Prevalence and Genetic Analysis of α- and β-Thalassemia and Sickle Cell Anemia in Southwest Iran
- DOI
- 10.2991/j.jegh.2018.04.103How to use a DOI?
- Keywords
- Deletion mutants; Khuzestan; sickle cell anemia; thalassemia
- Abstract
This prospective study assessed the prevalence and genetic analysis of α- and β-thalassemia and sickle cell anemia (SCA) in Southwest Iran. Hematological indices were measured in 17,581 couples living in Khuzestan Province, Southwest Iran. Individuals with mean corpuscular volume <80, mean corpuscular hemoglobin <27, hemoglobin A2 ≥3/5 were considered as β-thalassemia traits. Prevalence of minor β-thalassemia, α-thalassemia, SCA, iron deficiency anemia, and silent thalassemia were respectively identified in 995 (5.6%), 1169 (6.65%), 1240 (7.05%), 911 (5.18%), and 1134 (6.45%) individuals using a multiplex amplification refractory mutation system, and direct DNA sequencing of globin genes. Three codons IVS-II-1 (G → A; 26%; n = 13), IVS-I-1 (G → T; 16%; n = 8), and IVS-I-110 (G → A; 14%; n = 7) were the most frequent mutants and IVS-II-1 was the most common β-thalassemia mutation. Also, based on a gap-polymerase chain reaction assay, genotype frequencies of α-globin mutations were −α3.7 kb (50%; n = 25), Med/ααthal (12%; n = 6), and −α4.2/αα (10%; n = 5), which were the most frequent deletion mutants (72% in total). The most common deletion (50%) was −α3.7 kb. Our data suggest that the population of Southwest Iran is at high risk of α- and β-thalassemia caused by these deletion mutants and SCA. Our findings will be useful for developing an efficient control program and genetic counseling.
- Copyright
- © 2018 Atlantis Press International B.V.
- Open Access
- This is an open access article under the CC BY-NC license (http://creativecommons.org/licences/by-nc/4.0/).
1. INTRODUCTION
Thalassemia is a genetic abnormality involving mutations of the genes responsible for hemoglobin production in the blood. There are two broad types of thalassemia, α- and β-thalassemia; each of which has a different prevalence among certain ethnicities or population groups. Approximately 4.5 of every 10,000 live births throughout the world are affected by thalassemia (http://www.ironhealthalliance.com/disease-states/thalassemia/epidemiology-and-pathophysiology.jsp). α-Thalassemia is more frequent in Southeast Asia than in other areas of the world, and up to 40% of genetic traits have been found in thalassemia traits (TTs) (1–30%). People living in the Mediterranean, African, and South Asian areas are more likely to be affected by β-thalassemia. Genetic prevalence of β-thalassemia throughout the world is 2–18% (affected by a gene mutation) in the Eastern Mediterranean and 0–11% in Southeast Asia [1]. Approximately 5% of the global population have a variation in the α or β part of the hemoglobin molecule, although some of these are asymptomatic and known as silent traits. In fact, only 1.7% of the global population have signs as a result of the gene mutations, known as α-TT. However, tribal or ethnic groups are more likely to be affected and 5–30% of the population may be symptomatic among these groups [1].
Iran has an area of 1,648,000 km2, and like many other countries in the region, has a large number of patients with major thalassemia. α-Thalassemia is infrequent in Iran. Gene frequency of β-thalassemia is high, and it varies considerably from area to area. Its highest rate (>10%) is found around the Caspian Sea and Persian Gulf. Prevalence of β-thalassemia in other areas is between 4% and 8% [2]. In Southern Iran, the IVS-II-1 (G → A) mutation is the most frequent (31%) mutation for β-thalassemia. In Khuzestan Province, in Southern Iran, the frequency of β-thalassemia minor is also high and reaches 10% [2].
According to a study conducted by Zandian et al. [3] in Khuzestan, from 152 volunteer couples (342 individuals) from Arab ethnic groups in Dashte–Azadegan and Khorramshahr cities, 3.63% and 10.57%, respectively, had sickle cell trait; Of these, 84.21% had normal hematological indices [mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH)]. A few studies on genetic diversity of β-thalassemia mutations and sickle cell anemia (SCA) have been conducted in Iran, particularly in Khuzestan Province. Therefore we decided to carry out this cross-sectional and prospective study to assess the genetic diversity and prevalence of α- and β-thalassemia and their relationship with each other, detection of genetic diversity of α- and β-thalassemia minor, and detection of genetic diversity of SCA among 17,581 volunteer couples living in Khuzestan Province, Southwest Iran. Eventually data for the analysis of mutated genes, mutational pattern, as well as their prevalence were submitted to the Research Center of Thalassemia and Hemoglobinopathies, Jundishapur University of Medical Sciences, Ahvaz, Iran. Data were then submitted to Khuzestan Healthcare and Treatment Institute, Khuzestan, Iran to develop a control program in order to reduce the prevalence of thalassemia.
