Low pre-diagnosis attrition but high pre-treatment attrition among patients with MDR-TB: An operational research from Chennai, India
HDS and DN are joint first authors, both contributed equally.
- DOI
- 10.1016/j.jegh.2017.07.001How to use a DOI?
- Keywords
- MDR tuberculosis; Turnaround time; Diagnosis and treatment pathway; Delayed diagnosis; Drug susceptibility testing
- Abstract
Background: Worldwide, there’s concern over high pre-diagnosis and pre-treatment attritions or delays in Multidrug resistant tuberculosis (MDR-TB) diagnosis and treatment pathway (DTP). We conducted this operational research among patients with presumptive MDR-TB in north and central Chennai, India to determine attrition and turnaround times (TAT) at various steps of DTP and factors associated with attrition.
Methods: Study was conducted in Revised National Tuberculosis Control Programme setting. It was a retrospective cohort study involving record review of all patients with presumptive MDR-TB (eligible for DST) in 2014.
Results: Of 628 eligible for DST, 557 (88%) underwent DST and 74 (13%) patients were diagnosed as having MDR-TB. Pre-diagnosis and pre-treatment attrition was 11% (71/628) and 38% (28/74) respectively. TAT [median (IQR)] to test from eligibility for DST and initiate DR-TB treatment from diagnosis were 14 (9,27) and 18 (13,36) days respectively. Patients with smear negative TB and detected in first quarter of 2014 were less likely to undergo DST. Patients in first quarter of 2014 had significantly lower risk of pre-treatment attrition.
Conclusion: There was high uptake of DST. However, urgent attention is required to reduce pre-treatment attrition, improve TAT to test from eligibility for DST and improve DST among patients with smear-negative TB.
- Copyright
- © 2017 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Background
Tuberculosis (TB) is a major public health problem worldwide and the control of TB faces major threat from the increase in multidrug-resistant tuberculosis (MDR-TB). In recent times, access to Drug Susceptibility Testing (DST) for patients with TB has increased [1]. However, gaps in the diagnosis and treatment pathway (DTP) of MDR-TB remain. Worldwide studies have raised concern over high attrition and/or delays in MDR-TB DTP [2–8]. Of the estimated 300,000 MDR-TB among notified TB cases globally, around 123,000 (41%) patients with MDR-TB were diagnosed and of them 90% were initiated on treatment [1].
India with the highest burden of TB and MDR-TB accounts for more than one fifth of the global TB burden [1]. There were an estimated 71,000 MDR-TB cases among the notified TB patients in 2014 and only 25,748 cases were notified giving a case notification rate of 36% [1]. This further drops to 26% if we considered the total estimated incidence of MDR-TB in India (∼99,000 cases per year) [9]. The Revised National Tuberculosis Control Programme (RNTCP) has adopted the Stop TB strategy recommended Programmatic Management of Drug-resistant TB (PMDT) for effective delivery of drug resistant tuberculosis services since 2006 [10].
Prompt identification of presumptive MDR-TB patient (one who is eligible for DST), diagnosis of MDR-TB and initiation of treatment are crucial to prevent the transmission of disease and reduce related high morbidity and mortality [11]. RNTCP has limited cohort-wise information on whether all presumptive MDR-TB patients are identified and investigated for MDR-TB diagnosis as the cohort analysis under RNTCP begins from those who were offered DST. There is also paucity of data regarding the delays and factors causing pre-diagnosis and pre-treatment delay in the MDR-TB DTP. To our knowledge, there are only three published studies from India [3,12,13]. Systematic review of the DTP with representation from various states in India is required. Operational issues are unique and differ from region to region especially in a large country like India. Understanding these will aid programme managers working at national and local level to strengthen PMDT services.
Considering this, we conducted a multi-centre operational research (OR) across districts in India. Here we report the findings related to DTP among patients with presumptive MDR-TB in Chennai, India, for the year 2014. Specific objectives were to determine the i) number (proportion) with pre-diagnosis attrition and pre-treatment attrition ii) turn-around time (TAT) for various steps in DTP (including time to DST and time to initiate treatment) and iii) clinical and demographic factors associated with attrition.
