Glucocorticoids Use and Post-Hospitalization Outcomes among Patients with Coronavirus Disease 2019: A Systematic Review of Observational Studies with Meta-Analysis
- DOI
- 10.2991/dsahmj.k.211028.001How to use a DOI?
- Keywords
- COVID-19; glucocorticoids; mortality; meta-analysis
- Abstract
Background: A systematic review with meta-analysis was conducted to assess the association of glucocorticoids use with the likelihood of all-cause mortality in cases with Coronavirus Disease 2019 (COVID-19).
Methods: Databases and cross-referencing were searched for observational studies about patients with COVID-19 who had a definite outcome (recovered/discharged or death). The outcome of interest was all-cause mortality, and the main exposure was receiving glucocorticoids after hospital admission. Results were pooled from selected studies by random-effects mode. Heterogeneity was examined using Q and I2 statistic, and a potential small-study effect was assessed through testing of the funnel plot asymmetry. Eighteen observational studies involving 10,039 patients with COVID-19 were pooled for final meta-analyses.
Results: Overall, the pooled results demonstrated that the association of glucocorticoids use with the risk of mortality was not statistically significant among individuals with COVID-19 (odds ratio = 1.39; 95% confidence interval, 0.83–2.3299) with significant heterogeneity and substantial inconsistency (Q = 292.24, p < 0.01, I2 = 94%). Subgroup analysis showed non-significant results, except for studies that reported using methylprednisolone.
Conclusion: The risk of death among COVID-19 patients may be not associated with exposure to glucocorticoids after hospitalization, except for methylprednisolone, which may be decrease the risk of death. However, these findings should be interpreted with caution because of substantial inconsistency across studies.
- Copyright
- © 2021 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The Coronavirus Disease 2019 (COVID-19) infection has become a worldwide health concern since its emergence in December 2019. Globally, about 229,373,963 COVID-19 cases have been confirmed with 4,705,111 deaths recorded by September 22, 2021 [1]. The clinical manifestation of the disease varied from mild upper respiratory tract infection to severe symptoms developed in lower respiratory tract, while some cases remain asymptomatic [2].
To relieve these symptoms and to manage inflammatory responses of the infection, anti-inflammatory medications, such as glucocorticoids, could be used in some cases. Anti-inflammatory glucocorticoids may have a protective effect against the fatal consequences of inflammatory reaction to COVID-19 infection [3]. A study by Sung et al. [4] revealed that use of high doses of glucocorticoids resulted in improved health outcomes among patients with severe acute respiratory syndrome-associated coronavirus (SARS-CoV). However, some studies suggested that high doses of corticosteroids do not decrease the deaths rates of SARS-CoV; for example, in several studies it was observed that it could to lead to severe adverse reactions [5,6]. Another study showed that glucocorticoids tend to be significantly effective in the treatment of lung dysfunction, and it may decrease the length of mechanical ventilation use [6,7]. Other studies conducted on animals showed that use of glucocorticoids was effective in treatment of inflammation and reduction of mortality in severe cases [8,9].
Thus, the association of glucocorticoids use after hospitalization on health outcomes and mortality of COVID-19 cases is still controversial. It is important to determine if there is a link between glucocorticoid administration and risk of death after having COVID-19 infection, and then to determine the direction of this association. Therefore, the objective of this meta-analysis was to examine the relationship between glucocorticoids use and the risk of death after COVID-19 infection.
2. MATERIALS AND METHODS
The study was a systematic review and meta-analysis performed on the basis of a validated guideline [10].
2.1. Searches and Databases
Many databases, such as PubMed and Cochrane Library, and cross-referencing were used to search for relevant observational studies. The main focus of the search was the use of glucocorticoids among COVID-19 patients after hospitalization, and the search has no language, and publication status restrictions to avoid any potential source of bias. The mentioned databases were searched up to August 2021. Search strategies were modified based on the used database; however, particular keywords were used, such as “coronavirus disease 2019,” or “SARS-CoV-2,” and “Glucocorticoids,” and “Death” or “Mortality.” As glucocorticoids could be reported as a confounder, and mortality could be reported as a secondary outcome, a general search was applied on all studies that were conducted on COVID-19 patients. Cross-referencing was undertaken to consider all relevant studies. Duplicates were removed electronically using Endnote by the first author and then were removed manually by the second author.
