A Meta-analysis on Vitamin D Deficiency in Patients with Sickle Cell Disease
 , Fatima Abdelhakam Abdellatif MohamedElmugadam2, Hiba Faroug Mohamed Awadalla3, Abdalla Omer Obeid Mohamedali4, Mansour Osman Alhaj Alawad1, Ahmed Abdulgadir Noureddin1, Afnan Abugundul Ahmed Osman3, Esraa Mohamed Osman Mohamed1, Gehad Abdelmonem Abdalla Ibrahim5, Malaz Ahmed Osman Elsayed3
, Fatima Abdelhakam Abdellatif MohamedElmugadam2, Hiba Faroug Mohamed Awadalla3, Abdalla Omer Obeid Mohamedali4, Mansour Osman Alhaj Alawad1, Ahmed Abdulgadir Noureddin1, Afnan Abugundul Ahmed Osman3, Esraa Mohamed Osman Mohamed1, Gehad Abdelmonem Abdalla Ibrahim5, Malaz Ahmed Osman Elsayed3- DOI
- 10.2991/dsahmj.k.200717.001How to use a DOI?
- Keywords
- Vitamin D; sickle cell disease; meta-analysis
- Abstract
Vitamin D Deficiency (VDD) is reported to be more frequent with serious clinical outcomes in patients with Sickle Cell Disease (SCD). There is a wide disparity in data in the existing literature regarding the prevalence and risk of VDD in patients with SCD. These data require further summary and analyses for better accuracy. This review aimed to assess the association between VDD and SCD, and was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Medline/PubMed, World Health Organization Virtual Health Library, ScienceDirect, and Google Scholar were used for the systematic search. A random effects model was used to estimate the pooled prevalence, Risk Ratio (RR), and Standardized Mean Difference (SMD) estimates with the corresponding 95% Confidence Interval (CI) using OpenMeta Analyst software version 10.10 (Tufts Medical Center, Boston, MA, USA). Twenty-five studies fulfilled the eligibility criteria. The prevalence of VDD among patients with SCD was 63.8% (95% CI, 52.5–75.1). The risk of VDD among patients with SCD was more than two times that of the general population (RR = 2.129; 95% CI, 1.024–4.423; p < 0.001). Serum vitamin D levels were significantly lower in SCD patients than in their controls (SMD = −1.883: 95% CI, −3.006 to −0.760; p < 0.001). This review provides a comprehensive view of the association between vitamin D status and SCD.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Vitamin D is essential for normal development and maintenance of healthy bone in pediatric and adult populations, as well as the regulation of the immune system functions and inflammatory responses [1,2]. Recently, Vitamin D Deficiency (VDD) has become of public health importance as it contributes to adverse clinical manifestations [1]. In addition to rickets and bone disorders, VDD is related to several other health disorders such as cardiovascular diseases, asthma, infectious diseases, muscle weakness, diabetes mellitus, autoimmune thyroid diseases, and several malignancies [1–4].
Sickle Cell Disease (SCD) is one of the most common inherited disorders worldwide [5]. Several different genotypes cause SCD, although all include the HbS allele, the homozygous inheritance of the mutated beta S-globin chains (HbSS) is the most common form that refers to conditions characterized by the production of sickle hemoglobin, leading to a cascade of pathophysiological consequences including infarction, hemolysis, oxidative stress, hypercoagulability, and inflammation [5–7]. SCD is associated with protean clinical presentations such as chronic hemolytic anemia, painful crisis, acute chest syndrome, sequestration crisis, aplastic crisis, and acute cerebrovascular accidents [5–9].
Several studies have revealed that patients with SCD are at risk of multiple macro- and micronutritional deficiencies, and they have a higher prevalence and risk of VDD regardless of their age or ethnic background [10–12]. Several studies have explored the risk factors influencing vitamin D status, such as limitation of exposure to natural light, which affects vitamin D biosynthesis; dark skin, which acts as a natural barrier to UV irradiation penetrating the skin; obesity and high body mass index, in which adipose tissues sequester vitamin D; and poor dietary intake of vitamin D [13–15].
