Perception toward Biosimilars and Nonmedical Switching: A Cross-sectional Survey among Arab Rheumatologists
 , Humaid Al Wahshi2, Jamal Al Saleh3, Farida Al Balushi2, Mostafa Zayed4, Maha A. Omair5, Eduardo Mysler6
, Humaid Al Wahshi2, Jamal Al Saleh3, Farida Al Balushi2, Mostafa Zayed4, Maha A. Omair5, Eduardo Mysler6- DOI
- 10.2991/dsahmj.k.200727.001How to use a DOI?
- Keywords
- Rheumatoid arthritis; biosimilars; nonmedical switching
- Abstract
Objective: The aim of this study is to assess the level of knowledge and acceptance for biosimilars and nonmedical switching in Arab rheumatologists.
Methods: A cross-sectional survey was conducted during the Arab League Against Rheumatism conference using a structured questionnaire consisting of 17 questions.
Results: The participants were mainly females (50.7%), practicing in the Gulf region (65.7%) with a median [Interquartile Range (IQR)] age and years of practice as consultants of 44 (13) years and 10 (14) years, respectively. The median (IQR) self-perceived knowledge of biosimilars was 5.3 (4) out of 10. Most physicians agreed that the evidence published to grant biosimilars an approval for the studied indication was enough (40.6%), yet most of them believed it was not enough for extrapolation of indications (40.6%). The mean (standard deviation) likelihood to prescribe biosimilars in the future was 5.39 (2.6). The majority of rheumatologists (59.8%) believe that nonmedical switching could pose harm to patients. Most physicians agreed that nonmedical switching will lead to a significant saving in cost (58.5%) with the majority expecting a cost reduction between 30% and 50% to justify nonmedical switching.
Conclusion: This is the first study to evaluate acceptance of biosimilars and nonmedical switching on a diverse population of rheumatologists in the Middle East. Future educational activities task forces should target these topics.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Biosimilars are newly developed biological agents that have a similar structure to already approved biological originators agents with the equal efficacy and rate of adverse events [1]. The most important reason for developing biosimilars is to increase patients’ access to biotherapeutics through a reduced cost [2]. The Food Drug Authority (FDA) and the European Medicine Agency (EMA) have developed regulations and policies for developing and approving biosimilars. Even with the rigorous evaluation these agents undergo, many aspects are still evolving especially in the view of their long-term safety and immunogenicity [1].
Unlike the originator biologic, biosimilars only require pharmacokinetics/bioequivalence studies and at least one Phase III trials in a representing disease to obtain approval with the automatic extrapolation of approval for all indications of the originator biologic [3].
The Middle East is an important emerging market for biologics [4]. Rheumatologists practicing in different Arab countries are faced with different types of challenges in managing their patients owing to differences in payers, health system structures, access to therapy, socioeconomic status of patients, and others [5]. Complete adaptation of European and North American recommendation guidelines is not always practical; rather, an adolopment with regional experts is viewed as a more scientific method to implement good clinical practice [6,7]. One important step in the introduction of biosimilars into daily clinical practice is whether or not switching will be performed without the approval of the treating physician and consent of the patient. Patients and rheumatologists are required to have adequate knowledge about these agents as important stakeholders in developing regulations and guidelines on a national level. Moreover, they need to be patient advocates and be able to educate patients about their change of therapy [8].
The Arab League Against Rheumatism (ARLAR) is a multinational organization that comprises 17 societies with more than 500 members from 22 countries. Its mission is to represent Arab Rheumatologists and promote the pursuit of excellence in arthritis care, education, and research. Its meeting is organized every 2 years. The most recent meeting was held in February 23–25, 2018 in Muscat, Oman, in partnership with the Oman Society of Rheumatology (http://arlar.org/).
The aim of the current survey is to evaluate the level of knowledge and views of delegates attending the ARLAR meeting on biosimilars and nonmedical switching.
2. MATERIALS AND METHODS
During the ARLAR meeting, a cross-sectional survey was conducted using a structured questionnaire. The questionnaire consisted of 17 questions (Supplementary Material 1), which covered three main sections. Definitions of the term biosimilars and the concept of nonmedical switching were included in the survey, prior to addressing each section of the questionnaire. Multiple methods of distribution were used to optimize response; these included two e-mails sent 2 weeks apart, the ARLAR meeting smartphone application, and hard copies distributed by volunteers during the 3 days of the meeting.