2.2. Inclusion/Exclusion Criteria and Risk of Bias
The required inclusion criteria for meta-analysis of potential studies were as follows: (1) observational studies about COVID-19 cases having a definite outcomes (death or recovered), (2) cases were defined as patients who died in hospital after COVID-19 infection, (3) comparison group were recognized as patients who recovered or discharged from hospital after COVID-19 infection, (4) data reported for use of glucocorticoids, which is the main risk factor of interest, (5) data reported for definite outcomes after COVID-19 infection as the outcome of interest is either death or recovered (or discharged).
For the risk of bias, potential eligible studies were examined using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [11].
2.3. Statistical Analyses
Outcomes were reported as Odds Ratios (ORs) with their 95% Confidence Intervals (95% CIs). The ORs were calculated from the pooled studies, and then analyzed using log ORs, which were converted back to ORs for ease of interpretation. Cochran’s Q-test and the I2 statistic were used to test inconsistency and heterogeneity between included studies. An alpha value lower than 0.10 was evidence of a significant heterogeneity, and I2 higher than 50% was representative of significant inconsistency [12]. The results were pooled and a random-effects model was used to integrate between-study variance into the estimate because the primary analysis showed a significant heterogeneity [13]. Funnel plots symmetry were tested to assess any presence of potential small-study effect. Sensitivity analyses were performed through deleting one study at each time. To assess the robustness of the results, subgroup analysis was conducted based on the type of glucocorticoids used, severity of COVID-19 illness, setting, publication status, and the average age of patients across the included studies. All statistical analyses processes were carried out using Microsoft Excel 2013 (Microsoft Corp., Redmond, WA, USA) with MetaXL, version 5.0 [14].
3. RESULTS
3.1. Search Results
As presented in Figure 1, 18 observational cohort studies met all eligibility criteria after review of the full text of all screened articles [15–32]. The included studies involved 10,039 COVID-19 patients with a total of 1918 deaths and 8121 recovered patients.

Flow diagram describing the search.
3.2. Main Characteristics of the Pooled Studies
The main characteristics of the included studies are shown in Table 1. Nine of the included studies were performed in China [18,19,22,24,25,29–32], three studies in the USA [23,26,27], two studies in Italy [15,17], one study in France [16], two studies in Spain [20,21], and one study in The Netherlands [28]. Nine studies reported the type of glucocorticoids used [15,18,20,26–31]. Five studies specified that methylprednisolone was used for COVID-19 patients [18,20,28,30,31], one study reported using dexamethasone [15], and three studies reported using a combination of glucocorticoid (methylprednisolone, hydrocortisone, dexamethasone, and/or prednisone) [26,27,29]. Six studies included only severe cases of COVID-19, which was described as critically ill, Acute Respiratory Distress Syndrome (ARDS), or severe COVID-19-associated Cytokine Storm Syndrome (CSS) [17–19,21,28,30]. With regard to publication status, five studies have not been peer-reviewed yet [15,20,25,27,29] whereas other studies have been peer-reviewed and published [16–19,21–24,26,28,30–32].