2. MATERIALS AND METHODS
2.1. Study Design
This was a prospective study that was carried out in the Department of Pathology and Thalassemia Control Cell, Shafa Hospital in Ahvaz, the capital city of Khuzestan Province, Southern Iran (Latitude: 31.436015, Longitude: 49.041312) between February 2014 and December 2017. A total of 17,581 prospective couples from Ahvaz (Latitude: 31.3183272, Longitude: 48.67061869999998) and Abadan (Latitude: 30.347296, Longitude: 48.2934) cities of Khuzestan Province were screened for the presence of thalassemia or any structural variant. All participants and their family members with hematological indices were questioned about their medical history. A 5-mL intravenous blood sample was collected in ethylenediamine tetraacetic acid EDTA anticoagulant. Red cell indices were measured on an automated hematology analyzer (Sysmex KX 21; Sysmex Corporation, Kobe, Japan). Hemoglobin (Hb)A2 and HbF were studied by high-performance liquid chromatography (HPLC) used for chromatographic separation of human Hb [4,5].
2.2. Sample Collection and Preparation
Five milliliters of intravenous blood was collected in a vacuum collection tube containing EDTA, which was stored at 3–8°C for a maximum 7 days if processing were delayed. HbA2 calibrators and normal and abnormal controls were analyzed at the beginning of each run.
2.3. Thalassemia Screening
At the first, the men’s red cell indices were checked by complete blood count. If they had microcytosis (MCH < 27 pg or MCV < 80, the women’s red cell indices were tested too. When both were microcytic, their HbA2 concentrations were measured by HPLC (Model D10; Bio-Rad, France, using an ELITech Kit; ELITech Group, Puteaux, France). Tris-glycine buffer was used in order to measure HbA2 by HPLC. Electrophoresis was performed on cellogel at pH 8.5 in Tris-glycine buffer for 90 minutes, as described previously [4,5]. A concentration >3.5% was indicative of β-TT. Microcytic individuals with HbA2 concentration in the normal range (1.5–3.5%) were treated with iron and their indices rechecked. Patients who had TT (MCV < 80 fL, MCH < 27 pg/L, and HbA2 ≥ 3.5%) were examined using multiplex amplification refractory mutation system (M-ARMS) to detected α- and β-thalassemia mutations, direct DNA sequencing of α- and β-globin genes, and gap-polymerase chain reaction (PCR) for globin gene deletions, respectively.