All delegates and speakers in the ARLAR meeting were invited to participate in this survey. Participation was optional, and surveys with more than three missing answers were excluded. No honorarium was provided to the participating physicians. Ethical approval was acquired from King Saud University Institutional Research Board No. (E-17-2771; Riyadh, Saudi Arabia) dated December 17, 2017. The final approval of the conclusion was given by the scientific committee of ARLAR.
2.1. Demographics
This included questions regarding sex, age, region of origin, region of practice, specialty, degree, and years of practice. Owing to the diversity of the audience attending the meeting, the region of origin/practice was classified into the Gulf region (Oman, Saudi Arabia, Kuwait, Qatar, United Arab Emirates, Bahrain, and Yemen); Africa (Egypt, Tunisia, Morocco, Algeria and Sudan); Levant (Lebanon, Iraq, Iran, Palestine and Jordan); and Other countries (if not included in one of the three regions). Biosimilars were approved by the regulatory bodies in their respective countries as follows: Saudi Arabia and Iran, 2010; Egypt and India, 2012; and Jordan, 2013.
2.2. Biosimilars
This section started with the definition of biosimilars, stating “The FDA definition of a biosimilar: is a biological product that is highly similar to the reference product, notwithstanding minor differences in clinically inactive components; and No clinically meaningful differences exist between the biological product and the reference product in terms of the safety, purity, and potency.”
The section included six multiple-choice questions investigating the perception and experience of biosimilars. All questions were single answered questions, except for the last question, which could indicate more than one answer.
2.3. Nonmedical Switching
The section started with the definition of nonmedical switching. Nonmedical switching is defined as “switching of a therapy for reasons other than inadequate response or intolerance/development of adverse events.”
The section included six single answered questions in the knowledge and perception of nonmedical switching of biologics. Question 2 allowed multiple answers and it depended on the answer of the first question.
2.4. Statistical Analysis
Descriptive statistics was used for demographics and participants’ characteristics. Parametric and nonparametric tests were used to compare participants with different levels of responses. Analysis was performed using Statistical Package for Social Studies (SPSS 22; IBM Corp., New York, NY, USA).
3. RESULTS
3.1. Demographics
Out of 454 delegates and speakers invited, 91 (20%) completed the survey. The average time to complete the survey was 2–3 minutes. The median [Interquartile Range (IQR)] age was 44 (13) years. Of the responders, 67 fulfilled the inclusion criteria and were included in the analysis, generating a 15% response rate (Table 1). There were slightly more female than male participants in this survey (50.7%). Twenty-five participants (37.3%) originated from the Gulf region and 65.7% worked in the same region. Fifty-one participants (76.1%) were on a consultancy level in rheumatology, with a median of 10 years (IQR, 14) of practice as a consultant (Table 1).
| Participants (N = 67) | ||
|---|---|---|
| n | Percentage (%) | |
| Female sex | 34 | 50.7 |
| Country of origin | ||
| Gulf | 25 | 37.3 |
| Levant | 17 | 25.4 |
| Africa | 18 | 26.9 |
| Other | 7 | 10.4 |
| Country of practice | ||
| Gulf | 44 | 65.7 |
| Levant | 12 | 17.9 |
| Africa | 8 | 11.9 |
| Othera | 3 | 4.5 |
| Type of practice | ||
| MOH | 21 | 31.3 |
| University/academic | 19 | 28.4 |
| Private | 12 | 17.9 |
| Military | 6 | 9 |
| Other | 9 | 13.4 |
| Consultant | 51 | 76.1 |
Other (India, n = 1; UK, n = 6). MOH, Ministry of health.
Demographics of the surveyed attendees
3.2. Biosimilars
3.2.1. Level of knowledge
The median (IQR) level of self-perceived knowledge on biosimilars graded by participants was 5.3 (4). A negative correlation between the years of practice as a consultant and level of knowledge on biosimilars was significant (r = −0.308, p = 0.013).
3.2.2. Evidence published on biosimilar approval and extrapolation
When asked about whether the evidence published to biosimilars was adequate for an approval for the studied indication, 26 (40.6%) participants answered “yes,” 16 (25%) answered “no,” and the remaining 34.4% answered “I don’t know” (Figure 1a). The response ratio was reversed when participants were asked about the adequacy of the published evidence for extrapolation for other indication—that is, 26 (40.6%) participants answered “no,” 17 (26.6%) answered “yes,” and the remaining 21 (32.8%) replied that they did not know (Figure 1b).