Since vitamin D is involved in the regulation of calcium level and bone health, its deficiency could further impair bone comorbidity and contribute to the musculoskeletal disorders already present in patients with SCD [11,12,16]. A recent study has investigated the association of vitamin D status with inflammatory parameters and anti-inflammatory cytokines in patients with SCD, and showed the contribution of VDD to the uncontrolled, chronic inflammation and concurrent vaso-occlusion seen in the course of SCD [17]. Low serum vitamin D is associated with an increased level of proinflammatory cytokines and diminished level of anti-inflammatory cytokines when compared with healthy non-SCD individuals, and the levels also differ in SCD patients with vaso-occlusive crisis and those in steady state [18].
Moreover, some people with SCD who were treated for VDD had less painful crises than those who are not treated [18]. Also, recent trials suggest the potential benefit of vitamin D supplementation on the resolution of chronic pain symptoms, reducing the number of days with pain, and reduced use of analgesics [19]. It is reported that vitamin D supplementation can suppress proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor-α and upregulate the synthesis of the anti-inflammatory cytokine IL-10 [18]. A trial in SCD patients showed the significant effect of 3 months’ daily vitamin D supplementation in reducing the levels of proinflammatory cytokines IL-2, 6, 8, 17, and 18, and increasing the level of anti-inflammatory cytokine IL-11 [18].
Previous studies of VDD in patients with SCD have shown inconsistent, variable data. A previous systematic review found that VDD was highly prevalent among patients with SCD [20]. To the best of our knowledge, there is no meta-analysis of existing evidence on the various aspects of the association between SCD and VDD. These data require further summary and analyses for better accuracy. The results of this meta-analysis provide a comprehensive view and may contribute to better management of SCD.
2. MATERIALS AND METHODS
2.1. Search Strategy and Inclusion Criteria
The methodology was developed from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [21]. A systematic literature search was performed using the electronic databases of Medline/PubMed, Google Scholar, World Health Organization Virtual Health Library, and ScienceDirect. The search terms used were “vitamin D”, “hydroxycholecalciferol”, “25-hydroxyvitamin D”, “25-hydroxycholicalciferol”, “cholecalciferol”, and “sickle cell” to ensure no possible relevant articles were missed. Also, we reviewed the articles referenced by those identified articles in this search.
The inclusion criteria were: (1) cross-sectional, case–control, or cohort studies that assessed the prevalence and/or association between VDD and SCD or its complications; (2) studies with sufficient data for calculation of prevalence, Risk Ratio (RR), and/or Standardized Mean Difference (SMD) estimates; and (3) studies published in English up to November 2019. Studies were considered relevant if they assessed one or more of the following: prevalence of VDD amongst SCD patients; risk of developing VDD among SCD patients; and mean level of 25-hydroxyvitamin D among SCD patients compared to their controls.
We excluded case reports, editorials, letters, abstracts, and studies without sufficient data of interest. If two or more studies had the same patient population, the recent study with more complete data was included to avoid duplication.
The titles and abstracts of all articles retrieved from this search were screened for potential inclusion in this review. Then, studies deemed to be relevant were reviewed (full text) for inclusion according to the defined eligibility criteria. Quality of the included studies was assessed using the Newcastle–Ottawa Scale; a tool that determines the quality based on the selection of the study group, comparability of groups, and ascertainment of the exposure and outcomes [22].
Three independent reviewers extracted the relevant information using a designed data extraction form and any disparity among the reviewers was resolved by discussion and consensus. For qualitative and quantitative data synthesis, we extracted the following information from each article: authors; year of publication; study region; study design; primary aim of the study; strengths/additional findings of the study; main limitations of the study; age groups of the participants; reference level used for definition of VDD and/or severe VDD; number of SCD patients; number of controls (if present); number of SCD patients with VDD; number of controls with VDD (if present); and mean serum level of 25-hydroxyvitamin D with their corresponding SD values.
