], while B-cell depletion was markedly less effective. The average residual T-cell dose per million CD34+ cells was 5700; the typically targeted dose of 5 million CD34+ cells/kg of the recipient thus contained 28,500 T-cells/kg, well below the targeted dose of 50,000 T-cells/kg. Irrespective of the T-cell dose, T-cell add-back to reach the targeted dose was not requested by the clinicians. As shown in Figure 1, eight out of 13 products contained fewer than 10,000 T-cells/million CD34+ cells so that patients could have been transplanted with the CD3/CD19-depleted grafts alone. However, clinicians typically requested at least part of the concurrently CD34-selected graft, even if the T-cell content of the CD3/CD19-depleted graft was very low, with the aim of maximizing the HSPC dose.
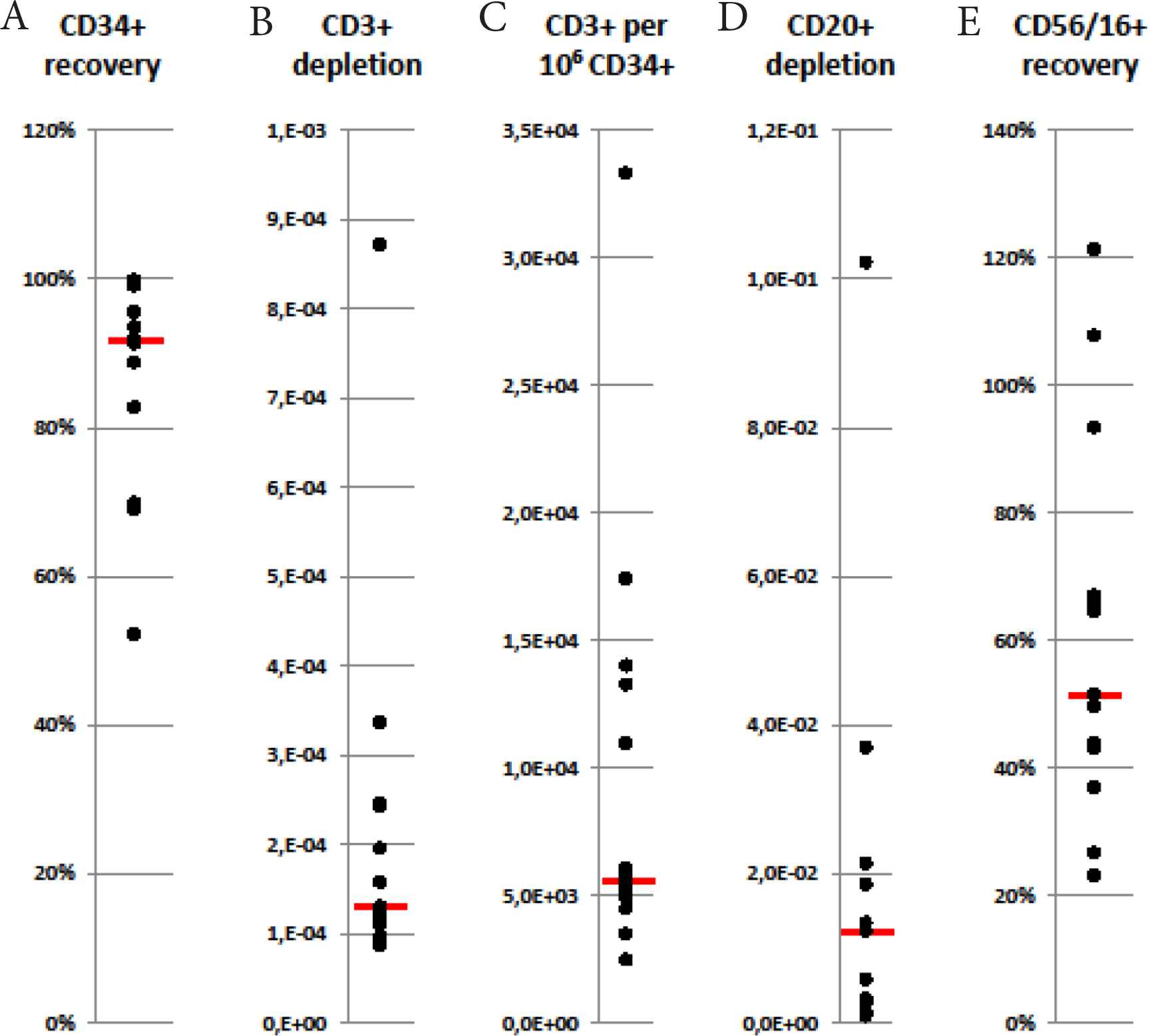
Outcomes of CD3/CD19-depletions: CD34+ cell recovery (A), CD3+ cell depletion (B), number of T-cells per 1 million CD34+ cells (C), CD20+ cell depletion (D) and CD56/16+ cell recovery (E) are depicted. Recovery and depletion are calculated as the total number of post-depletion cells divided by pre-depletion cells, thus accounting for all cell loss during processing and due to quality control sampling. T-cell dose per million CD34+ cells, arguably the most patient-relevant value, is calculated as post-depletion T-cells (total) divided by CD34+ cells (total/106). Each dot represents one depletion process, the bar marking the median (n = 13 depletions).
