The Effect of a Regimen of Antifungal Cream Use on Episodes of Acute Adenolymphangitis (ADL) among Lymphedema Patients: An Application Using Marginal Structural Models
- DOI
- 10.2991/j.jegh.2017.10.009How to use a DOI?
- Keywords
- Lymphatic filariasis; lymphedema management; adenolymphangitis; marginal structural models; time-varying confounding
- Abstract
Episodes of adenolymphangitis (ADL) are a recurrent clinical aspect of lymphatic filariasis (LF) and a risk factor for progression of lymphedema. Inter-digital entry lesions, often found on the web spaces between the toes of those suffering from lymphedema, have been shown to contribute to the occurrence of ADL episodes. Use of antifungal cream on lesions is often promoted as a critical component of lymphedema management. Our objective was to estimate the observed effect of antifungal cream use on ADL episodes according to treatment regimen among a cohort of lymphedema patients enrolled in a morbidity management program. We estimated this effect using marginal structural models for time varying confounding. In this longitudinal study, we estimate that for every one-unit increase in the number of times one was compliant to cream use through 12 months, there was a 23% (RR = 0.77 (0.62, 0.96)) decrease in the number of ADL episodes at 18 months, however the RR’s were not statistically significant at other study time points. Traditionally adjusted models produced a non-significant RR closer to the null at all time points. This is the first study to estimate the effect of a regimen of antifungal cream on the frequency of ADL episodes. This study also highlights the importance of the consideration and proper handling of time-varying confounders in longitudinal observational studies.
- Copyright
- © 2018 Atlantis Press International B.V.
- Open Access
- This is an open access article under the CC BY-NC license (http://creativecommons.org/licences/by-nc/4.0/).
1. INTRODUCTION
Patients with lymphatic filariasis (LF) often suffer from episodes of acute adenolymphangitis (ADL). These episodes are characterized by plaque-like areas of inflammation, swelling of the limbs, fever, malaise and chills [1], and are typically the first clinical sign of LF, occurring years after infection. ADL episodes are a recurrent clinical aspect of LF lasting 3–15 days each and can occur several times each year [2]. The frequency of ADL episodes has been shown to increase with more advanced lymphedema and ADL episodes are a risk factor for the progression of chronic LF symptoms such as lymphedema and elephantiasis [2–6].
Inter-digital entry lesions, thought to be caused by fungal infections [7,8], are often found on the web spaces between the toes of those suffering from lymphedema and may come and go over time. These entry lesions likely contribute to the occurrence of ADL episodes [7,9,10]. Etiologically, entry lesions are hypothesized to serve as a point of entry for bacteria, leading to ADL episodes [7,11]. In one study, inter-digital entry lesions were associated with more advanced lymphedema stage [10]. Recognition and treatment of inter-digital entry lesions with antifungal cream is a critical component of lymphedema management recommendations [10].
ADL: adenolymphangitis; CDC: Centers for Disease Control and Prevention; IPTW: inverse-probability-of-treatment weighting; IPCW: inverse-probability-of-censoring weighting; LF: lymphatic filariasis ; MDA: mass drug administration; MSM: marginal structural model; RR: rate ratios Several papers have illustrated the effectiveness of lymphedema management programs in decreasing the frequency of ADL over time [12–15], Two studies evaluating the association between overall compliance and ADL found no association [6,12]. Another study found that among patients with inter-digital entry lesions, those who were compliant overall to a lymphedema management program had a lower rate of ADL episodes over time [16], but did not find this relationship among those without inter-digital entry lesions.
Our objective was to estimate the effect of a treatment regimen of antifungal cream use on ADL episodes. Because inter-digital entry lesions act as a time-varying confounder and are also on the causal pathway between antifungal cream use and ADL episodes, we estimated this effect using marginal structural models and compared these results to traditionally adjusted models.
2. MATERIAL AND METHODS
2.1. Ethics Statement
This project was submitted for human subjects review to the Center for Global Health at the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA. It was approved by CDC and determined to be program evaluation. Permission for the survey was obtained from the Odisha State Department of Health and Family Welfare. Participants were asked to give their written informed consent prior to participation. For those unable to write, consent was documented by recording the person’s fingerprint or marking the signature line with an ‘X’ and by countersignature of survey personnel. For participants under 18 years of age, verbal consent of a parent or guardian was also obtained. Consent procedures were approved by CDC and the Odisha State Department of Health and Family Welfare.
