Evaluation and Improvement of in vitro Detection Methods of Thrombin Inhibitor Activity
- DOI
- 10.2991/efood.k.201002.001How to use a DOI?
- Copyright
- © 2020 The Authors. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Thrombin inhibitors are the main drugs for the prevention and treatment of thrombotic diseases. Although currently used thrombin inhibitors such as bivalirudin and argatroban show good therapeutic effects, the bleeding and poor oral bioavailability were reported to be their typical shortcomings [1,2]. Therefore, new thrombin inhibitors that can reduce these shortcomings are attracting more and more attention. At present, the chromogenic substrate method, Fibrinogen Clotting Time (FCT) assay method and spectrophotometry (Yang’s method) are widely used in the preliminary screening stage of thrombin inhibitors. Compared with the other two methods, the chromogenic substrate method is widely used as a rapid and accurate method for assaying thrombin inhibitors activity. Meanwhile, ethylene diamine tetraacetic acid (EDTA) is added to the whole detection system, so the effect of divalent metal ions on the results can be eliminated [3]. However, the chromogenic substrate method is only suitable for the detection of inhibitors that can combine with the active site of thrombin, rather than for inhibitors that only combine with thrombin exosite-I. Since the chromogenic substrate method reflects the activity of thrombin by measuring the absorbance value, it is not suitable for detecting colored inhibitors (This problem also exists in Yang’ method.). Although these shortcomings are perfectly avoided in the FCT assay method, the method still has fatal defects. The use of fibrin clotting time to characterize thrombin activity leads to certain requirements for ion concentration in the entire reaction system.
Yang et al. [4] proposed a method for assaying the activity of the thrombin inhibitor. The principle of this method is that the amount of fibrin clot formation is related to the activity of thrombin when the amount of fibrinogen is constant. Clot formation cause changes in absorbance at 405 nm and the inhibition rate of thrombin was calculated according to the following formula
Y – Hydrolysate inhibition rate (%).
Ae – Absorbance value without inhibitor.
Ai – Absorbance value with inhibitor.
Ao – Blank absorbance.
The method has been cited for 40 times and compensates for the shortcomings of the chromogenic substrate method, which can be used to detect both active site inhibitors and exosite-I inhibitors [5–7]. However, this method also showed some limits as followings. Firstly, there is a problem with the choice of absorbance. Yang’s method finally determines the absorbance at 405 nm. However, the entire reaction system appears white, opaque at the time of final solidification, rather than yellow or other colors. Therefore, the reason for the difference in absorbance is the solidification of the solution leading to an increase in turbidity rather than a change in color. However, absorbance of 350 nm or 650 nm were chosen in the similar methods [8,9]. Secondly, there is no direct relationship between thrombin inhibitory activity and the value of absorbance. Yang believes that the higher the absorbance, the higher the thrombin activity and the lower the inhibitor activity; conversely, the lower the absorbance, the lower the thrombin activity and the higher the inhibitor activity. However, the magnitude of the absorbance value is related to the concentration of divalent metal ions. Although divalent metal ions such as Mg2+ and Fe2+ have no significant effect on the coagulation of fibrinogen and the absorbance value of the clot (only affect the formation of thrombus), the impact of Ca2+ and Zn2+ cannot be ignored [10–12]. Yang did not mention the concentration of Ca2+ and Zn2+, only sodium ions were added to the buffer. Therefore, this method is prone to false positive results. In other words, samples with Ca2+ or Zn2+ chelating ability can be mistaken for thrombin inhibitors by using this method.
In order to confirm that there may be a false positive result and prove the significance of metal ions, the following experiments were carried out. The first experimental methods, buffers, calculation methods, etc. are the same as those proposed by Yang et al. [4]. In brief, 140 μL fibrinogen with a concentration of 0.1% and 40 μL sample were added into the plate wells (FEP101296, JET Biofil, China), incubated at 37°C for 5 min. 10 μL thrombin (12 U/mL) was added to start the reaction and the absorbance at 405 nm was measured every minute for a total of 10 min. As for the samples, three kinds of samples have been set up, a positive control, the experimental group, and the negative control, they were 0.5 mM bivalirudin, 7.5 mM EDTA (same concentration as chromogenic substrate method) and 50 mM Tris-HCl buffer, respectively. Results were shown in Figure 1. The results showed that both bivalirudin and EDTA had good thrombin inhibitory activity. In fact, EDTA has no thrombin inhibitory activity, just a metal ion chelating agent. The results indicate that if the sample has the same metal chelating properties as EDTA, the sample may be mistaken for antithrombin activity according to this experimental method in the study of Yang etc.