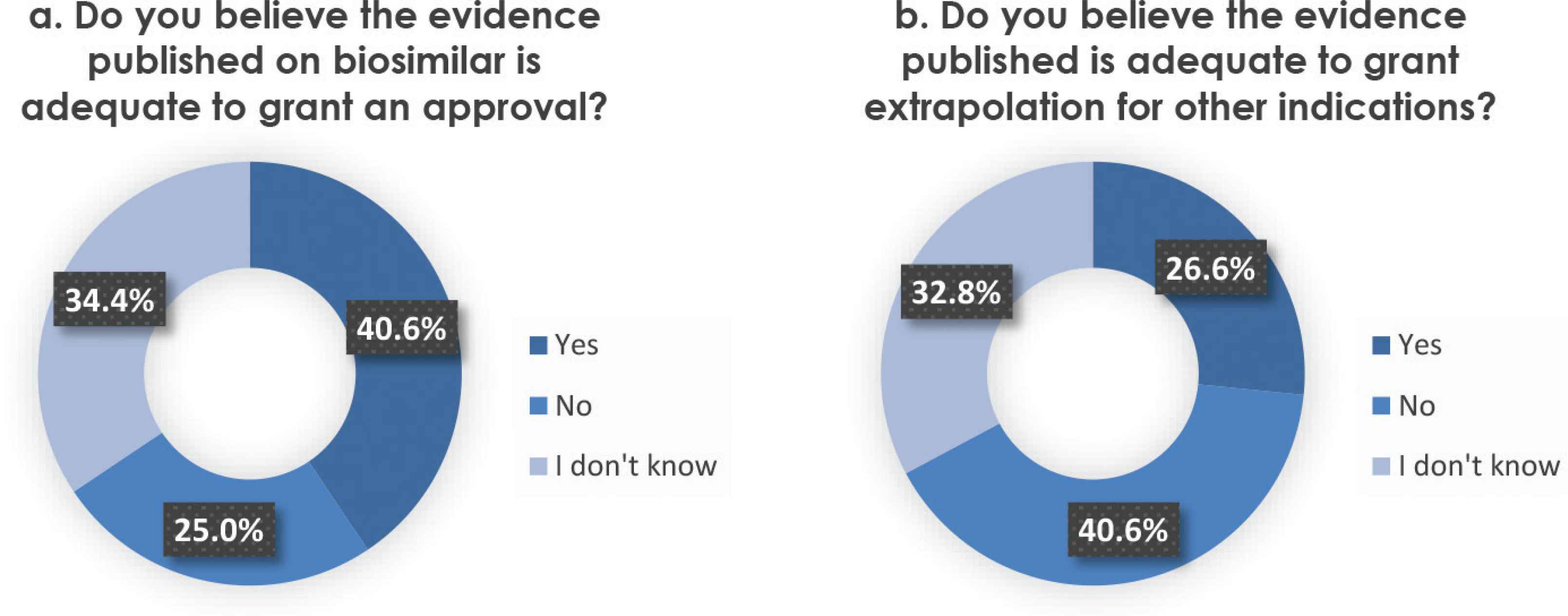
(a) Perception on Evidence published on biosimilar approval. Participants were asked whether they believed the published evidence is adequate to approve a biosimilar. (b) Perception on Evidence published on extrapolation of indications. Participants were asked whether they believed the published evidence is adequate to provide a biosimilar extrapolation for other indications.
3.2.3. Potentials and barriers of prescribing a biosimilar
Regarding the likelihood of prescribing a biosimilar to an eligible Rheumatoid Arthritis (RA) patient at the current moment, the mean (standard deviation) response of participants was 5.39 (2.604). Nineteen (29.7%) participants admitted they will definitely or highly likely to prescribe a biosimilar. By contrast, 11 (17.2%) participants selected the “not likely” or “less likely” option (Figure 2). There was a negative correlation between the numbers of years of practice and the likelihood of prescribing a biosimilar (r = −0.253, p = 0.044).