2.2. Study Population
Patients enrolled in an ongoing lymphedema management program implemented in Khurda district, Odisha State, India were recruited to partake in a study evaluating the impact of the program on ADL episodes over a 24 month period. In 30 selected villages in Bologarh block, 533 persons with lymphedema were identified in June 2009. Of these patients, 456 were approached to be in the lymphedema program. To be eligible, persons had to be at least 14 years of age and report lower leg swelling for at least 3 months, resulting in 375 eligible patients, of which 370 agreed to participate. The original sample size (of 370) was powered to detect a 5% decrease in ADL episodes over two years. See Ref. [16] for more details on the sampling and patient recruiting process.
All patients enrolled in the program were trained in basic lymphedema management by physician-trained volunteers, including daily washing of limbs with soap and water, daily exercise and elevation of the affected limb, and daily use of footwear outside the home. Patients were trained in the importance of early treatment and prevention of secondary bacterial and fungal infections with topical and oral antimicrobial agents. If inter-digital fungal infections were present, patients were instructed to use an antifungal cream on a daily basis. Patients were supplied with soap and antifungal cream for the first 6 months of the program; thereafter they were instructed to purchase these supplies at local stores and pharmacies.
Through a questionnaire, patients were interviewed regarding demographics, frequency of compliance to lymphedema management techniques, ADL history and treatment, access to supplies, MDA history, and perceived disability. Interviewers also completed a clinical assessment on each person to determine lymphedema stage using the 7-stage system [17] and to determine if inter-digital entry lesions were present. Interviews took place at baseline (prior to enrollment in July 2009) and at, 1, 2, 3, 6, 12, 18 and 24 months after enrollment in the program. Data cleaning and analysis were performed in SAS 9.3 (Cary, North Carolina, USA).
2.3. Outcome Definition
An ADL episode was defined by patient self-report of two or more of the following symptoms: redness, pain, or swelling of the leg or foot, with or without the presence of fever or chills [5,11]. Patients were asked how many times they had an ADL episode in the previous 30 days. The ADL rate per subject was calculated as the number of ADL episodes reported by each subject divided by 30 days.
2.4. Exposure Definition: Antifungal Cream Regimen
Use of antifungal cream was measured through patient self-report of how frequently they used antifungal cream in the previous 30 days. A person was considered to be compliant to antifungal cream use if he/she reported using antifungal cream at least once per day. Compliance with antifungal cream was treated as a dichotomous (0, 1) variable denoted as “cream k” at time point k.
To measure the effect of a cream regimen, we used the sum of cream compliance over time [18] through K-1, where K indicates the time point at which ADL was evaluated and k indicates any time point previous to K:
We lagged compliance to cream by one study time point in relation to the outcome to allow time for a potential effect of antifungal cream. We did not include baseline cream use in the sum because patients had yet to be enrolled in the lymphedema management program.
2.5. Statistical Analysis
Marginal structural models (MSM) were used to estimate the effect of a regimen of antifungal cream on ADL episodes, with inter-digital entry lesions as a time-varying confounder. MSMs are a class of causal models for the estimation, from observational data, of the causal effect of a time-dependent exposure in the presence of time-dependent covariates that may be simultaneously confounders and intermediate variables [19,20]. The parameters of MSMs can be estimated through inverse-probability-of-treatment weighting (IPTW), creating a pseudo population in which the time-varying covariate is no longer associated with treatment, yet does not improperly control away the effect of the time-varying covariate as an intervening variable in the causal pathway of interest.
Fig. 1 is a causal diagram illustrating the hypothesized causal relationship of interest through K = 3 months of follow-up. Since antifungal cream is indicated when inter-digital lesions are present and lesions are a risk factor for ADL episodes, the presence of inter-digital entry lesions at a given time point (Lk) confounds the relationship between cream use at each time point (Ak) and the number of ADL episodes in the preceding 30 days at K = 3 months of follow-up (Y). It is hypothesized that the use of antifungal cream at an earlier time point will prevent the future presence of inter-digital entry lesions, thus the presence of lesions is also on the causal pathway between cream use at the previous time point (Ak-1) and the number of ADL episodes at follow-up (Y). In Appendix A, Fig. A.1, we expand the causal diagram of Fig. 1 to represent the cumulative effect of cream use on the number of ADL episodes at K = 24 months of follow-up. Fig. A.2 incorporates censoring as another time-varying treatment in the causal diagram.