2. Methods
2.1. Study setting
2.1.1. General setting
Chennai is one of the metropolitan cities of India and is the capital of Tamil Nadu state. It has a population of approximately 7 million and is situated along the south-east coast with Bay of Bengal in the east. The study was conducted in North and Central regions of Chennai after consulting the programme managers. (Fig. 1) RNTCP infrastructure includes one District TB Centre (DTC), 26 sub-district level programme management units (Tuberculosis Units - TU) and 52 designated microscopic centres (DMCs) for sputum smear examination. Among 52 DMCs, 7 are located in medical colleges, 7 in district level hospital and 38 in primary/secondary level health centres and one in a private facility.
North and Central Chennai, Tamil Nadu, India.
2.1.2. PMDT services
In Chennai, DST services are provided in the Supra National Reference Laboratory (SNRL) situated at National Institute for Research in TB (NIRT). The diagnostic facility is accredited by the RNTCP for phenotypic (solid/liquid culture and DST) and molecular diagnostic techniques (Line Probe Assay – LPA and Cartridge-Based Nucleic Acid Amplification Test - CBNAAT). LPA is the rapid diagnostic test used in presumptive MDR TB cases in Chennai district. In 2014, if sputum sample was positive, LPA was used upfront. If the sample was sputum negative then culture was done, followed by LPA, if culture positive. The diagnostic facility is situated about 5 kilometres from DTC. MDR-TB treatment is provided at the Government Hospital for Thoracic Medicine, Tambaram (DR-TB centre), which is a tertiary level public health care facility, 30 kilometres from Chennai. Treatment is being provided according to RNTCP PMDT guidelines which are in the line with WHO recommendations (DR-TB centre) [11]. Patients with rifampicin resistance are treated with the standard regimen for MDR-TB. Therefore, MDR-TB in this study includes patients with rifampicin-resistance as well.
Patients with presumptive MDR-TB included all patients with retreatment TB, any TB patient who was smear-positive during follow-up (FUS+), new pulmonary TB patients who were contacts of known MDR-TB patients and all HIV-TB co-infected patients at diagnosis. As per guidelines, patients with presumptive MDR-TB were to be identified at DMCs, samples collected by TB Health Visitor and sent to SNRL along with a request for culture and DST form, a copy of which was maintained at the DMC. DR-TB supervisor at the district level maintained a list of such patients in ‘referral for DST’ register and ensured treatment initiation of patients with laboratory confirmed MDR-TB.
2.2. Study design and study population
It was a retrospective cohort study involving record review of all TB patients diagnosed at DMCs and registered for treatment under RNTCP, in North and Central Chennai and who met the presumptive MDR-TB criteria (DST-eligible patients) between 1 January 2014 and 31 December 2014. This included retreatment patients who were directly referred for DST from the DMC before being registered for TB retreatment regimen.
2.3. Data variables, sources of data and data collection
Data were collected during November 2015 and March 2016. A list of patients eligible for DST was prepared based on the information from the TB treatment register (at TU). For the presumptive MDR-TB criteria ‘FUS+’, we examined the laboratory registers in all DMCs of the district. For the presumptive MDR-TB criterion ‘new pulmonary TB patients who are contacts of known MDR-TB patients’, we contacted DR-TB treatment supervisor and referred to the list maintained by him. Wherever required, DR-TB treatment cards were referred to. Retreatment patients who were directly referred for DST were identified by matching our list with programme list of referred patients to include patients not found in former but found in latter.
Each eligible patient was tracked using TB registration number (if missing, name, age and gender in the ‘referral for culture and DST register’ at DTC and ‘culture and DST register’ at SNRL) and DR-TB treatment register at DR-TB centre. Data for each eligible patient (from eligibility, identification and referral by programme to testing and diagnosis) were reviewed for three months post the date of eligibility for DST. In cases where LPA test was invalid or patient was smear negative, the period of record review was extended for three months. Data for each eligible patient (from referral for treatment and treatment initiation) were reviewed for three months post the date of dispatch of DST result.