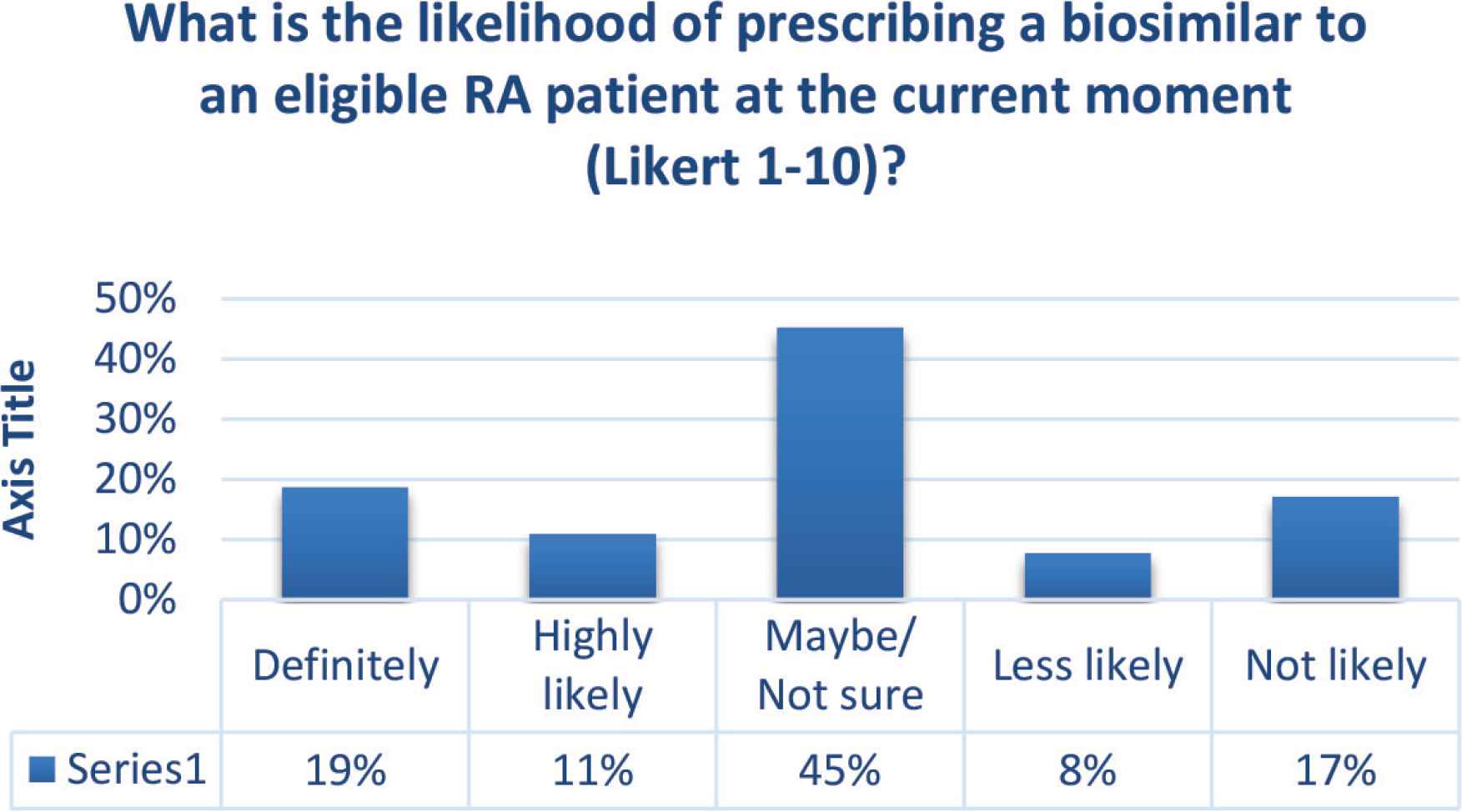
Likelihood of prescribing a biosimilar.
It was found that participants who answered “yes” for the question “whether the published evidence is enough to grant approval and extrapolation for other indications,” were more likely to prescribe a biosimilar compared with those who answered “no” (p = 0.043 and 0.003, respectively).
Thirty-two (50%) participants are willing to use biosimilars on a large scale after using it for 1–2 years on a small group of patients. Meanwhile, 22 (34.4%) and 10 (15.6%) participants preferred to wait for 3–6 years and 5–10 years, respectively (Figure 3). Males and participants who answered “yes” for the question asking about the evidence to grant biosimilar approval were more likely to prescribe biosimilars within 1–2 years (p = 0.033 and 0.005, respectively).

Participants willing to use biosimilars after using them for a certain period.
Barriers to biosimilars prescription included: no need to use biosimilars in my practice (90.6%), lack of data from local country/market (76.6%), lack of trust/confidence in the product manufacturer (75%), inadequate cost savings compared to branded biologics (71.9%), lack of national treatment guidelines recommending the use of biosimilars (59.4%), doubts on similarity to original molecule (54.7%), inadequate safety and efficacy profile/data (42.2%), and lack of long-term data (32.8%) (Figure 4).