Inclusion criteria for the iron deficiency anemia (IDA) group were Hb <13 g/dL for men and <12 g/dL for women, MCV < 80 fL and MCH < 27 pg for both sexes, and ferritin < 28 ng/mL for men and < 6 ng/mL for women [6,7]. Exclusion criteria for the IDA group included the presence of mutations associated with α-TTs and/or β-TTs. For inclusion in the β-TT group, individuals had MCV < 80 fL, MCH < 27 pg, and HbA2 > 3.5% [4,5]. α-TT was confirmed by the presence of mutations (Fig. 1).
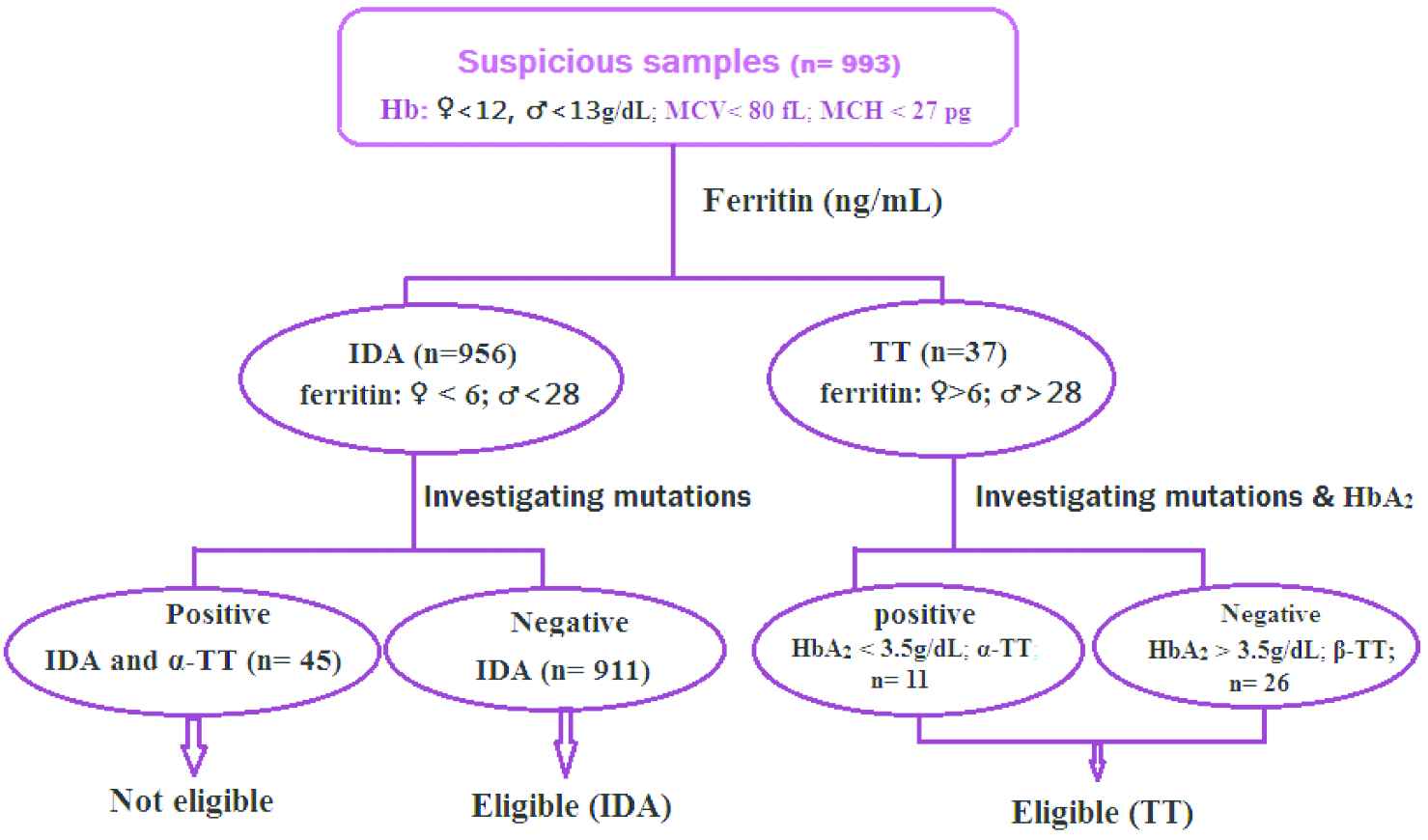
Inclusion and exclusion criteria. Hb, hemoglobin; IDA, iron deficiency anemia; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; TT, thalassemia trait
Silent α/β-thalassemia carriers have no signs or symptoms of the disease, but are able to pass it on to their children. In our study, silent thalassemia carriers showed a normal hematological picture or slight alterations in some hematological parameters (mild changes in erythrocyte morphology, or MCV below the normal mean), and normal Hb status or slight alterations in HbA2 level, while α/β-globin synthesis ratio was abnormal (>1 or <1). Genotype and some hematological parameters in some individuals with silent or α- or β-thalassemia are shown (see Table 1).
| Silent thalassemia | Hb (g/dL) | MCV | HbA2 | α/β ratio | Genotype | |
|---|---|---|---|---|---|---|
| β | α | |||||
| Silent β+ thalassemia (−101 C → T mutation) | 14.2 ± 1.6 | 85 ± 1.2 | 3.3 ± 0.3 | 1.1 ± 0.14 | −101/bA | αα/αα |
| Silent β+ thalassemia (IVS II 844 C → G mutation) | 14.4 ± 1.8 | 86 ± 1.5 | 3.4 ± 0.23 | 1.10 ± 0.03 | AA ♂ CP ♀ |
αα/αα αα/αα |
| Silent α+ thalassemia (−a2Nco I mutation) | 13.6 ± 0.2 | 75 ± 0.2 | 2.3 ± 0.23 | 0.88 ± 0.08 | βA/βA | αNco Iα/αα |
| Silent α+ thalassemia (a2Hph I mutation) | 13.5 ± 1.1 | 78 ± 1.2 | 2.4 ± 0.04 | 0.85 ± 0.12 | βA/βA | αHph Iα/αα |
Data are presented as mean ± SD; Hb, hemoglobin; MCV, mean corpuscular volume.
Distribution of some hematological parameters in subjects with silent α- or β-thalassemia
2.4. Thalassemia Mutation Analysis
Genomic DNA was extracted from peripheral blood lymphocytes using a QIAamp DNA Blood Mini Kit (QIAGEN, Valencia, CA, USA). β-Globin gene mutations were first characterized using an M-ARMS to detect common mutations in Southwest Iranian populations, including IVS-II-1 (G → A), IVS-I-1 (G → T), IVS-I-110 (G → A), CDs 36/37 (−T), IVS-I-5 (G → C), cd5, IVSI-6 (T → C), and cd39 (C + T), as previously described [8]. Uncommon β-thalassemia genes were further characterized by direct DNA sequencing of all coding regions and exon–intron boundaries to detect uncommon point mutations, as described previously [9]. α- and β-Thalassemia mutations were subsequently screened by gap-PCR to detect entire globin gene deletions, as previously described [10–13].