| Study | Design | Publication status | Country | Funding | Patients’ characteristics | Glucocorticoid type and dose | Sample size |
|---|---|---|---|---|---|---|---|
| Albani et al. [15] | Retrospective cohort | Not peer-reviewed | Italy | Nonfunded | Adult patients who had a positive test for SARS-CoV-2 | Dexamethasone with a dose of 8–10 mg for 4–10 days | 1403 |
| Bani-Sadr et al. [16] | A before–after study | Published (peer-reviewed) | France | Nonfunded | Patients with COVID-19 | NR | 257 |
| Bartoletti et al. [17] | Retrospective cohort | Published (peer-reviewed) | Italy | Nonfunded | Adult patients diagnosed with severe acute respiratory syndrome-SARS-CoV-2 | Any corticosteroid at dose of 0.5 mg/kg initiated within 72 h of hospital admission | 513 |
| Cao et al. [18] | Cohort study | Published (peer-reviewed) | China | Funded | Adult patients with severe COVID-19 (SARS-CoV-2 pneumonia and/or cases with chronic illness) | Methylprednisolone Sodium Succinate | 102 |
| Chen et al. [19] | Retrospective cohort | Published (peer-reviewed) | China | Funded | Adult patients with moderate to severe or critical COVID-19 | NR | 274 |
| Cusacovich et al. [20] | Retrospective cohort | Not peer-reviewed | Spain | Nonfunded | Patients who are adults and testing positive on SARS-CoV-2 | A daily dose of 125–500 mg of intravenous methylprednisolone from 2–5 days | 205 |
| Fernández-Cruz et al. [21] | Retrospective cohort | Published (peer-reviewed) | Spain | Nonfunded | Adult patients with COVID-19 pneumonia and complicated with ARDS and/or a hyperinflammatory syndrome where included | NR | 463 |
| Jiao et al. [22] | Retrospective cohort | Published (peer-reviewed) | China | Funded | Patients with COVID-19 | NR | 2044 |
| Keller et al. [23] | Retrospective cohort | Published (peer-reviewed) | USA | Nonfunded | Only COVID-19 patients who either died or had been discharged from the hospital | NR | 1806 |
| Liu et al. [24] | Retrospective cohort | Published (peer-reviewed) | China | Nonfunded | Adult patients with COVID-19 | NR | 349 |
| Luo et al. [25] | Retrospective cohort | Not peer-reviewed | China | Nonfunded | Adults COVID-19 patients who were discharged or died | NR | 403 |
| Majmundar et al. [26] | Retrospective cohort | Published (peer-reviewed) | USA | Nonfunded | Adults cases of COVID-19 pneumonia | Systemic corticosteroids in the form of methylprednisolone, prednisone, hydrocortisone, and dexamethasone | 205 |
| Rahman et al. [27] | Observational study | Not peer-reviewed | USA | NR | Patients that tested positive for SARS-CoV-2 infection | Parenteral formulations included methylprednisolone, hydrocortisone and dexamethasone and enteral prednisone | 136 |
| Ramiro et al. [28] | Prospective observational study | Published (peer-reviewed) | The Netherlands | Nonfunded | Patients with severe COVID-19-associated cytokine storm syndrome | High-dose intravenous methylprednisolone for five consecutive days (250 mg on day 1 followed by 80 mg on days 2–5) | 172 |
| Shang et al. [29] | Retrospective cohort | Not peer-reviewed | China | Funded | Adults COVID-19 patients with clear clinical outcomes (discharged or dead) | Injections and oral tablets of methylprednisolone, prednisone acetate, and dexamethasone. Median daily dose in the death group and survivors were 65 and 40 mg, respectively | 416 |
| Wu et al. [30] | Retrospective cohort | Published (peer-reviewed) | China | Funded | Patients with COVID-19 pneumonia who developed acute respiratory distress syndrome | Methylprednisolone | 84 |
| Yang et al. [31] | Retrospective cohort | Published (peer-reviewed) | China | Nonfunded | Patients with confirmed COVID-19 | Methylprednisolone (50–80 mg/day) | 175 |
| Zhou et al. [32] | Retrospective cohort | Published (peer-reviewed) | China | Funded | Adults COVID-19 patients who died or were discharged | NR | 191 |
NR, Not reported.
Characteristics of the included studies
3.3. Risk of Bias
Figure 2 shows that all studies provided background information and stated the objectives of their research, described the participants, indicated the number of participants, reported their results, and summarized the main findings of their outcomes in the discussion. However, few studies identified possible bias sources and discussed the generalizability of their findings.