Data variables, corresponding sources of data and operational definitions have been summarized in Table 1.
| Variables | Source | Operational definition |
|---|---|---|
| Date of eligibility for DST, presumptive MDR-TB criteria, age in completed years, sex, TB registration number, year of registration, DMC name, baseline smear status | Treatment register/laboratory register at DMC | Under ‘retreatment’ criterion, for patient with smear positive TB, date of smear examination was the date of eligibility. For patients with smear negative TB, date of treatment initiation was the date of eligibility. Under TB/HIV, those with HIV first and TB later, date of eligibility depended on whether the patient was smear positive or negative and we followed the above mentioned definition. For those with TB first and then HIV, date of HIV testing was considered. For patients with known MDR-TB contacts, date of TB registration was considered. Follow up smear positive at 5 months was considered as ‘retreatment’ and included as eligible patient if the date of eligibility under ‘retreatment’ criterion was in 2014. |
| Whether referred for DST, date of referral for DST | Referral for Culture and DST register (DTC) or copy of request for Culture and DST form (DMC) | If there was a record for referral maintained at DTC or DMC then it was considered as ‘identified/referred’. In case of discrepancy in dates, earlier date was considered. |
| Sample received at SNRL, date of sputum received at SNRL, whether DST was performed, type of DST, date of DST, DST result, date of DST result, date of dispatch of DST result to DTC | Culture and DST register at SNRL | Eligible patients were tracked through their TB registration numbers; in cases where it was not entered, name and address of the patient was used. If SNRL DST register showed ‘contaminated’ as the result, and no further sample was received then it was recorded as ‘sample received; DST not done’. |
| Whether patient referred to DRTB centre from DTC, date of referral to DRTB centre for treatment | Referral for Culture and DST register (DTC) or copy of referral for treatment form (DMC/DTC) | – |
| Whether treatment initiated, DR-TB treatment. date of treatment initiation | PMDT Treatment register at DRTBcentre | – |
MDR-TB: Multi drug-resistant tuberculosis, DMC: Designated microscopy centre, DST: Drug susceptibility testing, SNRL: Supranational reference laboratory, DTC: District tuberculosis centre, DRTB: Drug-resistant tuberculosis.
Source of data collection and operational definition of variables collected for patients with presumptive/confirmed MDR-TB, north and central Chennai, India (2014).
2.4. Data management and statistical analysis
Data collected in a structured form were double entered, validated and analyzed using EpiData (version 3.1 for entry and version 2.2.2.183 for analysis, EpiData Association, Odense, Denmark). Real time data capture was enabled through data entry in a shared dropbox folder (www.dropbox.com) [14]. The Union South-East Asia Office coordinated this process. Key analytic outputs were number (proportion) of patients with presumptive MDR-TB at each step of DTP (Fig. 2); Turnaround Time (TAT) in days for each step; and association between not getting tested, not getting initiated on treatment and various clinical and demographic factors. Frequency, proportion, median, inter-quartile range, and relative risks (0.95 CI) were used to summarize and infer the analytic outputs.
