Neupogen (Filgrastim) Induced Acute Hematuria/Proteinuria with 3 Days of Use in a 7-Year-old Boy Diagnosed as Aplastic Anemia
 , Hassan Eliwan2, Fatima Eltayeb Hago1, Al Husein Mohammed Chyad Hammoudi3
, Hassan Eliwan2, Fatima Eltayeb Hago1, Al Husein Mohammed Chyad Hammoudi3- DOI
- 10.2991/dsahmj.k.200704.001How to use a DOI?
- Keywords
- Filgrastim; neupogen; G-CSF; hematuria; adverse effect; pediatrics
- Abstract
Filgrastim, a granulocyte-colony stimulating factor (G-CSF), is used in patients with neutropenia caused by chemotherapy, bone marrow transplant, radiation poisoning, or any unknown etiology. It stimulates the bone marrow to increase the production of neutrophils. Although it has a good safety and efficacy profile, many patients experience different side effects ranging from mild symptoms such as abdominal pain, musculoskeletal pain, diarrhea, and headache, to severe symptoms/conditions such as splenic rupture, anaphylactic reaction, pulmonary hemorrhage, and hypoxia. Hematuria/proteinuria is one of the uncommon side effects seen in patient using Filgrastim for a longer period. However, to date there is no reported case of Filgrastim-induced hematuria with only 3 days of use. We report a case of a 7-year-old boy who developed hematuria with only three doses of Filgrastim and was diagnosed to have aplastic anemia.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Filgrastim is a recombinant human Granulocyte Colony-stimulating Factor (G-CSF) approved by the US Food and Drug Administration [1]. It is used to treat patients with neutropenia caused by chemotherapy [2–5], human immunodeficiency virus infection [6], bone marrow transplant [7–9], or in cases of congenital [2,7], cyclic [2,10], or idiopathic neutropenias [2,11]. It is a hematopoietic growth factor that influences leukopoiesis [12–14], and affects the proliferation and differentiation of neutrophils within the bone marrow [15,16] and possibly other sites (e.g., spleen) [17,18]. It binds directly to G-CSF receptors on neutrophil progenitor target cell surfaces [19,20] and stimulates neutrophil proliferation, thereby increasing neutrophil counts and activity [21,22].
Like most medications, it has side effects. The side effects commonly observed are fatigue, headache, malaise, constipation, abdominal pain, decrease appetite, anemia, thrombocytopenia, spleenomagaly, and arthralgia [2,23]. It may also cause rare but important or life-threatening symptoms/conditions such as acute respiratory distress syndrome, anaphylaxis, capillary leak syndrome, decreased bone mineral density, hemoptysis, pulmonary alveolar hemorrhage, pulmonary infiltrates, sickle cell crisis, splenic rupture, Sweet syndrome, and vasculitis (aortitis) [2,23]. Glomerulonephritis and/or hematuria are among the rarest side effects, and these complications may be attributed to prolonged use of Filgrastim [2,23,24]. Acute glomerulonephritis is defined as the sudden onset of hematuria and proteinuria and Red Blood Cell (RBC) cast in urine [25].
We report a 7-year-old boy who experienced one of its rarest side effects at an unexpected time interval. The patient developed a rare and atypical occurrence of acute hematuria after receiving only his third dose of Filgrastim.
2. CASE REPORT
A 7-year-old boy presented to a local hospital with purpuric and petechial rashes all over his body, especially in his extremities with easy bruising, but without fever, vomiting, or active bleeding. Blood investigations revealed a low platelet count (4000 platelets/μL). However, the hemoglobin (12.2 g/dL), White Blood Cell (WBC; 6.8 × 103/μL), RBC (4.1 × 106/μL), and neutrophil (3.5 × 103/μL) counts were normal. Hence, the patient was diagnosed to have immune thrombocytopenic purpura. He was given two doses of immunoglobulins, after which his platelet count rose to 17,000/μL on day 4 and then back to 4000/μL on day 5.
On day 5, the patient was transferred to our hospital. Upon presentation, he was awake, alert, still with petechiae but with no active bleeding; other observations included absence of fever, clear chest with no sound, abdomen was soft, relaxed, and no organomegaly. His laboratory workup was repeated, and results showed pancytopenia with a WBC count of 2.5 × 103/μL; RBC count, 3.48 × 106/μL; 4000 platelets/μL; hemoglobin, 10.2 g/dL; and neutrophils, 1.5 × 103/μL (Figure 1). The next day, he had an episode of epistaxis, for which he was given platelets transfusion and was started on tranexamic acid and ceftriaxone because of his low neutrophil count. On day 10, he spiked once while waiting for bone marrow aspiration to be done. Laboratory tests were repeated on day 11, and results showed the same downward trend for hemoglobin (8.1 g/dL) and neutrophils (0.18 × 103/μL), with a platelet count of only 8000/μL. Another platelet transfusion was given on the same day.