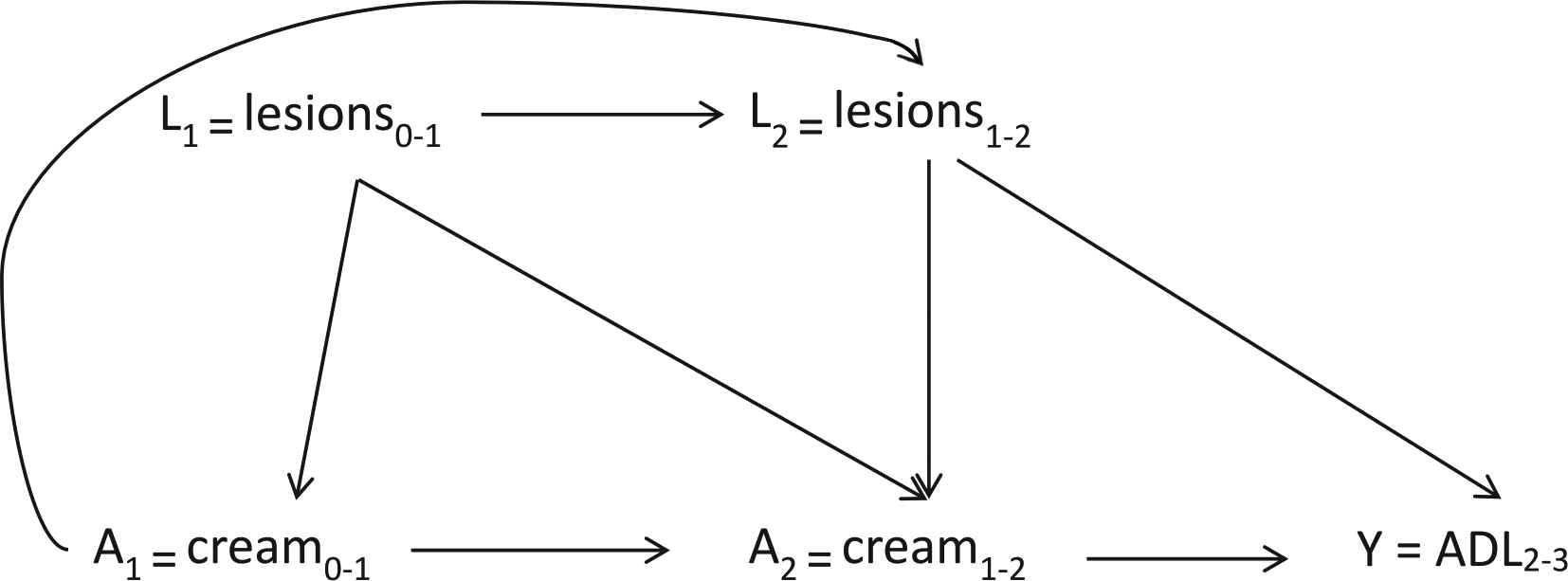
Causal diagram of the effect of a regimen of antifungal cream use (A) through k = 2 months on the outcome, ADL episodes, at K = 3 months. L represents the time-varying confounder, presence of inter-digital entry lesions.
We estimated the cumulative effect of cream use on ADL through several follow-up time points K: 3, 6, 12, 18, and 24 months. We modeled the number of ADL episodes as a Poisson variable and weighted each subject by their inverse probability of treatment (with antifungal cream) to control for the confounding effect of inter-digital entry lesions without controlling for it as an intermediate variable. We also weighted each subject by their inverse probability of censoring, adjusting for selection bias due to loss to follow-up [20]. All MSMs were fit to subjects who were uncensored up until the time point K of interest. Standard errors for the MSMs were calculated using robust estimates to appropriately adjust for the sampling weights. We used both stabilized and non-stabilized weights for the MSM.
We also estimated the effect of a regimen of antifungal cream on the expected number of ADL episodes controlling for inter-digital entry lesions using traditional Poisson models with lesions as a covariate in the model. Non-time-varying confounders, lymphedema status at baseline and patient report of ever receiving drugs such as albendazole and diethylcarbamazine during mass drug administration (MDA) were adjusted for in all models (MSM and traditional Poisson). Baseline lymphedema stage was categorized as advanced (stages 4–7) or early (stages 1–3). We modeled each outcome time point K separately; therefore, each model included one observation per subject.