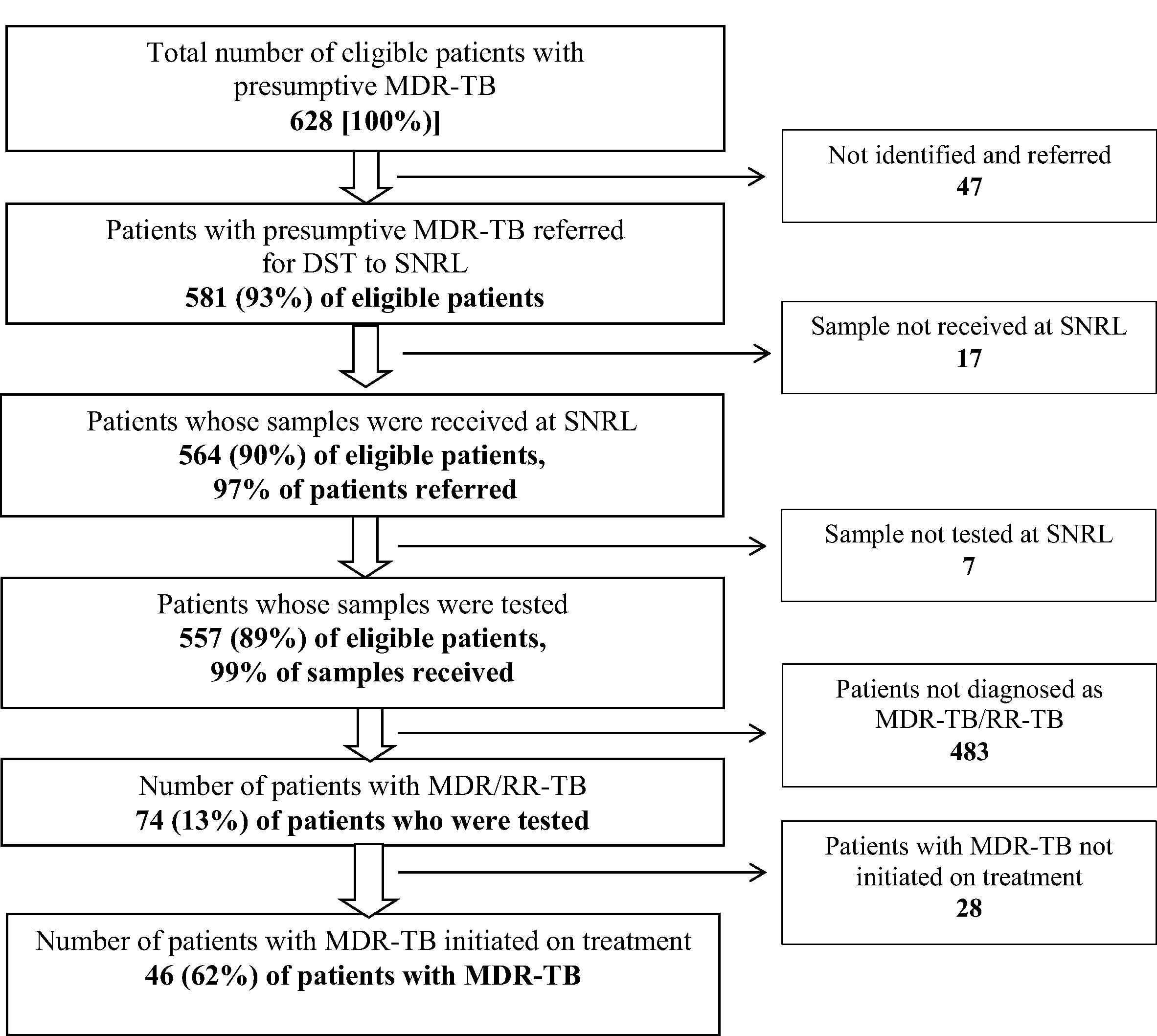
Flow of patients with presumptive/confirmed MDR-TB in the diagnosis and treatment pathway, North and Central Chennai, India (2014). MDR-TB: Multi drug-resistant tuberculosis; DST: Drug susceptibility testing, SNRL: Supra national reference laboratory.
2.5. Ethics
Ethics approval was obtained from the Ethics Advisory Group of The Union, Paris, France (EAG No: 54/15) and Institutional Ethics Committee, NIRT, Chennai, India (No: 090/NIRT-IEC/2016). Permission and support for the OR was sought from the State RNTCP programme managers and other relevant authorities before initiating the OR. As the OR involved retrospective review of RNTCP records, waiver for informed consent was sought and approved by the ethics committees.
3. Results
There were 628 patients eligible for DST: mean (SD) age was 44 (13) years and 520 (83%) were males. Nearly three-fourths of these patients were from DMCs located in primary/secondary level health facilities. Criteria for eligibility for DST was ‘retreatment TB’ in 497 (79%) cases (Table 2).