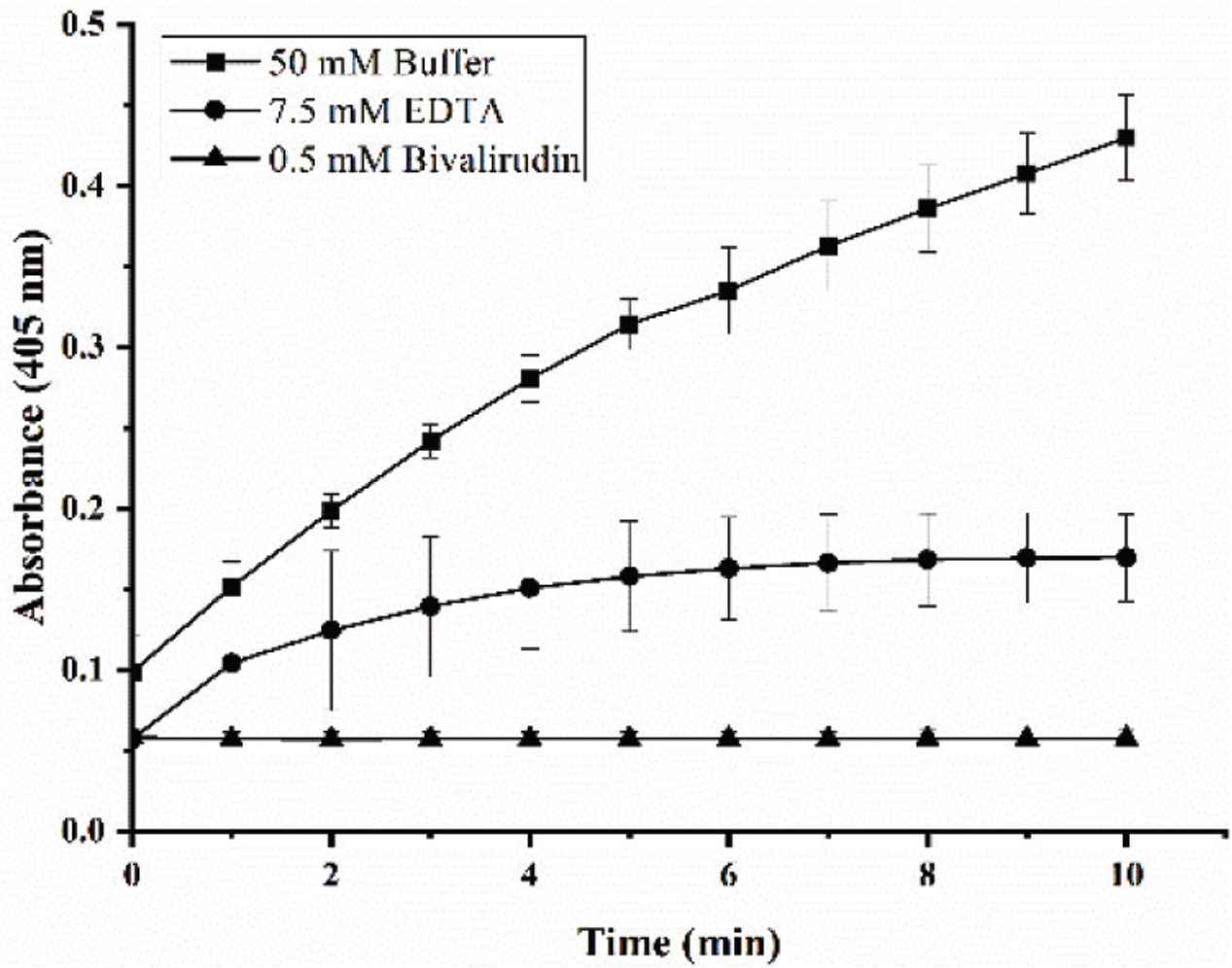
Effect of different samples on absorbance. 0.5 mM bivalirudin (▴) was added as positive control, 7.5 mM EDTA (⦁) was added as metal ion chelating agent without antithrombin activity and 50 mM Tris-HCl buffer (▪) was added as negative control.