2.5. Gap-PCR
Gap-PCR techniques (amplification using oligoprimers flanking deletion breakpoints) are used to detect different types of globin gene deletions, such as common α-thalassemia deletion mutations and α-gene duplication [11]. A typical gap-PCR test is illustrated for the diagnosis of α-thalassemia, and the primers can be multiplexed [14,15], as shown in Tables 2 and 3. The 3.7- and 4.2-kb α+-thalassemia deletions can be detected in one assay [11,12], and the Mediterranean thalassemia deletion (−MED) in the second assay [12]. Specific primer details are listed in Tables 2 and 3 for the multiplex diagnosis of the common α-thalassemia deletion genotypes.
| Primer | Description | Sequence | Annealing T(°C) |
|---|---|---|---|
| 1 | α2/3.7-F | CCCCTCGCCAAGTCCACCC | 64 |
| 2 | 3.7/20.5-R | AAAGCACTCTAGGGTCCAGCG | 64 |
| 3 | α2-R | AGACCAGGAAGGGCCGGTG | 64 |
| 4 | 4.2-R | CCCGTTGGATCTTCTCATTTCCC | 64 |
| 5 | 4.2-F | GGTTTACCCATGTGGTGCCTC | 64 |
Multiplex gap-PCR protocol for the diagnosis of −α3.7 and −α4.2 deletions
Primer sequences [1]
| Component | μL |
|---|---|
| α2/3.7-F (10 μM) | 1.0 |
| α2-R (10 μ M) | 0.25 |
| α2/20.5-R (10 μ M) | 1.0 |
| 4.2-F (10 μ M) | 1.0 |
| 4.2-R (10 μ M) | 1.5 |
| 10× buffer (750 mM Tris–HCl pH 8.8, 200 mM (NH4)2SO4, 0.1% Tween 20) | 2.5 |
| 25 mM MgCl2 | 1.5 |
| dNTPs (1 mM) | 5.0 |
| Betaine (5 M) | 3.75 |
| DMSO (10%) | 1.25 |
| Platinum Taq (5 U/mL) | 0.1 |
| DNA template (100 ng/mL) | |
| Water |
PCR mix
| PCR fragment size (bp) | Genotype | Product of primers |
|---|---|---|
| 2020 | α+-thalassaemia: −α3.7 | 1 + 2 |
| 1800 | Normal (αα) | 1 + 3 |
| 1628 | α+-thalassaemia: −α4.2 | 4 + 5 |
DMSO, dimethyl sulfoxide; PCR, polymerase chain reaction; T, temperature.
Gel electrophoresis conditions
Run PCR products out on 1.5% (1:1 Nusieve: agarose) gel for 2–3 h
Interpretation of results
Multiplex gap-PCR protocol for the diagnosis of −α3.7 and −α4.2 deletions
| Primer | Name | Sequence | Annealing T (°C) |
|---|---|---|---|
| 1 | MED(F) | CGATGAGAACATAGTGAGCAGAATTGCAGG | 60 |
| 2 | MED(R) | ACGCCGACGTTGCTGCCCAGCTTCTTCCAC | 60 |
| 3 | α2-R | AGACCAGGAAGGGCCGGTG | 64 |
| 4 | 4.2-R | CCCGTTGGATCTTCTCATTTCCC | 64 |
Multiplex gap-PCR protocol for the diagnosis of −MED deletions
Primer sequences [2]
| Component | μL |
|---|---|
| MED(F) (10 μM) | 0.4 |
| MED(R) (10 μM) | 0.4 |
| 10× buffer (750 mM Tris–HCl pH 8.8, 200 mM (NH4)2SO4, 0.1% Tween 20) | 2.5 |
| 25 mM MgCl2 | 1.5 |
| dNTPs (1 mM) | 4.0 |
| Betaine (5 M) | 3.75 |
| DMSO (10%) | 1.25 |
| Platinum Taq (5 U/mL) | 0.1 |
| DNA template (100 ng/mL) | 1.0 |
| Water | 6.2 |
PCR mix
| PCR fragment size (bp) | Genotype | Product of primers |
|---|---|---|
| 1010 | Normal (αα) | 3 + 4 |
| 875 | α+-thalassaemia: −MED | 1 + 2 |
PCR, polymerase chain reaction; T, temperature.
Gel electrophoresis conditions
Run PCR products out on 2% (1:1 Nusieve:agarose) gel for 1–1.5 h
Interpretation of results
Multiplex gap-PCR protocol for the diagnosis of −MED deletions
2.6. Statistical Analysis
Statistical analysis of hematologic indices and the results of molecular tests and mutation types among carrier couples was performed by one-way analysis of variance with SPSS software (SPSS Inc., Chicago, IL, USA).