2.2. Statistical Analysis
The statistical analyses were carried out using OpenMeta Analyst software version 10.10 [23]. The pooled summary prevalence, RR, and SMD were calculated from the random-effects model due to the notable heterogeneity. Statistical heterogeneity was assessed with the I2 statistics and the I2 values of 25%, 50%, and 75% were related to low, moderate, and high heterogeneity, respectively.
We conducted subgroup and meta-regression analyses to investigate the potential sources of heterogeneity and to determine the extent to which variables of interest moderated the overall results. The Chi-square test was used to assess the differences between the categorical subgroups and the significance level was set at 0.05. Publication bias was determined through Begg’s test, Egger’s test, and visual examination of the funnel plot [24,25].
According to the literature review [26,27] and the reference values used for defining VDD by most of the included studies, we considered a 25-hydroxyvitamin D serum level <20 ng/mL (<50 nmol/L) as VDD. In addition, we considered a 25-hydroxyvitamin D serum level <10 ng/mL as severe VDD in this analysis.
3. RESULTS
3.1. Studies Included
The schematic flow of study identification and selection process is presented in Figure 1, and the main features of the included studies are shown in Tables S1 and S2. Our search retrieved records for 490 published articles. Full texts of 40 studies were screened, and 15 studies were subsequently excluded because of insufficient data to estimate the outcomes of interest (Figure 1). A total of 25 studies published from 1993 to 2019 that met the eligibility for data were used for qualitative and quantitative syntheses: 10 studies from the Americas [12,28–36]; six studies from Asia [37–42]; five studies from Europe [43–47]; two studies from Africa [48,49], one study that used samples from both Jamaica and West Africa [50], and one study that used samples from both Brazil and Nigeria [51].