In order to demonstrate the effect of Ca2+ and Zn2+ on absorbance, the following experiment was carried out. Three kinds of buffer (pH = 7.4) were prepared for this experiment. Buffer A was 50 mM Tris-HCl with 0.12 mM NaCl. Buffer B was 50 mM Tris-HCl with 0.12 mM NaCl and 2 mM CaCl2. Buffer C was 50 mM Tris-HCl with 0.12 mM NaCl and 15 μM ZnCl2. Fibrinogen (1 mg/mL) and thrombin (12 U/mL) were dissolved in three kinds of buffer, respectively. The method was conducted according to Yang’s method [4], but the absorbance was determined at the wave length ranging from 350 to 750 nm. Results were shown in Figure 2. Although Zn2+ has a greater effect on fibrinogen turbidity than Ca2+, both ions influence absorbance. The result is consistent with previous report [10].
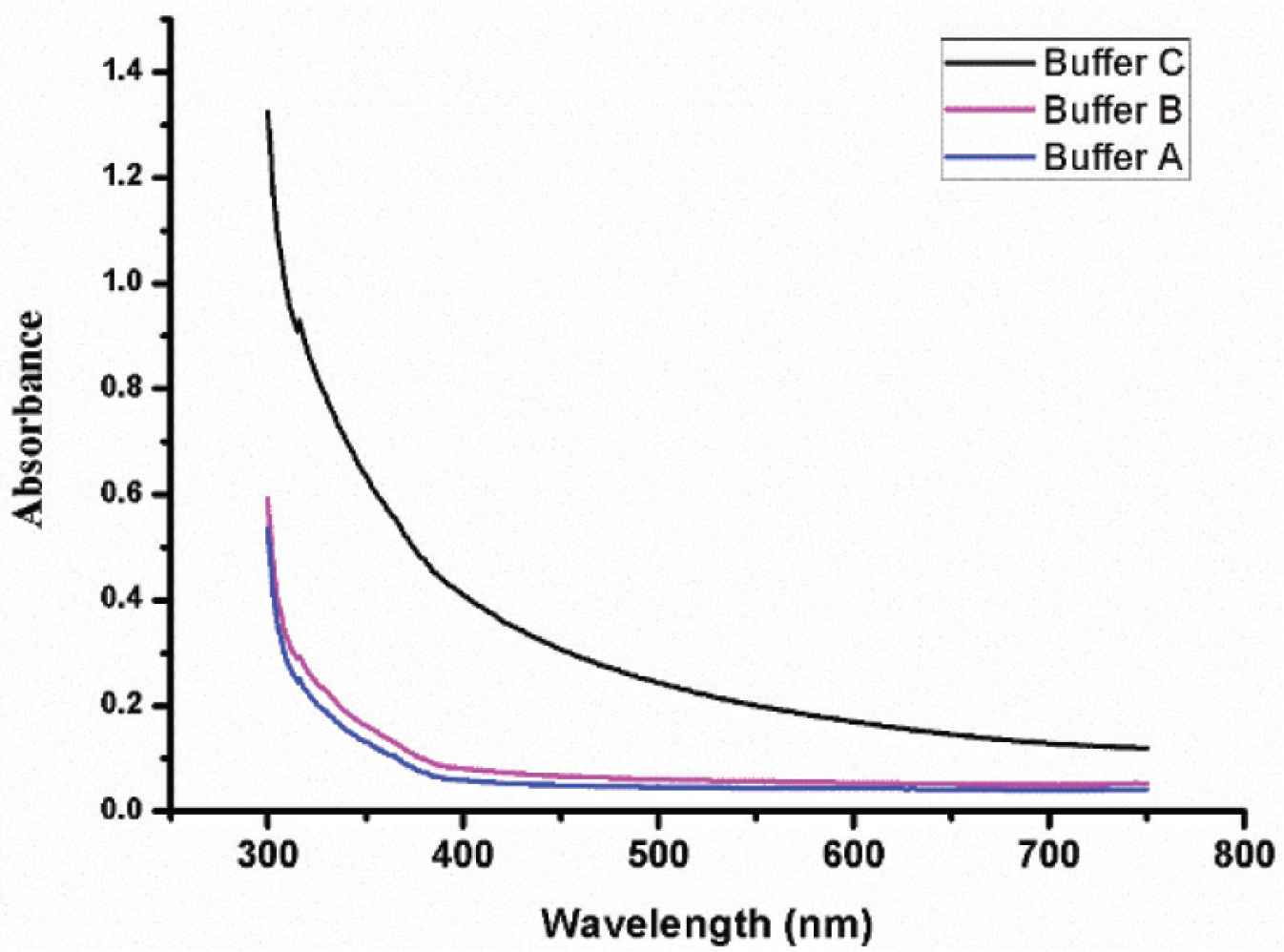
Effect of different buffers on absorbance. Buffer A (blue) was 50 mM Tris-HCl with 0.12 mM NaCl. Buffer B (magenta) was 50 mM Tris-HCl with 0.12 mM NaCl and 2 mM CaCl2. Buffer C (black) was 50 mM Tris-HCl with 0.12 mM NaCl and 15 μM ZnCl2.