2.6 Results
Three hundred seventy lymphedema patients were enrolled in the lymphedema management program at baseline. The mean age was 57 years and the majority (59%) of the cohort was female (data not shown). At baseline, about 27% of patients had inter-digital entry lesions, 14% had advanced stage lymphedema (stages 4–6), and patients reported having lymphedema symptoms for an average of 25 years (data not shown). A previous paper concerning this cohort [16] provides more detail on demographic and LF disease characteristics.
After 24 months of follow-up, 64% of patients remained uncensored (Table 1). On average, about 13% of patients had advanced lymphedema (stages 4–7) at any point during the study period. The mean number of reported ADL episodes per 30 days was 0.27 at 1 month, 0.14 at 6 months and 0.24 at 24 months. The distribution of the presence of inter-digital entry lesions and MDA history is also listed in Table 1. The percent of patients who had inter-digital entry lesions decreased slightly over the study period, while the percent of participants who ever participated in an MDA rose from 84% at 1 month to 89% at the end of follow-up.
| 1 month N (%) | 2 month N (%) | 3 month N (%) | 6 month N (%) | 12 month N (%) | 18 month N (%) | 24 month N (%) | |
|---|---|---|---|---|---|---|---|
| # Uncensored individuals | 351 (94.9) | 334 (90.3) | 312 (84.3) | 280 (87.6) | 261 (70.5) | 247 (66.8) | 235 (63.5) |
| Advanced lymphedema stage | 44 (12.5) | 39 (11.7) | 38 (12.2) | 36 (12.9) | 35 (13.4) | 32 (13.0) | 33 (14.0) |
| Mean number of adl episodes per month (max) | 0.27 (4) | 0.28 (4) | 0.24 (3) | 0.14 (5) | 0.26 (8) | 0.30 (7) | 0.24 (8) |
| Presence of inter-digital entry lesions | 112 (31.9) | 107 (32.0) | 84 (26.9) | 66 (23.6) | 77 (29.5) | 62 (25.1) | 62 (26.4) |
| Ever participated in mda | 294 (83.8) | 283 (84.7) | 266 (85.3) | 239 (85.4) | 223 (85.4) | 209 (84.6) | 210 (89.4) |
Frequency of clinical characteristics and history of MDA among uncensored lymphedema patients enrolled in a lymphedema management program. Khurda District, Odisha State, India, 2009–2011.
Table 2 shows the distribution of antifungal cream use (as a sum of compliance over time) among lymphedema patients at each time point. After 18 months of follow-up, about a third (28%) of patients had not been compliant to antifungal cream use at any point in the study period, while about a fifth (18.3%) had been compliant for at least one 30-day period. Another quarter (26.8%) of the cohort had been compliant for at least four 30-day periods during follow-up.
| Time point | N1 | Cumulative sum of compliance to cream use | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 | 5 | 4 | 3 | 2 | 1 | 0 | ||
| 18 months | 235 | 21 (8.94) | 23 (9.79) | 19 (8.09) | 30 (12.77) | 33 (14.04) | 43 (18.30) | 66 (28.09) |
| 12 months | 247 | 26 (10.53) | 25 (10.12) | 34 (13.77) | 30 (12.15) | 53 (21.46) | 79 (31.98) | |
| 6 months | 261 | 29 (11.11) | 41 (15.71) | 35 (13.41) | 61 (23.37) | 95 (36.40) | ||
| 3 months | 280 | 37 (13.21) | 52 (18.57) | 65 (23.21) | 126 (45.00) | |||
| 2 months | 312 | 45 (14.42) | 72 (23.08) | 195 (62.50) | ||||
N represents the number of uncensored patients at follow-up time K, the subsequent time point to the corresponding time point in the table. For example, N = 235 represents the number of uncensored patients through K = 24 months, from which we sum the number of times each subject used antifungal cream through 18 months;
The values displayed in the table represent the number (N) and percent of patients who were compliant to the cream use regimen at the specified time point.
Distribution of antifungal cream use regimens as cumulative sum of compliance to cream use over time2 among uncensored lymphedema patients. Khurda District, Odisha State, India. 2009–2011.