Flow chart for study selection process.
3.2. Prevalence of VDD among Patients with SCD
Most of the included studies (16 of 25 studies) provided sufficient data about VDD in patients with SCD based on serum level of 25-hydroxyvitamin D < 20 ng/mL (50 nmol/L). Based on that definition, the pooled prevalence of VDD in patients with SCD was 63.8% (52.5–75.1%) (Figure 2). No evidence of publication bias was detected based on visual examination of the funnel plot and from the results of Begg’s test (p = 0.31) and Egger’s test (p = 0.40). There were 10 studies with sufficient data to estimate the prevalence of severe VDD based on serum level of 25-hydroxyvitamin D < 10 ng/mL (25 nmol/L). The pooled prevalence of severe VDD in patients with SCD was 27.0% (95% CI, 15.9–38.0).
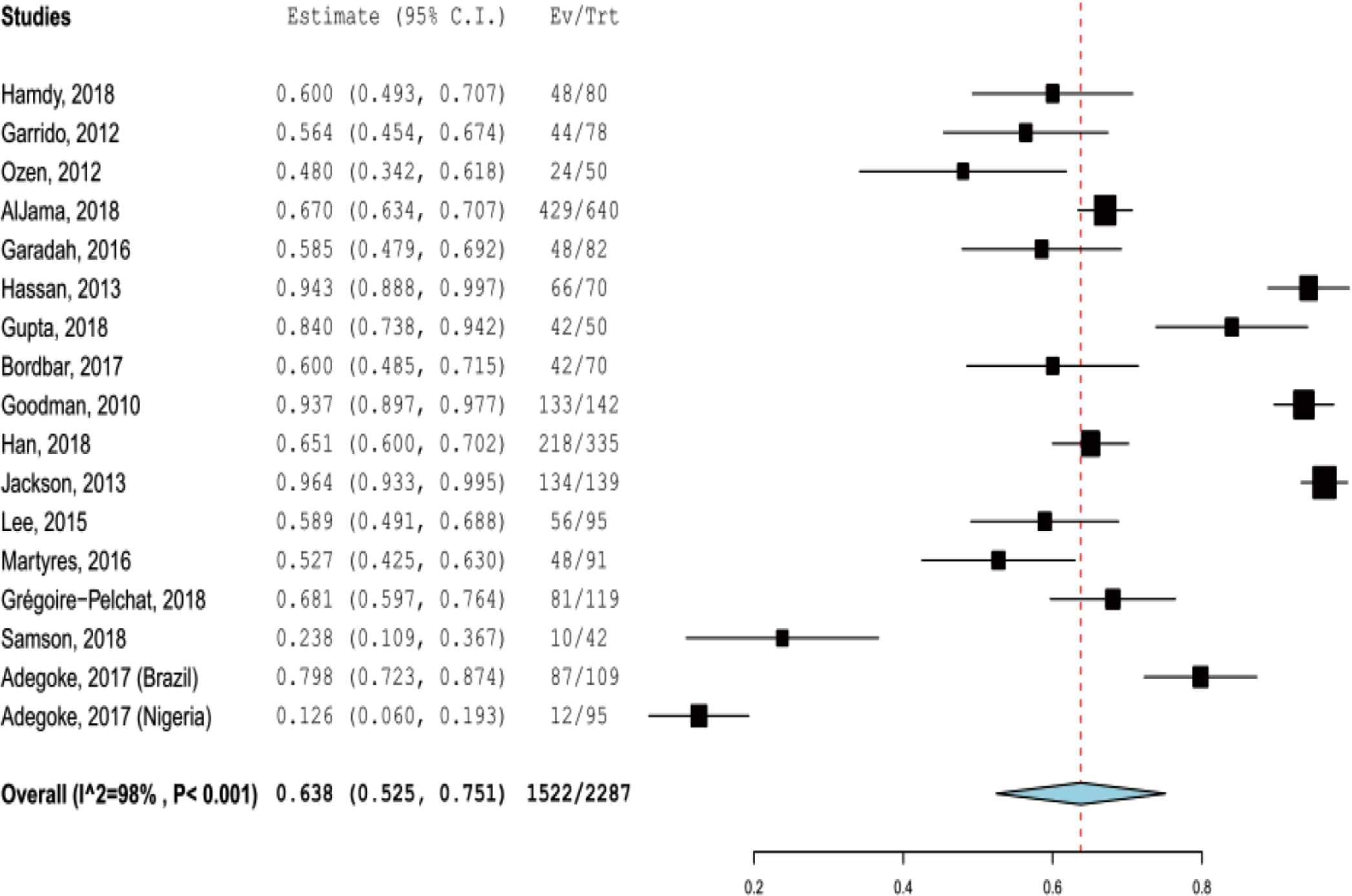
Pooled prevalence of vitamin D deficiency in patients with sickle cell disease.
In subgroup analysis based on age groups, there were 14 studies of children with SCD and the pooled prevalence of VDD was 56.2% (36.7–75.7). The pooled prevalence of VDD from the seven studies of adults with SCD was 75.5% (57.5–93.5). The prevalence of VDD was significantly associated with age groups (χ2 = 28.3, p < 0.001). The meta-regression showed that publication year was not correlated with the prevalence of VDD (coefficient = −0.024, p = 0.134).
3.3. Risk of VDD among Patients with SCD Compared to the General Population
There were five studies with sufficient data to calculate the risk of having VDD among patients with SCD compared to the general population. The pooled RR of VDD from the random-effects model was 2.129 (95% CI, 1.024–4.423) for patients with SCD compared with the general population (Figure 3). Publication bias was detected based on the visual examination of the funnel plot.