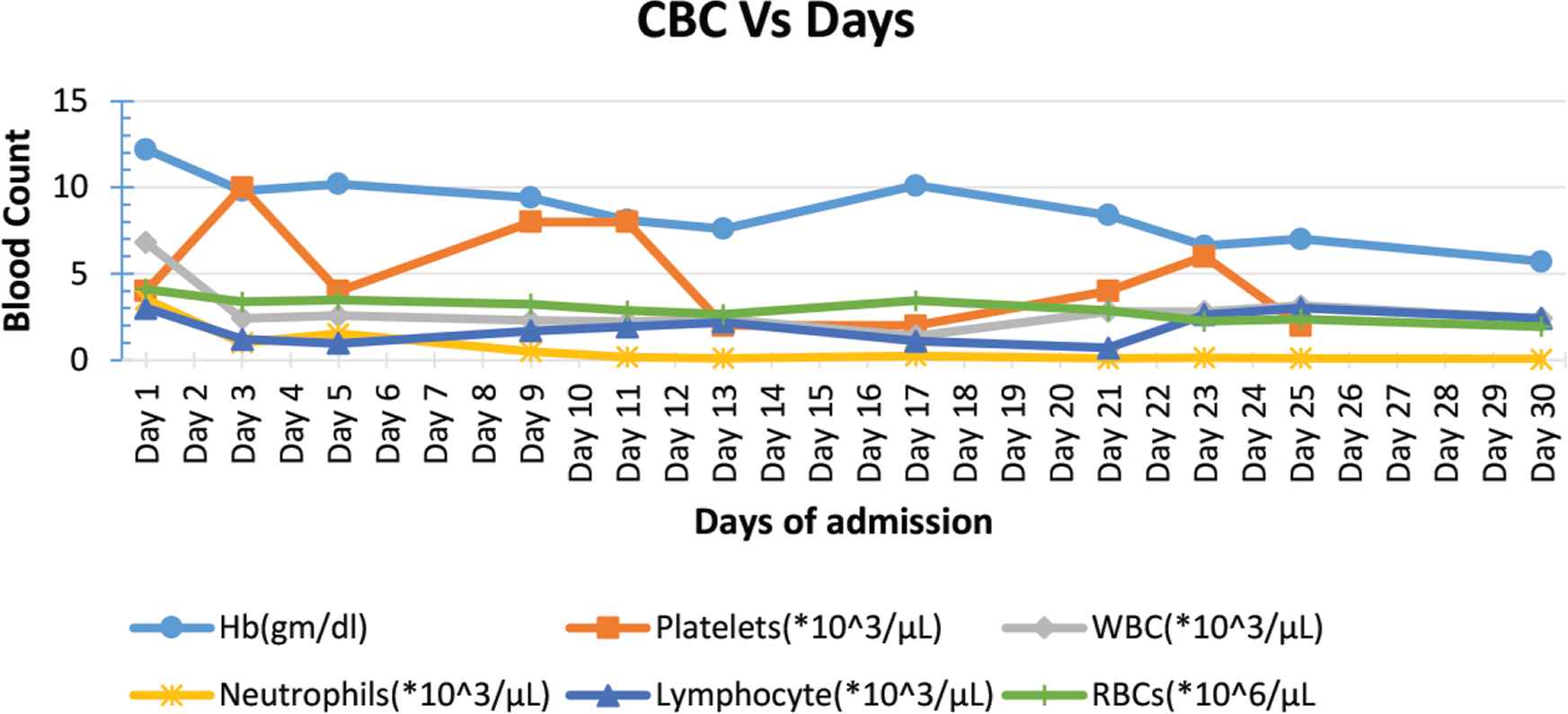
Complete blood cell count over days of admission. Hb, hemoglobin.
On day 14, bone marrow aspiration was performed, and then the patient was started on high doses of dexamethasone for 4 days. On day 17, upon completion of dexamethasone doses, Complete Blood Count (CBC) was repeated, and results were as follows: hemoglobin, 10.1 g/dL; platelets, 2000/μL; and neutrophils, 0.24 × 103/μL (Figure 1).