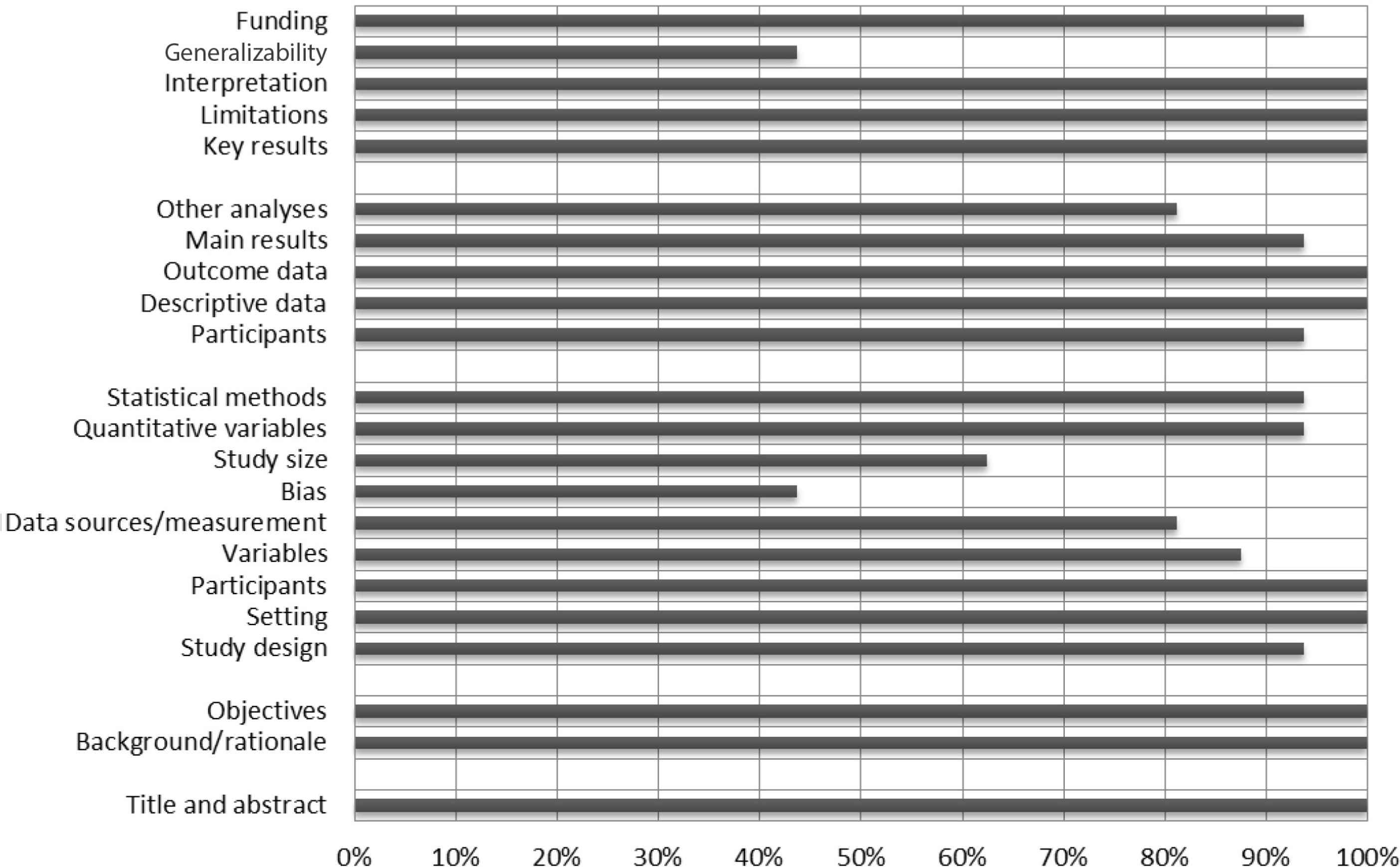
A summary risk of bias results using STROBE checklist.