Table 3 shows the estimated rate ratios (RR) obtained from the MSM using stabilized weights, MSM using non-stabilized weights, the traditionally adjusted Poisson regression controlling for lesions at each previous time point without weighting, and the crude Poisson regression. All models estimate the effect of cumulative antifungal cream use through K-1 on the number of ADL episodes reported in the previous 30 days at each specified follow-up period, K.
| Time (K) | Marginal structural model Ia,b RR (95% CI) | Marginal structural model IIb,c RR (95% CI) | Traditional adjustmentd RR (95% CI) | Crude model RR (95% CI) |
|---|---|---|---|---|
| 3 months | 1.08 (0.70, 1.67) | 0.94 (0.64, 1.37) | 1.12 (0.77, 1.64) | 1.76 (1.30, 2.38) |
| 6 months | 1.04 (0.60, 1.81) | 0.83 (0.45, 1.51) | 0.92 (0.47, 1.82) | 1.23 (0.89, 1.71) |
| 12 months | 0.63 (0.36, 1.09) | 0.81 (0.40, 1.60) | 0.84 (0.54, 1.30) | 1.27 (0.97, 1.67) |
| 18 months | 0.77 (0.62, 0.96) | 0.55 (0.39, 0.76) | 0.86 (0.59, 1.26) | 1.07 (0.90, 1.26) |
| 24 months | 0.80 (0.63, 1.03) | 0.74 (0.54, 1.01) | 0.83 (0.62, 1.11) | 1.08 (0.88, 1.32) |
Poisson model using stabilized inverse probability of treatment and inverse probability of censoring weights;
Additionally adjusted for lymphedema status (advanced, stages 4–7 vs. early, stages 1–3) and history of MDA;
Poisson model using non-stabilized inverse probability of treatment and inverse probability of censoring weights;
Adjusted for presence of inter-digital entry lesions, lymphedema status (advanced, stages 4–7 vs. early, stages 1–3) and history of MDA.
Model results of the association between a regimen of antifungal cream and the rate of ADL episodes at follow-up time point K among a cohort of lymphedema patients enrolled in a lymphedema management program, Khurda District, Odisha State, India, 2009–2011.
The stabilized MSM results suggest that an increase in the number of times a patient was compliant to antifungal cream was associated with a decrease in the frequency of ADL episodes at 12, 18, and 24 months. However, the confidence intervals for the risk ratio at 12 and 24 months include the null value. The results at 18 months suggest that for every one-unit increase in the number of times one was compliant to cream through 12 months, there was a 23% (RR = 0.77 (0.62, 0.96)) decrease in the number of ADL episodes at 18 months. Using the MSM parameter estimates (Table 4), at 24 months a patient who was compliant to antifungal cream use at all six previous time points (months 1, 2, 3, 6, 12, and 18) had an average number of ADL episodes 73% lower than the number of ADL episodes for a patient who was not compliant to cream use at any of the six previous time points (−0.22 × 6 = −1.32; RR = exp(−1.32) = 0.27).
| Time (K) | Marginal structural model Ia,b
|
Marginal structural model IIb,c
|
Traditional adjustmentd
|
Crude model
|
|---|---|---|---|---|
| 3 months | 0.08 (−0.36, 0.51) | −0.07 (−0.45, 0.32) | 0.12 (−0.27, 0.50) | 0.56 (0.26, 0.87) |
| 6 months | 0.03 (−0.52, 0.59) | −0.20 (−0.79, 0.40) | −0.08 (−0.76, 0.60) | 0.21 (−0.12, 0.54) |
| 12 months | −0.47 (−1.02, 0.09) | −0.22 (−0.91, 0.47) | −0.18 (−0.62, 0.26) | 0.24 (−0.03, 0.51) |
| 18 months | −0.26 (−0.48, −0.05) | −0.60 (0.39, 0.76) | −0.15 (−0.53, 0.23) | 0.07 (−0.10, 0.23) |
| 24 months | −0.22 (−0.47, 0.03) | −0.31 (−0.62, 0.06) | −0.18 (−0.47, 0.10) | 0.07 (−0.13, 0.28) |
Poisson model using stabilized inverse probability of treatment and inverse probability of censoring weights;
Additionally adjusted for lymphedema status (advanced, stages 4–7 vs. early, stages 1–3) and history of MDA;
Poisson model using non-stabilized inverse probability of treatment and inverse probability of censoring weights;
Poisson model adjusted for presence of inter-digital entry lesions, lymphedema status (advanced, stages 4–7 vs. early, stages 1–3) and history of MDA.