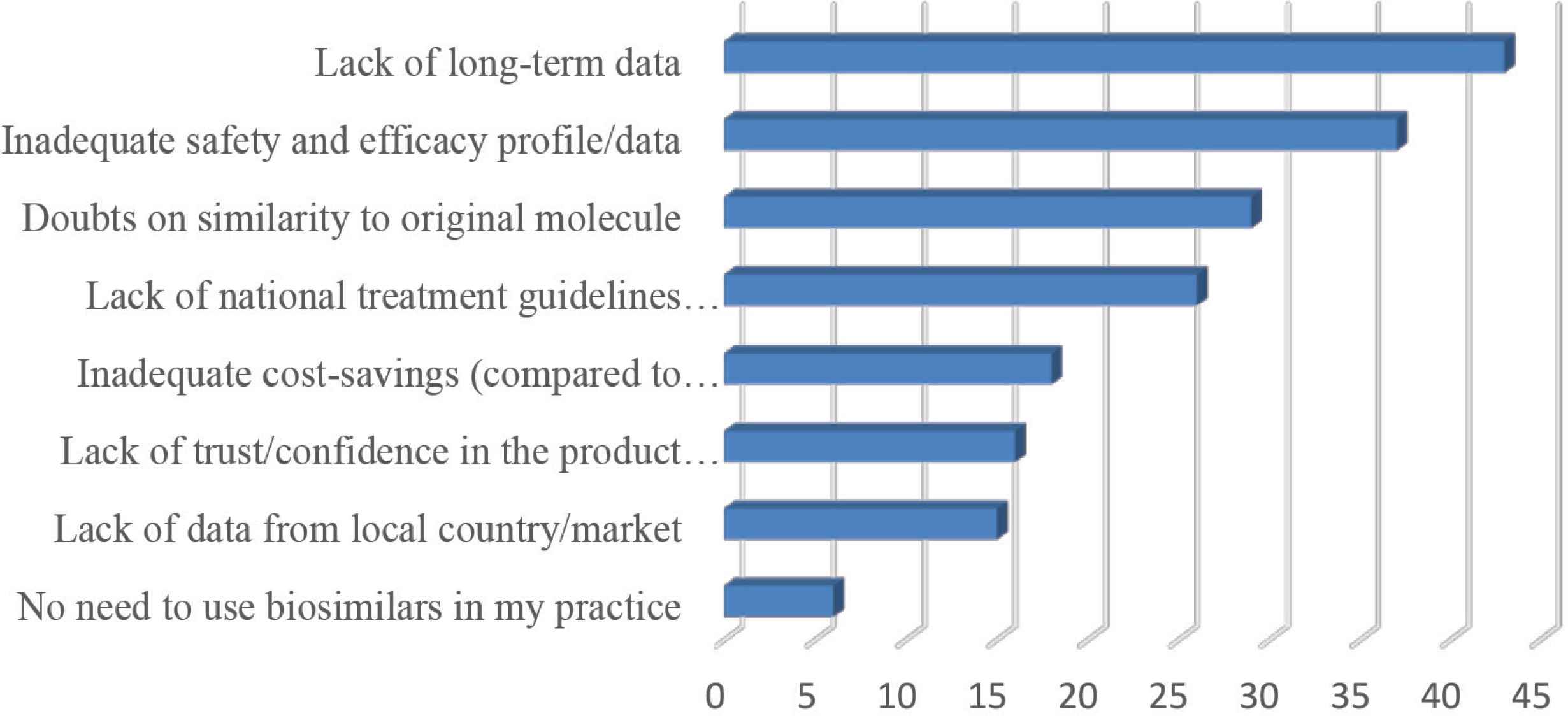
Barriers to prescribing a biosimilar (multiple answers allowed).
3.3. Nonmedical Switching
3.3.1. Factors affecting current and future practice
Nonmedical switching was performed by 21 (32.8%) participants. Of those participants, eight (38.1%) chose patient preference as the cause, seven (33.3%) chose drug unavailability, and six (28.6%) chose lack of medical coverage/insurance. Forty (62.5%) participants believed that nonmedical switching can be harmful to patients.
Forty (48.8%) participants agree that nonmedical switching can be harmful to patients, whereas 33 (40.2%) participants did not believe nonmedical switching of biosimilars would cause harm to patients.
Regarding the participants’ view of the likelihood of practicing nonmedical switching to biosimilars, 22 (26.8%) participants believed that nonmedical switching will definitely or highly likely be practiced in their countries once biosimilars were available. By contrast, 25 (30.5%) physicians saw that it was not or less likely for nonmedical switching to take place in their countries.