3.4. Pooled Results
Overall, the random-effects model revealed no significant association between glucocorticoids use and risk of death among COVID-19 patients (OR = 1.39; 95% CI, 0.83–2.32) (Figure 3). Furthermore, significant heterogeneity and inconsistency (Q = 292.24, p < 0.01, I2 = 94%) were observed across included studies. Asymmetry of funnel plot indicated the possibility of publication bias, potential small-study effects, or both, as shown in Figure 4.

Forest plot of the association between glucocorticoids use and death in COVID-19 patients.
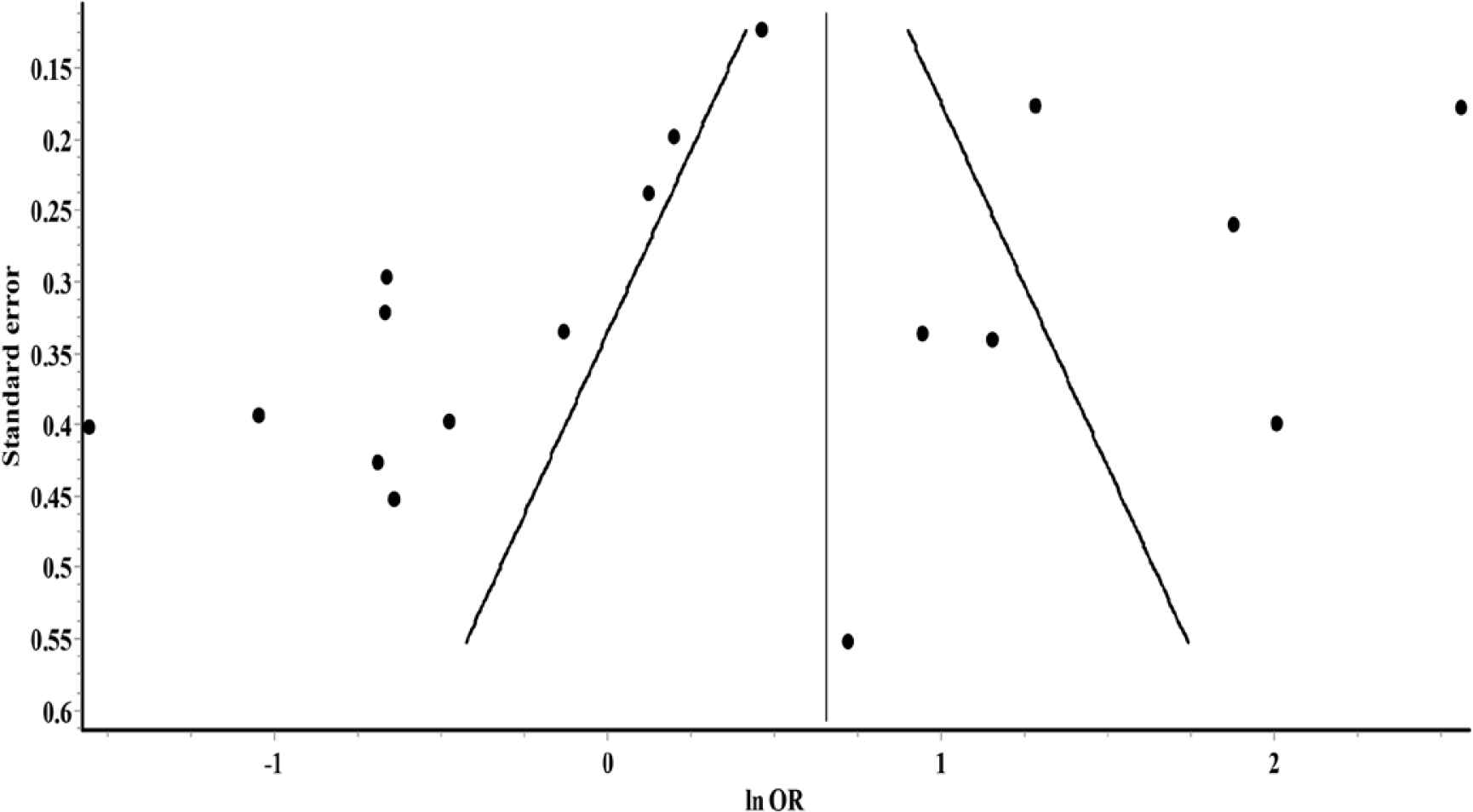
Funnel plot for small-study effects.