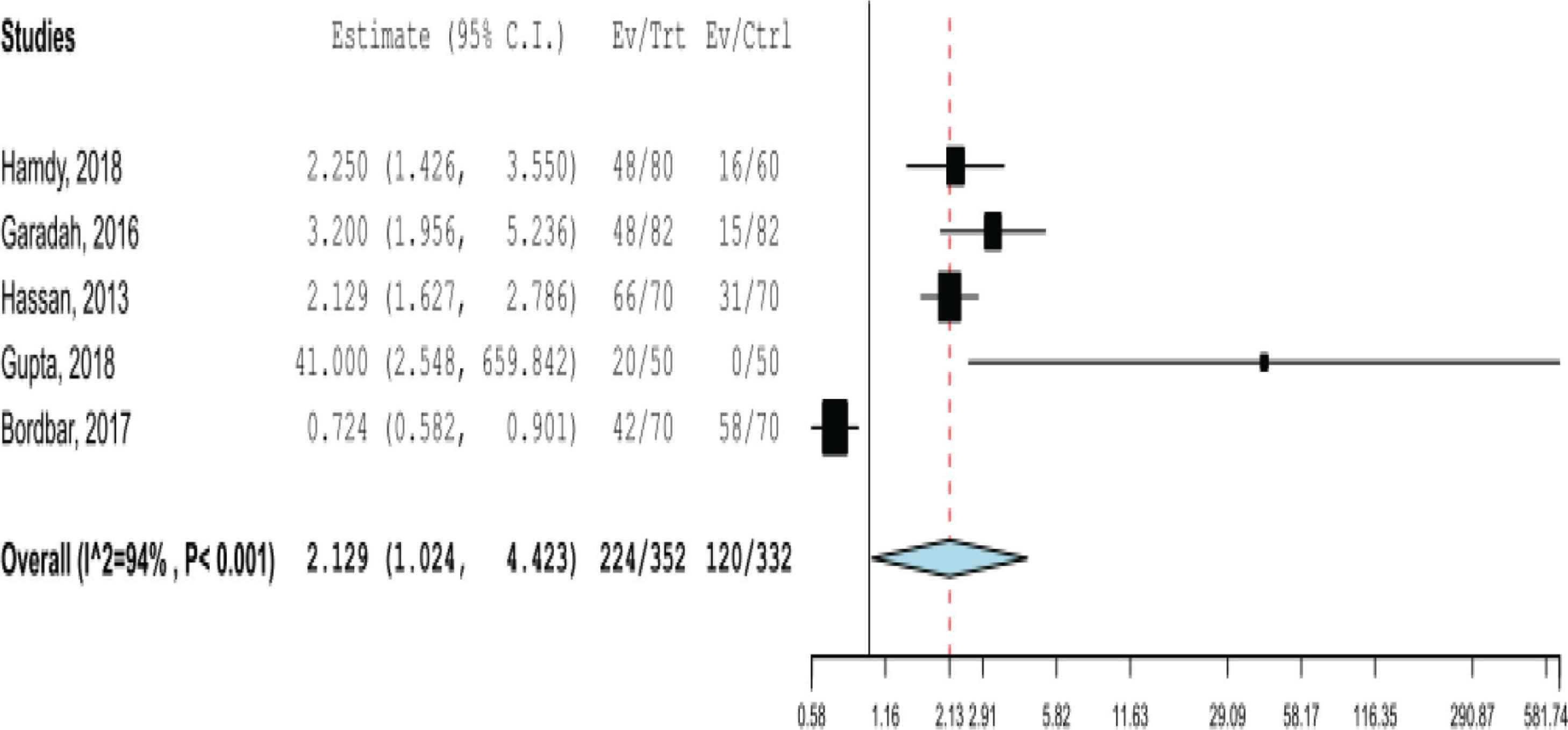
Pooled risk ratio of having vitamin D deficiency in patients with sickle cell disease.
3.4. SMD of Serum 25-Hydroxyvitamin D Level
There were four studies (including five study groups) with sufficient data to calculate the SMD of 25-hydroxyvitamin D estimates between patients with SCD and their controls. The pooled effect size showed that the serum 25-hydroxyvitamin D levels in SCD patients were significantly lower than their controls, with SMD = −1.883 (95% CI, −3.006 to −0.760; p < 0.001) (Figure 4). No evidence of publication bias was detected on visual examination of the funnel plot and from the results of Begg’s test (p = 0.35) and Egger’s test (p = 0.32).