On day 19, the patient was started on Filgrastim (G-CSF) at 5 μg/kg/dose once daily. However, later in the evening he developed abdominal pain, which was an expected side effect of Filgrastim. Other causes of abdominal pain were ruled out by abdomen ultrasound, and surgical referral was done, which was unremarkable. On day 21, the CBC test was repeated, and results continued to show pancytopenia with neutrophils decreasing to as low as 0.09 × 103/μL. Hence, the dose of Filgrastim was increased to 7.5 μg/kg/dose. The next day, the patient complained of red urine (hematuria), which was confirmed by urine analysis, showing RBC >50/hpf (Table 1). The intervention was made by reducing the dose of Filgrastim. As a result, his hematuria improved symptomatically, which was confirmed by repeated urine analysis.
| Urine analysis parameter | Day 2 of admission | Day 21 of admission (Day 3 of Filgrastim) | Day 22 of admission (Day 4 of Filgrastim) |
|---|---|---|---|
| Urine color | Yellow | Bloody | Light reddish |
| Appearance | Slightly turbid | Hazy | Hazy |
| Urine gravity | 1.02 | 1.015 | 1.005 |
| Urine pH | 7.5 | 8 | 6 |
| Urine nitrate | Nil | Nil | Nil |
| Urine protein | Nil | + | Nil |
| Urine glucose | Nil | Nil | Nil |
| Urine ketone | Nil | Nil | Nil |
| Urine WBCs | 0–2/hpf | 2–5 hpf | 0–2 hpf |
| Urine leukocyte | Nil | Nil | Trace |
| Urine RBCs | 1–2/hpf | >50/hpf | 10–15/hpf |
| Urine bacteria | Nil | Nil | Nil |
| Urine epithelial cell | Nil | Nil | Nil |
| Urine blood | Nil | +++ | +++ |
Urine analysis prior to and during Filgrastim use
3. DISCUSSION
In this report, the authors present a case of a 7-year-old boy who was administered Filgrastim for a total of 5 days, and reported the development of hematuria and proteinuria on day 3 of the therapy. These side effects are worth noting because they are among the rarest to occur after following Filgrastim therapy, and more significantly, in this case, they occurred after the patient received his third dose. This is atypical and is worth consideration given that these side effects reported in the literature occur only after long-term use of Filgrastim and has not previously been reported in the literature occurring this early in the course of the treatment.
This case is unique, because of the early occurrence of hematuria and proteinuria in Filgrastim therapy. While the evidence has shown the occurrence of side effects after long-term use of Filgrastim, there is no prior evidence showing these Filgrastim-related side effects.
In their study, Dale et al. [26] enrolled a total of 853 patients with severe chronic neutropenia who received G-CSF almost daily or on alternate days. Glomerulonephritis and/or persistent hematuria were reported in 25 patients. Of these 25 patients, the symptoms of 12 patients were associated with underlying conditions. In the remaining 13 patients, symptoms resolved in five out of 10 patients for whom the dose of Filgrastim was reduced or discontinued. In two of the remaining three patients for whom the same dose was continued, the renal symptoms resolved [26].
Meanwhile, Sotomatsu et al. [24] reported a single case of a 12-year-old boy diagnosed with severe chronic neutropenia at the age of 5 months. He was started with G-CSF at the age of 5 years. Seven years after starting G-CSF, he developed hematuria for the first time at the age of 12 years, and a month later he was diagnosed as having rapid progressing glomerulonephritis. Filgrastim was continued with steroids, cyclophosphamide, warfarin, and dipyridamole. His creatinine levels decreased and creatinine clearance was improved but proteinuria persisted [24].
4. CONCLUSION
Filgrastim may cause early-onset hematuria and proteinuria during the course of treatment. Stopping and discontinuing the Filgrastim therapy may not be the best intervention for patients who are expected to take a longer course of this medication. Reducing the dose or continuing the same dose combined with monitoring the patients with the Kidney Function Test (KFT) is preferred. A kidney biopsy may be recommended in cases of worsening KFTs.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SA and HE contributed in conceptualization, designing and writing which includes review and editing the manuscript. SA, FH and AH contributed in formal analysis and writing the original draft. HE supervised the project. However, all the authors review the manuscript and approve the final draft of the manuscript.
REFERENCES
Cite this article
TY - JOUR AU - Sohail Azam AU - Hassan Eliwan AU - Fatima Eltayeb Hago AU - Al Husein Mohammed Chyad Hammoudi PY - 2020 DA - 2020/07/07 TI - Neupogen (Filgrastim) Induced Acute Hematuria/Proteinuria with 3 Days of Use in a 7-Year-old Boy Diagnosed as Aplastic Anemia JO - Dr. Sulaiman Al Habib Medical Journal SP - 92 EP - 94 VL - 2 IS - 3 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200704.001 DO - 10.2991/dsahmj.k.200704.001 ID - Azam2020 ER -