3. RESULTS
Among all volunteers, 995 (5.6%) had β-thalassemia minor (435 female and 560 male). Of these, 695 cases (69.85%) belonged to the city of Abadan and 300 (30.15%) to Ahvaz. Average value of HbA2 among female and male populations in the city of Abadan was 4.97% and 5.23%, respectively. In Ahvaz, the average value of HbA2 was 4.3% in women and 5.01% in men. The mean hematological indices among TTs are shown in Table 4.
| Blood indices | Type of mutation | p | ||||
|---|---|---|---|---|---|---|
| No mutation | α | β | α and β | |||
| MCV (fL) | Female | 77.60 ± 6.09 | 74.65 ± 5.86 | 62.72 ± 5.13 | 67.5 ± 4.75 | 0.001 |
| Male | 77.14 ± 6.07 | 75.11 ± 6.10 | 64.02 ± 5.99 | 68.39 ± 7.25 | 0.001 | |
| Total | 77.40 ± 6.03 | 75.40 ± 6.04 | 63.64 ± 5.72 | 68.36 ± 6.22 | >0.001 | |
| MCH (pg) | Female | 24.99 ± 2.38 | 23.7 ± 2.32 | 20.01 ± 2.42 | 21.51 ± 1.71 | 0.001 |
| Male | 24.83 ± 2.37 | 24.34 ± 2.36 | 20.56 ± 2.70 | 22.57 ± 2.34 | 0.001 | |
| Total | 24.92 ± 2.36 | 24.3 ± 2.34 | 20.29 ± 2.57 | 22.56 ± 2.04 | >0.001 | |
| RBC | Female | 4.77 ± 0.37 | 5.04 ± 0.14 | 5.43 ± 0.60 | 5.22 ± 0.65 | 0.001 |
| Male | 5.65 ± 0.51 | 5.79 ± 0.47 | 6.29 ± 0.57 | 6.19 ± 0.70 | 0.001 | |
| Total | 5.14 ± 0.61 | 5.40 ± 0.57 | 5.89 ± 0.73 | 5.76 ± 0.82 | >0.001 | |
| HbA2 (%) | Female | 2.57 ± 0.37 | 2.60 ± 0.53 | 4.97 ± 0.89 | 5.39 ± 0.93 | 0.001 |
| Male | 2.91 ± 0.96 | 2.95 ± 1.08 | 5.23 ± 1.23 | 4.87 ± 1.30 | 0.001 | |
| Total | 2.72 ± 0.711 | 2.77 ± 0.86 | 5.11 ± 1.08 | 5.08 ± 1.1 | >0.001 | |
Mean MCH and MCV among β-TTs were less than those of α-thalassemia traits (mean difference: −4.01 and −11.76, respectively, p = 0.001). Mean HbA2 and RBC increased among the TTs (mean difference: 2.36 and 0.62, respectively, p = 0.001). Mean MCH and MCV among α- and β-TTs were less than in individuals with normal indices (mean difference: 2.36 and 9.04, respectively, p = 0.001). Mean MCH, MCV, HbA2, and RBC among α-TTs were not significantly different (p > 0.001). Comparisons are expressed according to one-way analysis of variance; Data are presented as mean ± SD; HbA2, hemoglobin A2; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; RBC, red blood cell; SD, standard deviation; TT, thalassemia trait.
Mean of hematological indices among thalassemia traitsa
In a molecular study of 50 individuals (5%) randomly selected using M-ARMS, eight codons from the β-globin defects accounted for 90% of the total β-thalassemia mutations: IVS-II-1 (G → A; 26%; n = 13), IVS-I-1 (G → T; 16%; n = 8), IVS-I-110 (G → A; 14%; n = 7), CDs 36/37 (−T; 10%; n = 5), IVS-I-5 (G → C; 8%; n = 4), IVSI-6 (T → C; 6%; n = 3), cd5 (6%; n = 3), and cd39 (C + T; 4%; n = 2); the most common mutation was IVS-II-1 (Fig. 1). Prevalence of α-TTs was 6.65% (n = 1169). Also, 50 individuals were tested for α-thalassemia gene mutations based on a gap-PCR assay. Genotype frequencies of α-globin mutations were −α3.7 kb (50%; n = 25), Med/ααthal (12%; n = 6), and −α4.2/αα (10%; n = 5), which were the most frequent deletion mutants (72% in total). The −α3.7 kb deletion was the most common deletion (Fig. 2).