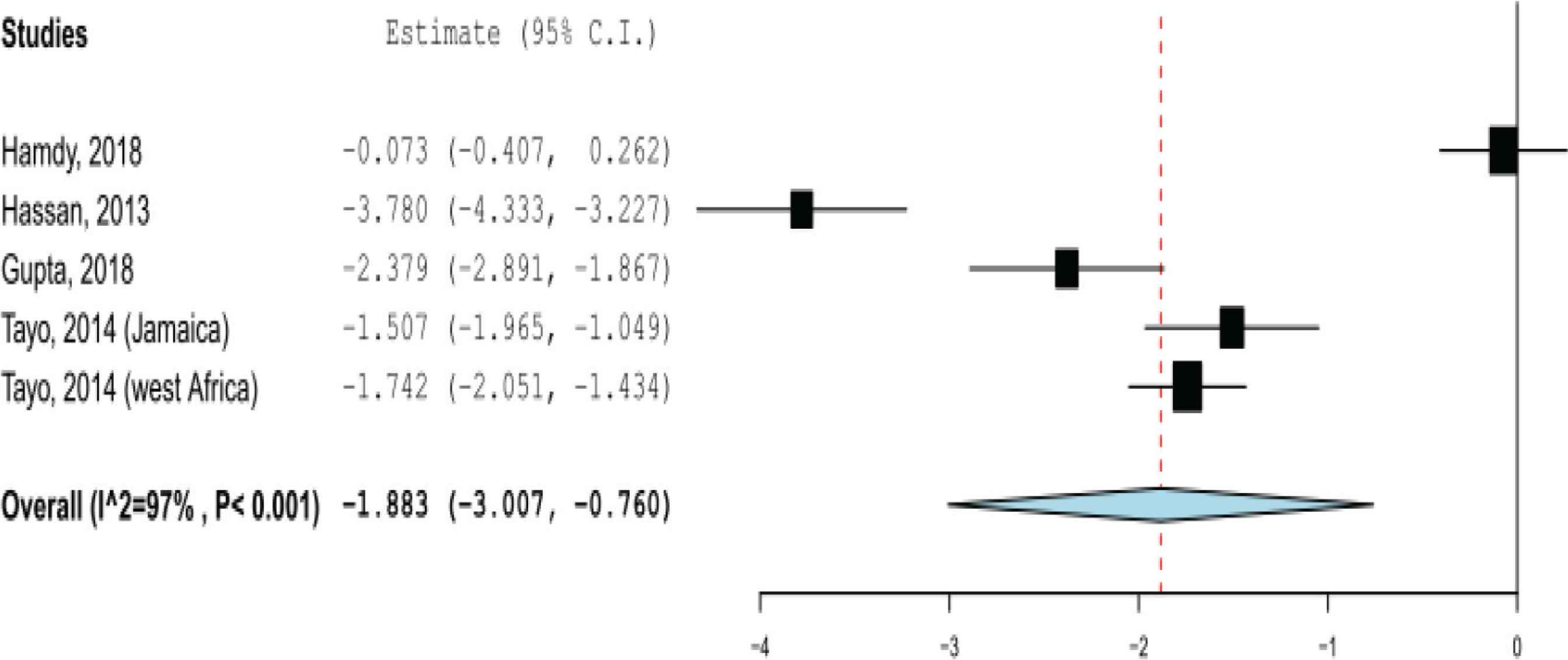
Pooled standardized mean difference of 25-hydroxyvitamin D estimates between patients with sickle cell disease and their controls.
4. DISCUSSION
In this review, we have an updated estimate of the global prevalence of VDD in patients with SCD. A spectrum of definitions of VDD has been reported in the literature based on serum level of 25-hydroxyvitamin D, which is considered the most accurate marker for vitamin D status [2,4,26,27]. However, most of the included studies in this review used the definition of VDD as serum level of 25-hydroxyvitamin D < 20 ng/mL (50 nmol/L), which is a common definition in the literature [4], and it is consistent with the definition of VDD based on the reports of The United States Endocrine Society, American Academy of Pediatrics, and Institute of Medicine [1].
Existing evidence from the reviewed studies shows that 63.8% of the SCD patients had VDD, and 27.0% of them had severe VDD. We have found that the prevalence of VDD is higher in adults than in children. This pattern of a relationship with age is well established [28,31,36]. However, it is unclear that VDD follows a specific geographical pattern because it is a global health problem. Moreover, variation was found between studies conducted within the same country as noted in the American and Canadian studies included in this analysis.