In order to eliminate the occurrence of false positives due to the influence of ions on the absorbance, the clotting time is measured to replace the absorbance at 405 nm. In brief, the fibrinogen and thrombin were all dissolved in 50 mM Tris-HCl buffer with 100 mM NaCl and 2 mM CaCl2. The concentrations of fibrinogen and thrombin are 1 mg/mL and 10 U/mL, respectively. FCT assay was determined in accordance with references with minor but critical modifications [13]. As for as FCT, 200 μL fibrinogen and 50 μL buffer or sample were incubated for 60 s at 37°C, 50 μL thrombin was added and the clotting time was measured with a coagulometer (MC4, Merlin, Germany). The clotting time of blank (50 mM Tris-HCl buffer), EDTA (7.5 mM), inactive peptide as negative control (1.25 mM), thrombin exosite-I inhibitor (0.6 mM) and bivalirudin (0.6 mM) were measured. The detection index changes from absorbance to solidification time, which can effectively avoid the influence of Zn2+ on the experimental results. Meanwhile, adding enough Ca2+ to the reaction system can avoid the false positive result caused by the difference in Ca2+ concentration. As shown in Figure 3, the FCT of thrombin exosite-I inhibitor and bivalirudin shows significant difference compared with control, but inactive peptide and EDTA are not. Calculate the Relative Standard Deviation (RSD) value of the test results of the two methods, the original method RSD value exceeds 10% (RSD value was 13.3%), and the new method RSD value is <5% (RSD value was 3.0%). When the thrombin activity increased to 12 U/mL, the RSD value was 1.9%. When the thrombin activity decreased to 6 U/mL, the RSD value was 9.2%.
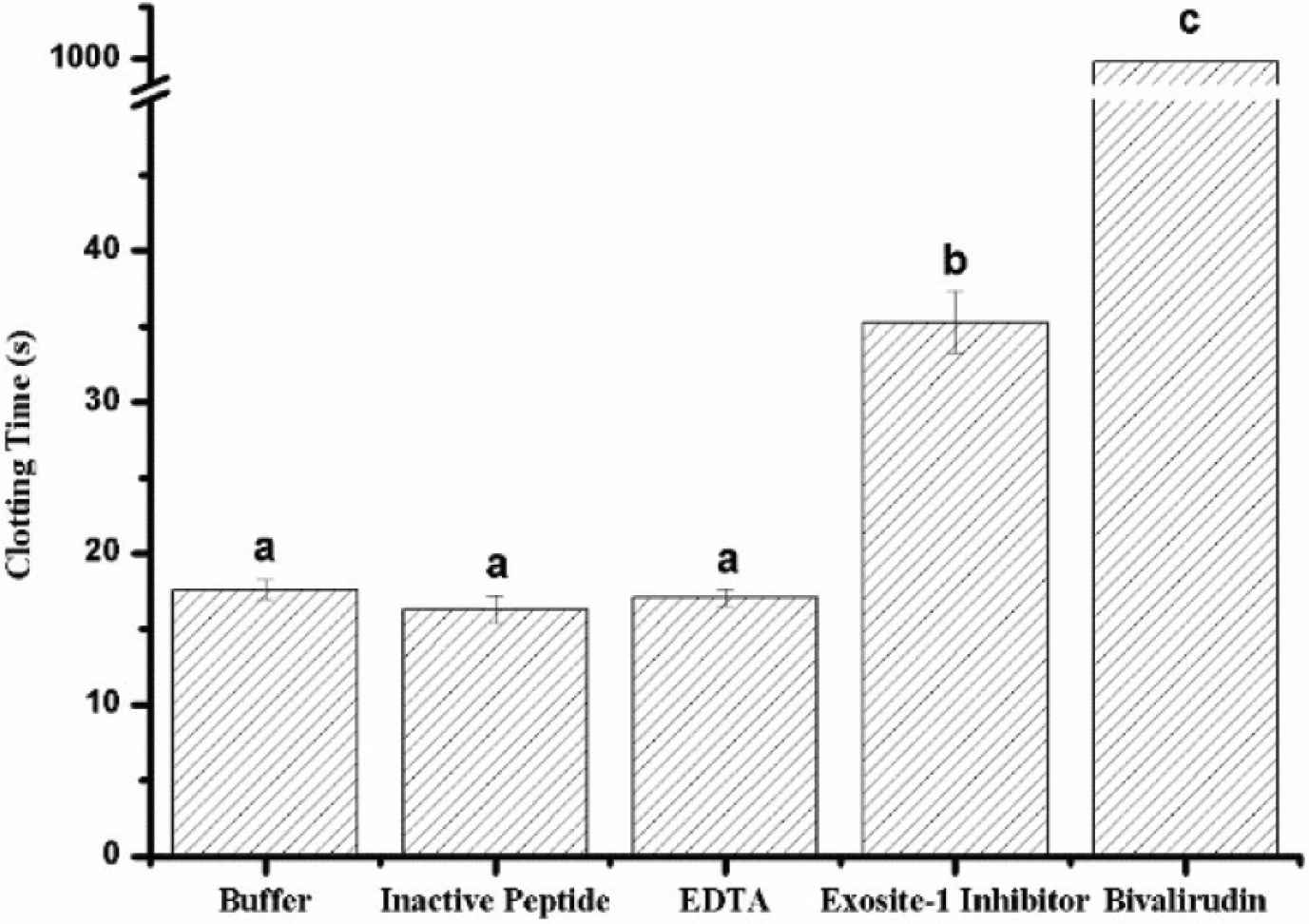
Fibrinogen clotting time of different samples. Thrombin exosite-I inhibitor and bivalirudin (bivalent direct thrombin inhibitor) have significant difference compare with buffer (blank) but inactive peptide (negative control) and EDTA are not.
2. CONCLUSION