3.5. Subgroup Analyses and Sensitivity Analyses
Results for subgroup analyses are reported in Table 2. Across 18 included studies, variations were observed in the type of glucocorticoids medication, severity of COVID-19 illness, setting, publication status, and the average age of patients. As was found, results were statistically significant and in the direction of a decreased risk of death when methylprednisolone only was used as glucocorticoid; however, results were not significant for other glucocorticoids. With regard to COVID-19 severity and publication status, there were no significant association between glucocorticoids use and risk of death across subgroups. For setting, there was a statistically significant association between glucocorticoids administration after hospitalization and risk of mortality, and these results were in the direction of an increased risk of death among hospitalized patients with COVID-19 in China, but results were not significant in the USA and European countries.
| Group | Studies (#) | OR (95% CI) | Cochran Q | Q(p) | I2(%) |
|---|---|---|---|---|---|
| Glucocorticoids medications | |||||
| Methylprednisolone only | 5 | 0.50 (0.27–0.92) | 11.81 | 0.02 | 66 |
| Other medications* | 4 | 1.40 (0.55–3.60) | 27.66 | <0.001 | 89 |
| COVID-19 severity | |||||
| Studies included only severe cases | 5 | 0.92 (0.42–2.05) | 29.80 | <0.001 | 87 |
| Studies included all COVID-19 cases | 13 | 1.61 (0.87–2.99) | 233.47 | <0.001 | 95 |
| Country | |||||
| China | 9 | 2.79 (1.36–5.71) | 110.10 | <0.001 | 93 |
| USA | 3 | 0.78 (0.46–1.31) | 3.54 | 0.17 | 44 |
| European countries | 6 | 0.72 (0.41–1.25) | 38.38 | <0.001 | 87 |
| Publication status | |||||
| Peer-reviewed | 13 | 1.22 (0.61–2.41) | 228.45 | <0.001 | 95 |
| Not peer-reviewed | 5 | 1.90 (0.76–4.76) | 62.83 | <0.001 | 94 |
| Average age group (years) | |||||
| ≥65 | 7 | 1.06 (0.41–2.75) | 192.60 | <0.001 | 97 |
| <65 | 11 | 1.66 (0.91–3.02) | 97.30 | <0.001 | 90 |
Other medications: using dexamethasone only, or using a combination of glucocorticoids (methylprednisolone, hydrocortisone, dexamethasone, and/or prednisone). OR, odds ratio; CI, confidence interval; COVID-19, coronavirus disease 2019.
Subgroup analyses for association between glucocorticoids use and risk of death among COVID-19 patients
For sensitivity analyses, we deleted one study each time and results have remained the same as the pooled results with no significant OR and statistically significant heterogeneity and inconsistency across all deletions.
4. DISCUSSION
This systematic review with meta-analysis was undertaken to examine the association of glucocorticoids use with risk of death among hospitalized patients with COVID-19. In general, the findings of this systematic review with meta-analysis of 18 epidemiological studies suggest that glucocorticoids use may not be associated with risk of death in patients with COVID-19. These findings remained the same with each removal of a study from the model in the sensitivity analyses. Moreover, there were unexplained heterogeneity and inconsistency in the included results although the heterogeneity across studies was incorporated into the model by using an RE model. Variations in patients’ characteristics, type of glucocorticoids, and publication status were observed, and thereby subgroup analyses were conducted based on those variations.