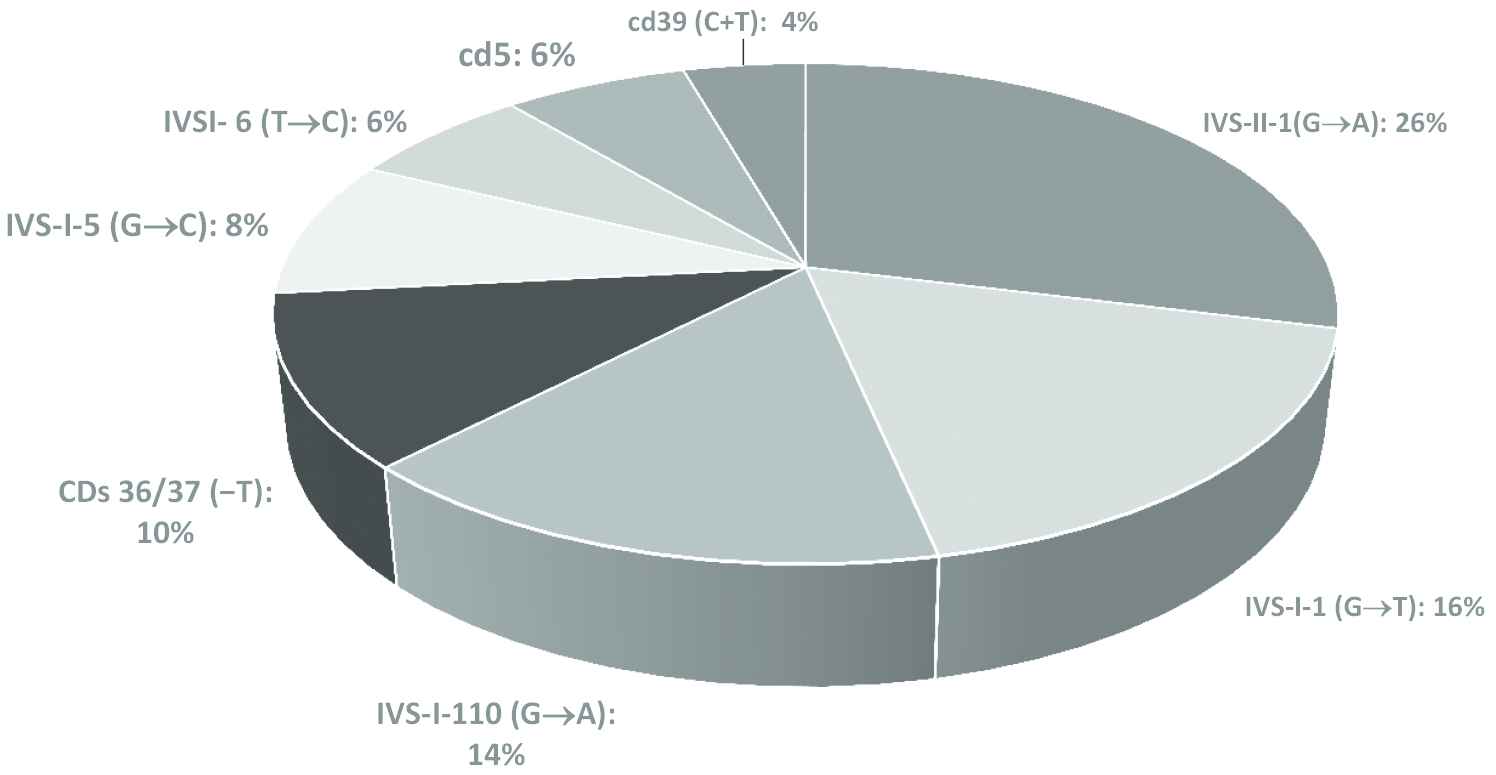
Genotype frequencies of β-thalassemia mutations
Prevalence of SCA, IDA, and silent thalassemia was 1240 (7.05%), 911 (5.18%), and 1134 (6.45%) respectively, as shown in Table 5 and Fig. 2. Distribution of SCA haplotypes among people in Southwest Iran is shown in Table 6. Genotype and hematological characteristics of subjects with silent α- or β-thalassemia are presented in Table 1.
| City | SCA | β-Thalassemia minor | IDA | Silent β+-thalassemia | Silent α+-thalassemia |
|---|---|---|---|---|---|
| Abadan | 888 (76) | 695 (69.8) | 530 (3) | 711 (4) | 112 (0.63) |
| Ahvaz | 280 (24) | 300 (30.2) | 381 (2.16) | 224 (1.27) | 87 (0.49) |
Data are presented as n (%); IDA, iron deficiency anemia; SCA, sickle cell anemia.