3.3.2. Price reduction perceptions
In our survey, 48 (58.5%) participants believed that nonmedical switching will lead to a significant saving in cost, whereas 34 (41.5%) participants did not agree that they will have any impact on cost. When asked what is the expected cost reduction that would justify nonmedical switching; five (7.5%), 12 (17.9%), 27 (40.3%), three (4.5%), and 16 (23.9%) participants selected a 10%, 20%, 30%, 40%, and 50% difference between the biosimilar and its originator, respectively (Figure 5).
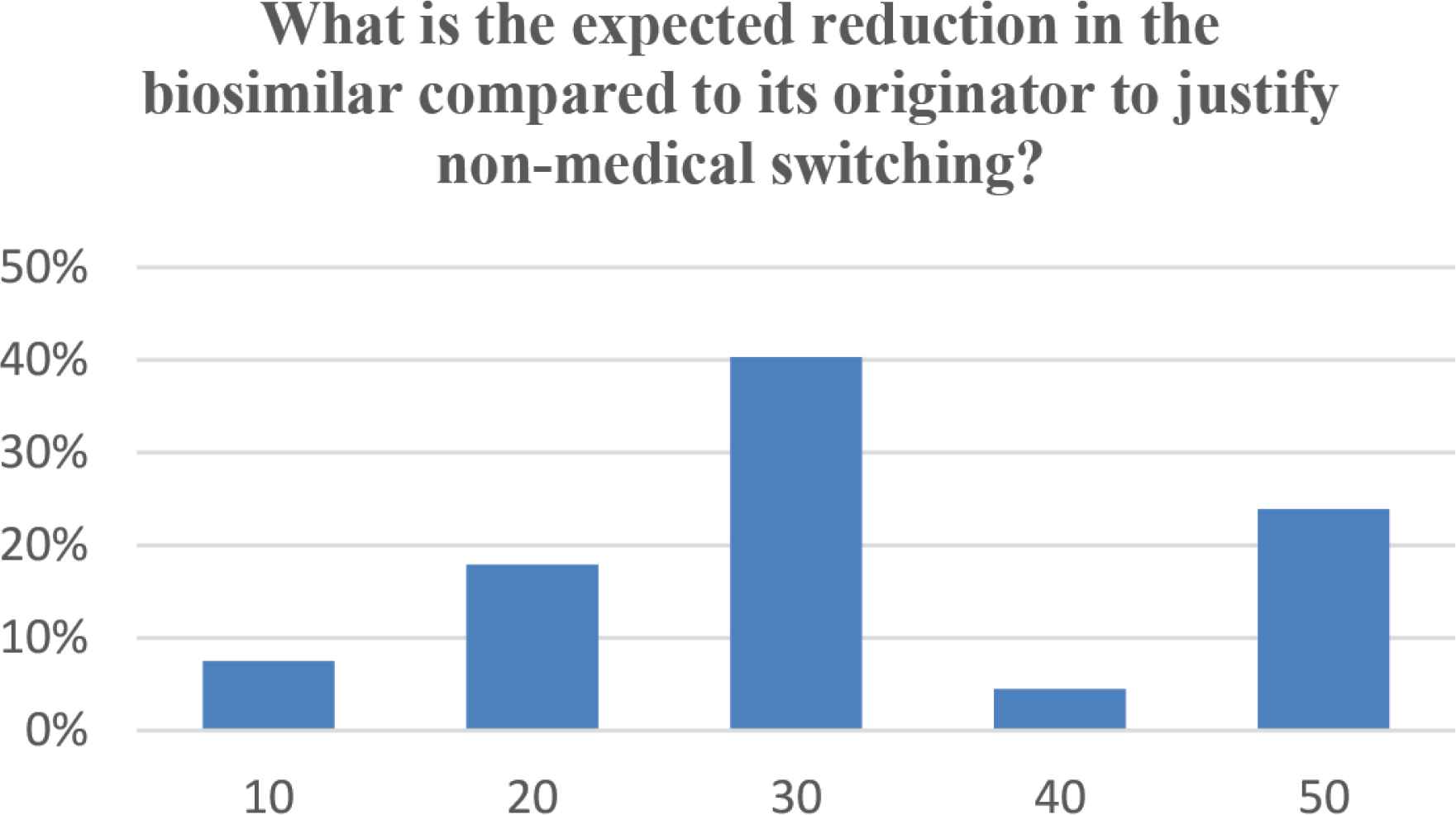
Expected cost reduction (%) of biosimilars compared to its originator to justify nonmedical switching.