Our subgroup analyses were conducted on the basis of COVID-19 severity, publication status, or patients’ average age, and suggested a continued non-significant association of glucocorticoids use with mortality risk among COVID-19 patients. By contrast, subgroup analysis of studies that were conducted in China showed that glucocorticoids use could be associated with risk of death, whereas findings were not significant for studies in the USA and European countries. However, there was a substantial heterogeneity and inconsistency among included studies in each of the stratified analyses.
For stratified analysis that included studies that reported using methylprednisolone only as glucocorticoid medication [18,20,28,29,31], methylprednisolone use was associated with a significant (50%) decrease in the risk of death among hospitalized patients with COVID-19 infection. One of these studies, which were conducted among COVID-19 patients with an average age of 67 years, showed that a course of high-dose of methylprednisolone may enhance respiratory recovery, and thereby this could lower mortality in patients with COVID-19-associated CSS [28]. A study by Wu et al. [30] showed that among COVID-19 cases, treatment of patients who developed ARDS with methylprednisolone decreased risk of death by 62% as compared with those who did not receive methylprednisolone. Yang et al. [31] found that that early use of methylprednisolone can significantly reduce risk of death among patients with severe COVID-19 younger than 65 years, and this effectiveness was hypothesized to be attributable to excessive immune response and cytokine storm.
The results of this meta-analysis should be interpreted while considering several limitations. First, some of the included studies are small in size. Thus, there is a need for further studies to address this issue for a better understanding of the influence of glucocorticoids therapy on health outcomes of COVID-19 patients. In addition, the included studies were significantly heterogenic with regard to sample size, characteristics of included patients, medications used, and other unobserved factors. Finally, variables and covariates vary across included studies and some of these studies have adjusted estimates whereas others have unadjusted estimates. Therefore, we calculated crude estimates from frequency of events in the included studies.
Regardless of these limitations, the present study has several strengths. To the best of our knowledge, this is one of the few systematic reviews with meta-analyses that examine the association between glucocorticoids use and health outcomes (death or recovery) in patients with COVID-19. Although there was a meta-analysis conducted by Lu et al. [33] whose main aim was to summarize evidence of the effectiveness and safety of glucocorticoids therapy on patients with COVID-19, they included studies about SARS-CoV along with other studies about Middle East Respiratory Syndrome coronavirus (MERS-CoV) in their meta-analysis. Considering the differences between SARS-CoV, MERS-CoV, and COVID-19 that were discussed in previous research [34], the results of our meta-analysis are robust because it only included studies in patients infected with COVID-19. Also, in this meta-analysis, a RE model was used to incorporate between-study variance into the model, which results in more conservative findings as compared with a fixed-effect model [35]. This finding provides evidence about the urgency of further detailed research, especially observational studies, to address the issue with regard to inconsistency of findings about the impact of glucocorticoids use in COVID-19 patients.
5. CONCLUSION
In summary, evidence from the included studies showed that glucocorticoids use may not be associated with risk of death among COVID-19 patients, except for methylprednisolone, which may be associated with a decreased risk of death in COVID-19 patients. However, these results should be interpreted carefully considering the inconsistency and heterogeneity among these pooled results, which may vary widely.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
EOA conceived the study. EOA and HOA contributed to the design, analysis, and interpretation of results. All authors were involved in the drafting of the manuscript. All authors edited and gave final approval to the version published. All authors state that they had complete access to the study data that support the publication.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Ebtihag O. Alenzi AU - Hanaa O. Alenizi PY - 2021 DA - 2021/11/05 TI - Glucocorticoids Use and Post-Hospitalization Outcomes among Patients with Coronavirus Disease 2019: A Systematic Review of Observational Studies with Meta-Analysis JO - Dr. Sulaiman Al Habib Medical Journal SP - 133 EP - 139 VL - 3 IS - 4 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.211028.001 DO - 10.2991/dsahmj.k.211028.001 ID - Alenzi2021 ER -