Frequency distribution of SCA, β-thalassemia minor, IDA, and silent β- or α-thalassemia among thalassemia carriers in Southwest Iran
| Ethnic groups | Frequency (%) |
|---|---|
| Arab–Indian | 0.37 |
| Benin | 0.17 |
| Bantu | 0.113 |
| Senegal | 0.18 |
Distribution of sickle-cell anemia haplotypes among different ethnic groups in Southwest Iran
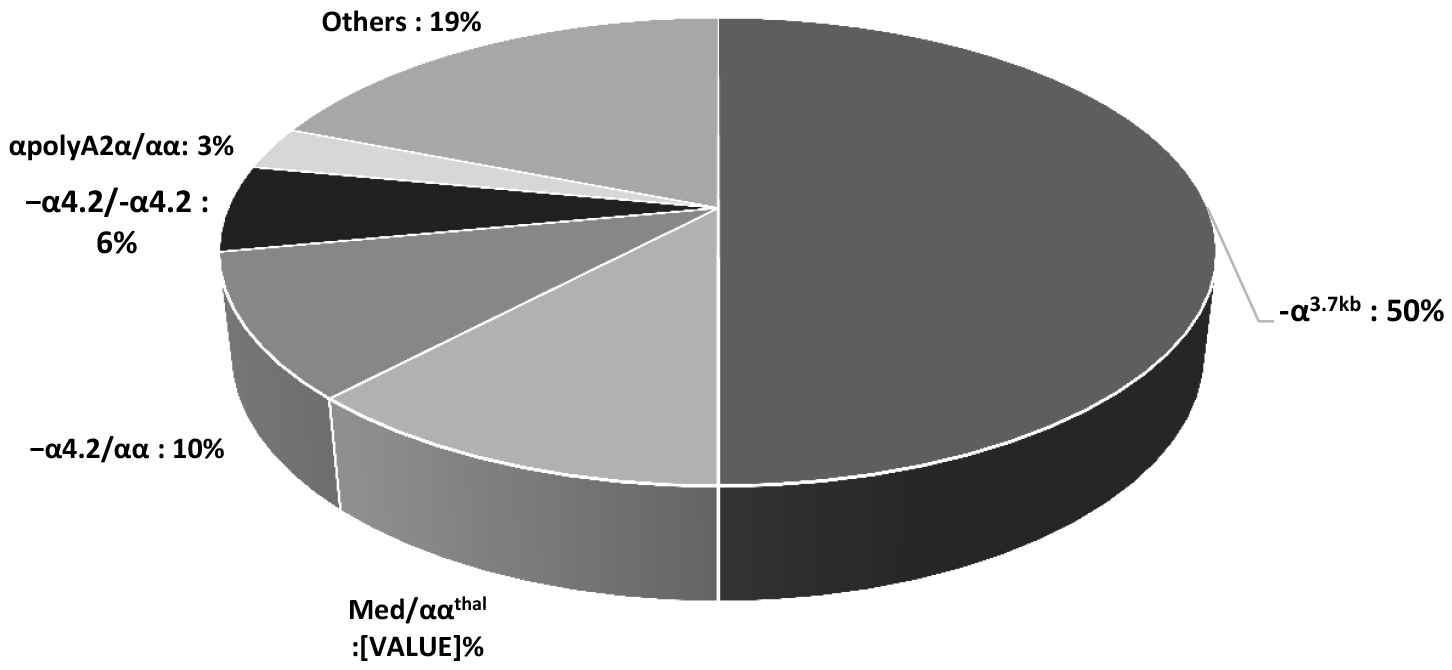
Genotype frequencies of α-thalassemia mutations
4. DISCUSSION
Thalassemia is common in Iran due to a tradition of inbreeding, a conservative religious culture, and a large number of ethnic groups in different areas of the country. Genetic prevention programs based on hospital-based screening and prenatal diagnosis were started in 1997, and Iranian laws were modified between 1998 and 2005 to permit abortion of affected fetuses [16]. Some research on the clinical and laboratory presentations of β-thalassemia syndromes has been conducted in Iran, and unfortunately, no systematic large-scale investigation has been performed to clarify the status of thalassemia in Iran [17,18].
There are numerous gene mutations responsible for β-thalassemia in Iran [19]. These mutations are originally of Iranian, Mediterranean, Turkish, Kurdish, Egyptian, Indian, Asian Indian, Tunisian, Chinese, and Afro-American origin [20]. This notable heterogeneity may be due to Islamic education, which emphasizes fraternity and hospitality, and urges Muslims to accept newcomers with open arms. Iran hosts the largest number of refugees in the world; mostly from Iraq and Afghanistan during the past two decades. This massive ethnic/genetic commixture has led to an unexpectedly high number of different mutations of β-thalassemia in this country. Iran, particularly Khuzestan Province, is a thalassemia hot zone [20].