4. DISCUSSION
The economic burden of biologics healthcare and the loss of patency of some biologics have led to the introduction of biosimilars and the process of nonmedical switching [9]. Agencies such as the FDA, EMA, and World Health Organization have developed requirements for the approval of a given biosimilar. There is a minor difference between these agencies’ guidelines and difference in terminologies, but all agencies have the same requirements in biosimilarity establishment [10]. Unlike the originator biologic, the approval of a biosimilar only requires pharmacokinetics/bioequivalence studies and one Phase III trials in a representing disease to get an approval with the automatic extrapolation of approval for all indications of the originator biologic [3]. Despite the rigorous evaluation these agents undergo, many aspects are still evolving especially in view of their long-term safety and immunogenicity. With the given information, regulatory bodies in different countries have adapted different strategies [3].
Familiarity of biologics was reported by half of our total participants, that is, almost one in every two rheumatologists participating in this study. This relative lack of knowledge in the Arab region is related to several factors including the availability of biosimilars, educational level and exposure, and years of practice. This was highly variable in relation to other international surveys; for example, in a recent Belgian survey, almost all physicians had knowledge of the concept of biosimilars [3]. Another survey in 2014, which was conducted on 470 physicians from Europe (France, Germany, Italy, Spain, and the UK), reported that only 22% were very familiar with biosimilars. The majority (54%) of participants had a basic understanding, whereas 24% of participants had never ever heard of biosimilars [11]. Cohen et al. [12] also reported on the need for education on biologics, biosimilars, and biosimilarity for US physicians in 2016. This concludes that the knowledge and familiarity of physicians on biosimilars may vary per region and by time.
In our study, there was a negative correlation between the years of practice as a consultant and level of knowledge on biosimilars that was significant. This was consistent with the survey conducted by Narayanan and Nag [13]. They found barriers to the uptake of biosimilars among rheumatologists, both overall, and based on number of years in practice [13].
The majority of physicians in our survey believed that the evidence published to biosimilars was adequate for its approval, although the Belgian Health Care Knowledge Center (KCE) 199 study discussed that a lack of information on the approval of biosimilars by the EMA was present [14]. Interestingly, the exact same number of participants did not agree that this evidence was adequate for extrapolation of other indications. In relation to our results, the Belgian survey conducted in 2016 showed that 56% of rheumatologists found that extrapolation could only be performed if efficacy and safety are proven to be similar in one of the indications, and if the medicine works via the same mechanism as in the other indications. Another group (39%) stated that indications should never be extrapolated. Studies by Beck et al. [15] and Hemmington et al. [16] also found that rheumatologists raised questions about the extrapolation of indications in biosimilars. Our data, consistent with previous results, reflect the concern of rheumatologists on the legitimacy of extrapolation of indications in biosimilars.
Physicians’ confidence in prescribing biosimilars was evaluated in our study, in which almost half of our participants needed only 1–2 years of experience with biosimilars to have trust in using them on a large scale of patients. The KCE 199 study detected a lack of faith, confidence, and motivation to prescribe toward biosimilars [14]. Another survey by the European Crohn’s and Colitis Organization (ECCO) reported that less than half of ECCO members (39%) felt confident about prescribing biosimilars [17].
Further investigating factors affecting biosimilar prescription in the Arab region, we found the main barriers included the following: no need to use biosimilars in practice, lack of data, and lack of trust in the manufacturer. Inadequate cost savings, lack of national treatment guidelines on the use of biosimilars, doubts regarding similarity to originator, inadequate safety and efficacy profile, and lack of long-term data were also cited. A study in France showed that the most common barriers were extrapolation to all indications, lack of information about tolerability, increasing patients’ worries and concerns, lack of clinical trials, and patients’ wishes to be treated with the reference medicinal product [15]. This was similar to another survey, in which the main reasons for not prescribing biosimilars were that they were less studied than the originator, and no clinical trials had been conducted in the respective indication [3]. From our view, factors affecting biosimilar prescription varied in relation to country and practice. Yet, the main concern that was common in most surveys was the lack of data supporting the efficacy and safety of biosimilars.
In the United States, a biosimilar is defined legally to be interchangeable with the reference product if (1) the biological product is biosimilar to the reference product, and (2) it can be expected to produce the same clinical result in any given patient [14]. There is a difference between interchangeability and nonmedical switching, where the latter is defined in our study as “switching of a therapy for reasons other than inadequate response or intolerance/development of adverse events.” In our study, almost one in every three physicians had already practiced nonmedical switching in the Arab region. The main factors causing switching were patient preference, drug unavailability, and lack of medical coverage/insurance. In the literature, other reasons for nonmedical switching were states, such as patients’ desire for alternative formulations (subcutaneous vs. infusion), less frequent dosing regimens, cost-related reasons which could be patient-driven, as a means of reducing out-of-pocket costs, or payer-driven, such as through a pharmacy benefits manager seeking to reduce costs [18].