Parameter estimates from models estimating the association between a regimen of antifungal cream and the rate of ADL episodes at follow-up time point K among a cohort of lymphedema patients enrolled in a lymphedema management program, Khurda District, Odisha State, India, 2009–2011.
The non-stabilized MSM results also suggest that an increase in the frequency of antifungal cream use was associated with a decrease in the frequency of ADL episodes at 6, 12, 18, and 24 months. Again, the RR was only significant at 18 months. The results of the traditional regression suggest a slightly negative association between the frequency of cream use over time and the frequency of ADL episodes at a subsequent time point. However, none of these were statistically significant.
2.7. Discussion
Using marginal structural Poisson models, our results suggest that among a cohort of lymphedema patients enrolled in a morbidity management program, increased use of antifungal cream over time is associated with a decreased frequency of ADL episodes after 18 months in a lymphedema management program. The estimated effect was found after adjusting for both time-varying and non-time-varying confounders. Assuming no unmeasured confounding and correctly specified models, our findings suggest a protective effect of antifungal cream for ADL episodes following 18 months of use.
Based on the MSM, we estimate that for every one-unit increase in the number of times a patient was compliant to antifungal cream use during the follow-up period, there was a 20% decrease in the rate of ADL episodes at 24 months, although this finding was not statistically significant. The MSM estimates were consistently further from the null than those of the traditionally adjusted regression. We suspect the traditional models may be controlling away some of the effect of antifungal cream by controlling for the presence of inter-digital entry lesions, both a confounder and intermediate on the causal path.
Exploring the cumulative use of antifungal cream over time, our results suggest that the protective effect of cream strengthens as the follow-up period for ADL episodes is expanded to 18 months and then recedes at 24 months. The same pattern is seen in the traditionally adjusted Poisson model. In both the stabilized and non-stabilized MSM’s, only the confidence interval at 18 months does not contain the null, while all of the confidence intervals for the traditionally adjusted and crude models contain the null. This may be due, in part, to the loss of statistical power to detect a significant estimated effect and to the movement of the RR’s closer to the null. Since the models used only uncensored individuals through each outcome time point, they may be under powered to detect a significant change in the frequency of ADL episodes. Specifically, the models estimating the effect of a cream regimen on ADL episodes at 18 and 24 months only included 247 and 235 of the original 370 individuals respectively. Furthermore, it is biologically plausible that continued application of antifungal cream over time, with continued fungal killing, is necessary for a protective effect. The attenuation of the RR at 24 months may also be due in part to the measurement error in this study: self-report of both exposure and outcome and averaging of the compliance measure over varying time intervals.
Within the context of lymphedema management programs, our study suggests that consistent use of antifungal cream may decrease the frequency of ADL episodes among lymphedema patients. Our findings provide some evidence for the effectiveness of long-term antifungal cream use in decreasing ADL episodes, while also providing evidence that is consistent with entry lesions as an entry point for bacterial pathogens that in turn lead to ADL episodes [9,21]. Antifungal cream use to treat inter-digital entry lesions is currently recommended as part of the morbidity management and disability prevention arm of the World Health Organization’s Global Programme to Eliminate Lymphatic Filariasis. [22], which also includes washing the affected limbs with soap and water, exercise and elevation of the affected limb, and use of footwear outside of the home. Current and future lymphedema management programs should continue to emphasize the importance of patient awareness of inter-digital entry lesions and the use of antifungal creams to treat them [10].
This is the first study to explore the effects of a regimen of antifungal cream on ADL episodes and the first to use MSM’s to control for time-varying confounding by inter-digital entry lesions. Although slight, the differences between the MSM’s and traditionally adjusted models indicate that consideration of time-varying confounding is important when attempting to estimate this causal effect. We believe that within the context of the time-varying nature of inter-digital entry lesions, the MSM is the most appropriate way to estimate this effect.
Our results should be considered in the context of the following limitations. First, the study lacks a control population (who do not receive cream), therefore, we can only evaluate cream use in the context of user compliance. Because antifungal cream is considered a standard of care in lymphedema management programs, it would have been unethical to not provide it to a subset of patients. Second, the study did not conduct a blood test to determine the causal agent of the ADL episode. Thus, it is not known whether the ADL episodes are caused by fungal or bacterial agents. If the latter, the antifungal cream would conceivably not have an impact on ADL episodes. It is also possible that the ADL episodes may be due to a coinfection with both fungal and bacterial agents, thus the impact of antifungal cream may be attenuated by the role of the bacteria.