Yao et al. [21] demonstrated that the Li people in Hainan Province have a high incidence of −α4.2 and −α3.7 thalassemia. Our study showed that Khozestan Province has high genotype frequencies of α-globin mutations, including −α3.7/αα (−α3.7 kb: 50%; n = 25), Med/ααthal (12%; n = 6), and −α4.2/αα (−α4.2 kb; 10%; n = 5), which were the most frequent deletion mutants (72% in total). The −α3.7 kb deletion was the most common α-thalassemia deletion among the α-TTs. This can be explained by the fact that this group of α-thalassemia deletions is common in Asia, where a high prevalence of α+-thalassemia has been observed.
Recent studies have revealed the presence of >47 different β-globin gene mutations responsible for β-thalassemia in Iran. IVS-II-1 (G → A) mutation followed by IVS-I-5 (G → C), codons 8/9 (+G), IVS-I-110 (G → A), IVS-I-1 (G → A), 25-bp deletion, IVS-I-6 (T → C), codon 5 (−CT), and codon 39 (C → T) mutations were the most frequent mutations, which accounted for 85% of the total β-thalassemia defects in Iran [19,22].
Rezaee et al. [23] attempted to study the origin of β-thalassemia mutations in different parts of Iran. They demonstrated that β-thalassemia mutations in different regions have different distribution patterns. These β-thalassemia mutations are indicative of the ancestral origin of the people who migrated to Iran from other regions of the world. In this study [23], in the southwest near the Arabian Peninsula, codons IVS-I-1 (G → A), 8/9 (+G), IVS-I-110 (G → A), IVS-II-1 (G → A), IVS-I-6 (T → C) and IVS-I-5 (G → C) were common mutations that caused 86% of cases of β-thalassemia. Codon IVS-I-1 is the most frequent mutation in Southwest Iran.
Karimi et al. [24] carried out a prospective study demonstrating that the IVS-II-1, IVS-I-110, IVS-I-1, and FSC 8/9 mutations were the most prevalent in the country, and IVS-II-1 was the most frequent in Southern Iran (having the highest rate of 24%).
In our study, the thalassemia syndromes (α- and β-thalassemia mutations, sickle cell disease) were assessed using M-ARMS, direct DNA sequencing of α- and β-globin genes, and gap-PCR among the 17,581 volunteer couples living in Khuzestan Province. Eight codons out of the β-globin defects accounted for 90% of the total β-thalassemia mutations: IVS-II-1 (G → A; 26%; n = 13), IVS-I-1 (G → T; 16%; n = 8), IVS-I-110 (G → A; 14%; n = 7), CDs 36/37 (−T; 10%; n = 5), IVS-I-5 (G → C; 8%; n = 4), IVSI-6 (T → C; 6%; n = 3), cd5 (6%; n = 3), and cd39 (C + T; 4%; n = 2. IVS-II-1 (G → A) mutation was the most frequent (26%) mutation for β-thalassemia in Khuzestan Province. Our study has confirmed the previous studies.
Prevalence of α-TTs was 6.65% (n = 1169) and −α3.7/αα (−α3.7 kb) was the most common α-thalassemia deletion (50%; n = 25), which was consistent with Zandian et al. [25]. It indicates the importance of identification of this gene in couples to prevent the occurrence of hemoglobin H (HbH) disease and hydrops fetalis.
Our study demonstrated the distribution of SCA haplotypes among different ethnic groups in Southwest Iran. The Arab ethnic group has the highest frequency of SCA traits among all ethnic groups, confirming the results of Zandian et al. [26].
5. CONCLUSION
The genetic basis and clinical severity of α- and β-thalassemia are heterogeneous among Iranians due to the presence of multiple ethnic groups in the country. The β-thalassemic IVSII-1 (G → A) mutation had the highest frequency in Southwest Iran. As a Mediterranean mutation, it might reflect its independent origin, genetic admixture, and or gene flow from neighboring countries. A broad spectrum of α-thalassemia alleles has been detected among Iranians and −α3.7 kb was the most prevalent thalassemia mutation. The results of this study could be useful to upgrade the program for prevention of neonatal thalassemia in Iran.
CONFLICTS OF INTEREST
Dr. Forozan H. Nezhad, First author, has received research grants from Ahvaz Jundishapur University of Medical Sciences. Other co-authors report no conflicts of interest relevant to this article.
REFERENCES
Cite this article
TY - JOUR AU - Forozan H. Nezhad AU - Khojasteh H. Nezhad AU - Parastoo M. Choghakabodi AU - Bijan Keikhaei PY - 2018 DA - 2018/12/31 TI - Prevalence and Genetic Analysis of α- and β-Thalassemia and Sickle Cell Anemia in Southwest Iran JO - Journal of Epidemiology and Global Health SP - 189 EP - 195 VL - 8 IS - 3-4 SN - 2210-6014 UR - https://doi.org/10.2991/j.jegh.2018.04.103 DO - 10.2991/j.jegh.2018.04.103 ID - Nezhad2018 ER -