Based on the analysis above, an assay method of the inhibitory activity against thrombin was established as following. The reaction system was operated in coagulometer at 37°C, and the pH 7.4. The concentration of fibrinogen and thrombin was 0.1% and 10 U/mL, respectively. 200 μL fibrinogen and 50 μL Tris-HCl buffer (blank) or sample were added into automated coagulation cup with a magnetic bead and incubated for 60 s. Then, 50 μL thrombin was added into automated coagulation cup and the clotting was measured. Tris-HCl buffer (50 mM Tris, 0.1 M NaCl and 2 mM CaCl2, pH = 7.4) was used in the present method. The concentration of the sample is recommended to be within 5 mM, not to exceed 10 mM and the thrombin concentration cannot lower than 10 U/mL. Compared with Yang’s method, although the method in our study cannot calculate the thrombin inhibition rate of inhibitors, it can still be used as a rapid detection method for large-scale screening and characterization of inhibitors. The improved method no longer uses absorbance as the detection index and adds enough necessary ions to the entire system to avoid the influence of the difference in metal ion content on the results. Meanwhile, a lower RSD value indicates that it has better repeatability than Yang’s method. Compared with the chromogenic substrate method, this method has a wider detection range and can also accurately characterize thrombin inhibitors which only inhibit thrombin exosite-I. Therefore, this method can be used in the extensive screening and preliminary characterization of thrombin inhibitors.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Hanxiong Liu AU - Shuzhen Cheng AU - Zhenyu Wang AU - Ming Du PY - 2020 DA - 2020/10/12 TI - Evaluation and Improvement of in vitro Detection Methods of Thrombin Inhibitor Activity JO - eFood SP - 377 EP - 379 VL - 1 IS - 5 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.201002.001 DO - 10.2991/efood.k.201002.001 ID - Liu2020 ER -