Third, the frequency of antifungal cream use and ADL episodes was based on self-report and may be subject to recall bias. Patients may have inaccurately recounted the number of ADL episodes they experienced over the last 30 days or may have had a difficult time identifying distinct episodes. Fourth, compliance to antifungal cream use was parameterized as a dichotomous variable based on patient report of using cream on average at least once per day over a 30-day period. This parameterization and the way in which cream use was measured, as an average over 30 days, may have resulted in additional measurement error. Furthermore, patients enrolled in the lymphedema management program may have demonstrated a social desirability bias, leading them to overestimate frequency of antifungal cream use.
The results of this study may also be subject to another form of measurement error. The presence of inter-digital entry lesions was observed through a physical examination by a volunteer and supervisor at each time point. Yet inter-digital entry lesions are a condition that may come and go over a matter of days, so that the observation of lesions at a certain time point may not indicate whether a patient had lesions during the entire time interval between data collection points. Conversely, cream use and ADL episodes were measured through a retrospective self-report that represents an average frequency over the time interval. The frequency of cream use and ADL episodes at any given time point represent an average over the previous 30 days, while the presence of inter-digital entry lesions represents their actual occurrence at the data collection time point, resulting in a lack of clarity in the temporal sequence of cream and entry lesions. The difference in the way these variables were measured may have biased the estimate of effect for which we were unable to control in the analysis.
Although only 64% of patients enrolled in the lymphedema management program remained uncensored for the full duration of data collection, we took into account the possibility of selection bias due to loss to follow-up by using inverse probability of censoring weights in the MSMs. These weights create a pseudo population of patients in which censoring is not associated with previous cream use or the presence of inter-digital entry lesions. Thus, we hope to properly control for any selection bias arising from censoring in estimating the effect of cream use on ADL episodes.
3. CONCLUSIONS
This is the first study to illustrate the potential preventative effects of antifungal cream on ADL episodes among lymphedema patients enrolled in a morbidity management program. Although the effect estimate was only statistically significant at the 18-month time point, it is certainly feasible that a stronger beneficial effect (with greater statistical significance) could be seen with longer-term or more consistent use of the antifungal cream. This finding is especially important within the context of the recent recommendation by the global LF elimination program that all LF endemic countries provide available, quality morbidity management services [22]. It may encourage future funding of lymphedema management programs with specific support of the provision of antifungal cream to enrolled patients, as well as the practice of consistent long-term use. This study also highlights the importance of the consideration and proper handling of time-varying confounders that are also affected by previous exposure status in longitudinal observational studies. Future research with greater power and more reliable measurement of inter-digital entry lesions, antifungal cream use, and ADL episodes is needed to further elucidate the potential benefits of consistent use of antifungal cream when inter-digital entry lesions are present.
APPENDIX A
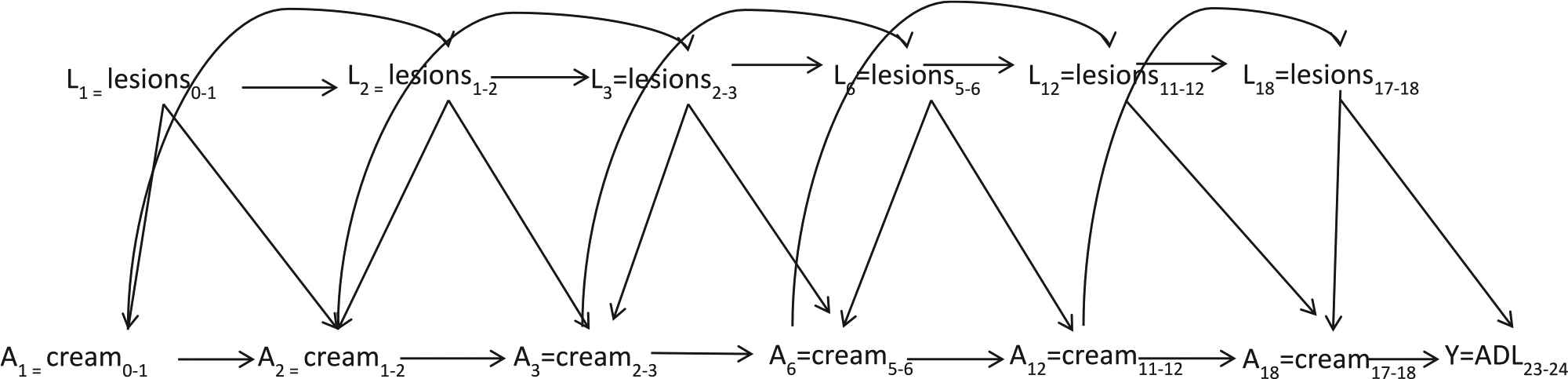
Causal diagram of the effect of antifungal cream use through 18 months on ADL episodes between 23 and 24 months illustrating the relationships between time-varying confounders L, cream treatment A, and the outcome of interest Y, ADL between 23 and 24 months.