The majority of participants in our study believed that nonmedical switching can be harmful to patients, and almost one in every four participants saw that nonmedical switching will definitely or highly likely be practiced in their countries once biosimilars were available. The rest of the physicians had a belief that nonmedical switching will cause harm to treated patients. In another survey on Belgian rheumatologists, 28% believed that originators and biosimilars are never to be interchanged whatsoever [3]. This suggests the need for more data on nonmedical switching between biosimilars and their reference product to prove the safety and efficacy of this practice.
Developing a biosimilar comes at a similar cost to developing the bio-originator. Therefore, the primary driver of cost savings may arise from a reduced requirement for studies to prove efficacy and safety directly [19]. However, because of the high costs of biologics, they can become a significant financial burden in many world markets, including some countries in the Middle East [9].
In our survey, more than half of the participants believed that nonmedical switching will lead to a significant saving in cost. When asked “what was the expected cost reduction that would justify nonmedical switching,” the majority stated that a 30% and 50% difference between the biosimilar and its originator, respectively, would justify nonmedical switch. Until now, evidence provides no meaningful data about the cost consequences of switching. Yet in 2017, van Overbeeke et al. [3] elaborated more about the influence of price on the preference for originators or biosimilars. When prices were equal, no rheumatologists preferred the biosimilar. In another context, when the prices were equal, more rheumatologists (73%) preferred the originator compared to when the originator was more expensive (38%) [3].
There is a current lack of knowledge in the concept and proper use of biosimilars among Arab rheumatologist societies. Most physicians agreed that the evidence published to grant biosimilars an approval for the studied indication was enough, yet most of them believed it was not enough for extrapolation of indications. There was a likelihood of prescribing biosimilars in the future, yet there were barriers that need to be addressed to increase confidence in their prescription. Most importantly, Arab physicians needed more time to gain experience in dealing with biologics. The majority of rheumatologists in our study believe that nonmedical switching could pose harm to patients. Most physicians agreed that nonmedical switching will lead to a significant saving in cost, yet they also believed that a relatively large cost reduction between the biosimilar and its originator is required to justify nonmedical switching.
4.1. Future Perspectives and Limitations
The current study is the first to assess the self-perceived level of knowledge and acceptance of Arab rheumatologists toward the use of biosimilars and nonmedical switching. The lack of proper knowledge of biosimilars suggests the need for more extensive educational programs for rheumatologists and the urgent need to create national guidelines to orient all physicians about concept and the proper use of biosimilars. The results will aid at designing educational activities and involvement of rheumatologists as important stakeholders in the process of introducing biosimilars into health systems. Future studies need to be performed on individual countries and include other stakeholders such as pharmacists, patients, and payers.
One of the most important limitations was the low response rate and the lack of validation of the questions used. The results of this regional survey might not be representative for the Arab region because of the different availability status of biosimilars in Arab countries. Several limitations arose from the design and method of investigation of the survey. In this study, the knowledge of participants regarding all terminology was investigated through different questions that could be interpreted differently.
CONFLICTS OF INTEREST
MAO has received speaker’s fees/grants from Pfizer, Abbvie, Amgen, Roche, Brystol Myers Squibb, Janssen, Actelion, New Bridge, GlaxoSmithKline, and Hekma. The other authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTION
MO, HA, JA, FA and EM contributed in project conception. MO, HA, JA, FA and EM contributed in data collection. MO, MAO and MZ contributed in statistical analysis. All authors contributed in manuscript preparation.
FUNDING
This study was supported by the
SUPPLEMENTARY MATERIAL
Supplementary data related to this article can be found at
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Mohammed A. Omair AU - Humaid Al Wahshi AU - Jamal Al Saleh AU - Farida Al Balushi AU - Mostafa Zayed AU - Maha A. Omair AU - Eduardo Mysler PY - 2020 DA - 2020/08/07 TI - Perception toward Biosimilars and Nonmedical Switching: A Cross-sectional Survey among Arab Rheumatologists JO - Dr. Sulaiman Al Habib Medical Journal SP - 179 EP - 185 VL - 2 IS - 4 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200727.001 DO - 10.2991/dsahmj.k.200727.001 ID - Omair2020 ER -