We did not analyze four studies identified during our search in this meta-analysis because of the different cut-off points of VDD used [12,28,31,38]. Rovner et al. [12] found that 33% of patients with SCD had 25-hydroxyvitamin D < 11 ng/mL and the risk of VDD among patients with SCD was more than five times greater than in the controls. Buison et al. [28] found that 65% of the included children with SCD had low vitamin D status based on 25-hydroxyvitamin < 11 ng/mL (27.5 nmol/L), and showed that children with SCD were at higher risk for low vitamin D status than healthy children were. Winters et al. [36] detected VDD in 32% of pediatric SCD patients and 49% of adult SCD patients, based on serum 25-hydroxyvitamin < 11 ng/mL. Chapelon et al. [43] assessed VDD in French children with SCD and found that 76% of them had 25-hydroxyvitamin < 12 ng/mL.
Several peculiar characteristics of SCD could explain the high prevalence and increased risk of VDD among patients with SCD, such as: impaired absorption by the damaged intestinal mucosa as a complication of SCD; decreased level of vitamin D binding protein due to the inflammatory condition in SCD; increased physiological demands due to the fast turnover in the process of erythrocytosis in SCD; and reduced levels of nutritional status, physical activity, and exercise in patients with SCD [12,28,30,40,44,46,49].
A few of the reviewed studies assessed the correlation between vitamin D status and SCD-related health complications. Hamdy et al. [49] showed a significant correlation between serum vitamin D level and the biomarkers of hemolysis (lower level of hemoglobin and a higher level of aspartate aminotransferase, lactate dehydrogenase, and bilirubin), and the clinical course and complications of SCD (frequency of blood transfusions and hospitalization, occurrence of vaso-occlusive crises, and recurrent infections). Similarly, Mandese et al. [44] demonstrated the significant negative correlation between levels of vitamin D and clinical indicators of the SCD severity (hospitalization and number of days of hospital admissions). A similar association between the VDD and the vaso-occlusive crisis of SCD was shown also by Lee et al. [32] and Gupta and Katariya [40]. By contrast, some of the included studies did not find a significant association between VDD and these complications [28,31,47].
The findings of this study need to be considered in the context of some limitations. The inclusion of studies published only in English may have compromised representativeness. We could not assess the association between VDD and SCD-related health complications because of the data limitation and heterogeneity among the included studies. Also, we could not assess the possible risk factors of VDD among patients with SCD. Data on SMD and risk of VDD among patients with SCD were obtained from few studies. There is still a need for large prospective studies with appropriate controls to assess the unanswered questions in this study.
5. CONCLUSION
We have summarized data of several studies exploring the vitamin D status in patients with SCD. VDD occurs with increased frequency in patients with SCD. The results of this study could have important implications and provide a reference for the management of patients with SCD. Healthcare providers need to be aware of the clinically important association between VDD and SCD to ensure effective management.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SM conceptualized the research idea. SM, FM, HA, AO and EM undertook database searches. SM and AM undertook data extraction and analysis. SM, MA, AN, GI and ME interpreted the results and drafted the manuscript. All authors revised, read, and approved the final manuscript.
SUPPLEMENTARY MATERIALS
Supplementary data related to this article can be found at
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Sagad Omer Obeid Mohamed AU - Fatima Abdelhakam Abdellatif MohamedElmugadam AU - Hiba Faroug Mohamed Awadalla AU - Abdalla Omer Obeid Mohamedali AU - Mansour Osman Alhaj Alawad AU - Ahmed Abdulgadir Noureddin AU - Afnan Abugundul Ahmed Osman AU - Esraa Mohamed Osman Mohamed AU - Gehad Abdelmonem Abdalla Ibrahim AU - Malaz Ahmed Osman Elsayed PY - 2020 DA - 2020/07/21 TI - A Meta-analysis on Vitamin D Deficiency in Patients with Sickle Cell Disease JO - Dr. Sulaiman Al Habib Medical Journal SP - 95 EP - 100 VL - 2 IS - 3 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200717.001 DO - 10.2991/dsahmj.k.200717.001 ID - Mohamed2020 ER -