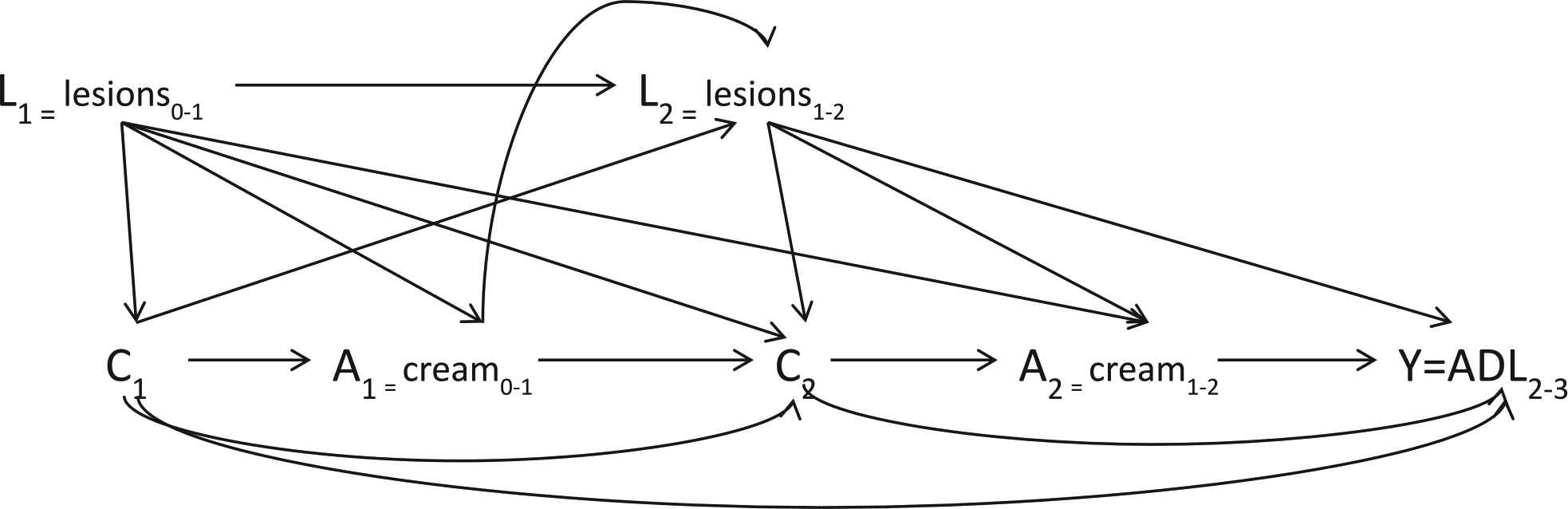
Causal diagram of the effect of antifungal cream use through 2 months on ADL episodes between 2 and 3 months illustrating the relationships between time-varying confounders L, cream treatment A, censoring C, and the outcome of interest Y, ADL between 2 and 3 months.
REFERENCES
Cite this article
TY - JOUR AU - K.E. Mues AU - M. Klein AU - D.G. Kleinbaum AU - W.D. Flanders AU - L.M. Fox PY - 2018 DA - 2018/12/31 TI - The Effect of a Regimen of Antifungal Cream Use on Episodes of Acute Adenolymphangitis (ADL) among Lymphedema Patients: An Application Using Marginal Structural Models JO - Journal of Epidemiology and Global Health SP - 176 EP - 182 VL - 8 IS - 3-4 SN - 2210-6014 UR - https://doi.org/10.2991/j.jegh.2017.10.009 DO - 10.2991/j.jegh.2017.10.009 ID - Mues2018 ER -