The first take-home message, therefore, is that CD3/CD19 depletions are requested quite infrequently, in spite of the fact that our center exclusively serves at least three transplant programs with these products. With respect to the possibility of a learning (or unlearning) effect, for none of the outcome parameters considered, i.e. CD34 cell recovery, T- and B-cell depletion, was an order effect suggested, and the two inefficient T-cell depletions were observed in years 2 and 8. The preparations were done by highly experienced GMP personnel; the operators performing the two inefficient depletion runs were, if anything, the more experienced in the team. Operators had maintained proficiency by regularly performing the much more frequent CD34 selections with the same technology. As mentioned above, a root cause analysis failed to suggest a reason for the inefficient depletions, whether process- or product-inherent.
A second message is that, despite conscientious use of well-validated standard operating procedures, the degree of depletion is quite variable, and a relevant number of depletions fail to achieve desired outcomes. We posit that failure to consistently produce largely T- and B-cell-free transplants with the CD3/CD19 depletion technology is likely an underreported phenomenon which is, at least in part, responsible for the relatively poor adoption of this product.
Thirdly, inefficient depletions can be rescued by repeating the process, which was previously also suggested by Huenecke et al. [22], although significant excess HSPC attrition should be regularly expected. Because of the paucity of selections, it is not possible to stock “rescue antibody” and kits. These must thus be ordered as immediate delivery which, besides considerable costs, will see the product age for an additional day, with the expected additional cell loss. Thirdly, it is apparent that T-cell- and particularly B-cell-depletion by CD3/CD19-depletion is markedly less efficient than by CD34 selection, by approximately one and two common log. Depletion of T-cells in CD34 selected grafts was shown in several studies to lie in the range of −5 to −6 log depletions [22,33,34], whereas T-cell depletion in CD3/CD19 depleted grafts did not exceed −4 log depletion [22].
Even though the manufacturer does not disclose the antibody clone for either CD34+ selection or CD3/CD19 depletion, given the availability of the technology, implicates that the monoclonal antibodies used for immunomagnetic manipulations are of high and consistent quality. Moreover, the CD3 and CD19 antigens are strongly and selectively expressed on T- and B-cells, respectively, much stronger than the CD34 antigen is on stem/progenitor cells, rendering weak labeling as a cause of failed depletion a less likely possibility. One difference potentially affecting marking and retention probability is the frequency of the cells being labeled in both procedures. CD34+ cells account for approximately 1% of all nucleated cells in an apheresis product, whereas T- and B-cells can add up to 40% and more of the WBCs in a given apheresis product. Overloading of the column with labeled cells was shown to influence the outcome of the procedure [35].
It is impossible to apply established criteria for robust processes [36,37] in pharmaceutical manufacturing to procedures done as seldom as 13 times in 8 years. Nevertheless, failure to achieve the desired T-cell depletion in 15% (two out of 13) of the manufactured products suggest inconsistency in the process, especially because a root cause analysis did not yield any result, and since essentially similar processes (CD34+ cell selection) provide more consistent results.
Clinical evidence with CD3/CD19-depleted grafts teaches us that with use of anti-thymocyte globulin during conditioning and effective GvHD prophylaxis severe GvHD is typically avoided, and post-transplant lymphoproliferative disease is not an excessively frequent occurrence [19–21]. The quality of the typical CD3/CD19 depleted graft is thus obviously suitable.
In summary, we are reporting inconsistent outcomes for T- and B-cell depletion by CD3/CD19 depletion with the CliniMACS technology, despite a rigorous manufacturing process, which is undesirable given the irreplaceability of these products.
CONFLICTS OF INTEREST DISCLOSURE
HB has received research funding and speakers’ fees from Miltenyi Biotech, manufacturers of the technology used here. EW and ES have no declarations.
AUTHORS’ CONTRIBUTION
EW and HB analyzed the data and co-wrote the manuscript. ES bears the overall responsibility for the work. All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
The consistently excellent, dedicated work of the cell therapy laboratory operators is acknowledged.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Eliza Wiercinska AU - Erhard Seifried AU - Halvard Bonig PY - 2021 DA - 2021/08/02 TI - CD3/CD19 Depletion for T-cell Reduction of Allogeneic Transplants: Mostly Efficient, but not Robust JO - Clinical Hematology International SP - 103 EP - 107 VL - 3 IS - 3 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.210725.001 DO - 10.2991/chi.k.210725.001 ID - Wiercinska2021 ER -