| Variable | Patient with presumptive MDR-TB | |
|---|---|---|
| Number | Percentage | |
| Total | 628 | 100 |
| Age (years) | ||
| • <14 | 3 | 1 |
| • 14–44 | 305 | 49 |
| • 45–64 | 293 | 47 |
| • >/= 65 | 27 | 4 |
| Gender | ||
| • Male | 520 | 83 |
| • Female | 108 | 17 |
| Health facility | ||
| • Primary/Secondary level | 459 | 73 |
| • District level | 151 | 24 |
| • Medical college/Others | 18 | 3 |
| Presumptive MDR-TB criteria | ||
| • Retreatment | 497 | 79 |
| ○ Relapse | 198 | 32 |
| ○ Loss to follow up | 215 | 34 |
| ○ Failure | 29 | 5 |
| ○ Others | 55 | 9 |
| • Follow up smear + | 73 | 12 |
| • New patient with TB/HIV | 58 | 9 |
| • New pulmonary TB with known MDR-TB contact | 0 | 0 |
| Site of Tuberculosis | ||
| • Extra pulmonary | 32 | 5 |
| • Pulmonary-smear negative | 77 | 12 |
| • Pulmonary-smear positive | 512 | 82 |
| • Pulmonary-smear missing | 7 | 1 |
| Quarter (Q) | ||
| • Q1 – 2014 | 179 | 29 |
| • Q2 – 2014 | 186 | 30 |
| • Q3 – 2014 | 142 | 23 |
| • Q4 – 2014 | 121 | 19 |
MDR-TB: Multi drug-resistant tuberculosis, TB: Tuberculosis, HIV: Human Immunodeficiency Virus.
Clinical and demographic profile of patients with presumptive MDR-TB, North and Central Chennai, India (2014).
Of the eligible, 93% (581/628) were identified and referred by the programme. Of the referred, 97% (564/581) reached SNRL and 99% (557/564) of them got tested. Pre-diagnosis attrition was 11% (71/628). Non referral from the peripheral health facilities to the DST facility contributed to 66% (47/71) of the pre-diagnosis attrition. (Fig. 2) TAT for various steps from eligibility to diagnosis has been summarized in Table 3. TAT [median (IQR)] to refer from eligibility and to get tested from date of eligibility were 7 (4, 17) and 14 (9, 27) days.
| Variable | Number of Patients^ | Days Median (IQR) |
|---|---|---|
| Patients with presumptive MDR-TB | 628 | – |
| Days to refer from date of eligibility** | 558 | 7 (4, 17) |
| Days to receive sample at SNRL from referral | 560 | 0 (0, 0) |
| Days to test at SNRL from sample receipt | 550 | 5 (3, 7) |
| Days to dispatch result from SNRL from testing | 550 | 3 (1, 6) |
| Days to initiate DR-TB treatment from result dispatch | 43 | 16 (9,34) |
| Days to test at SNRL from date of eligibility | 551 | 14 (9, 27) |
| Days to initiate DR-TB treatment from testing | 41 | 18 (13, 36) |
| Days to initiate DR-TB treatment from date of eligibility** | 24 | 38 (28, 72) |
MDR-TB: Multi drug-resistant tuberculosis, DMC: Designated microscopy centre, SNRL: Supranational reference laboratory, DRTB: Drug-resistant tuberculosis centre.
Includes patients who completed the respective process and whose respective dates were available.
date of eligibility for drug susceptibility for testing.
Turnaround time for various steps in diagnosis and treatment pathway of patients with presumptive / confirmed MDR-TB, North and Central Chennai, India (2014).
Among 534 patients tested, 74 (13%) had MDR-TB. Pre-treatment attrition was 38% (28/74). (Fig. 2) TAT [median (IQR)] to initiate treatment from diagnosis was 18 (13, 36) days (Table 3).
Factors and their association with not getting DST are shown in Table 4. Patients with extra pulmonary TB, smear negative pulmonary TB and those identified in first quarter of 2014 had significantly higher risk of not getting tested. Factors and their association with pre-treatment attrition are shown in Table 5. Patients in first quarter of 2014 had significantly lower risk of pre-treatment attrition.
| Variable | Total [N] | Not tested for DST [n (%)] | RR (0.95 CI) |
|---|---|---|---|
| Total | 628 | 74 (12) | |
| Age (years) | |||
| • <14 | 3 | 1 (33) | 3.6 (0.7, 18.7) |
| • 14–44 | 305 | 41 (13) | 1.5 (0.9, 2.3) |
| • 45–64 | 293 | 27 (9) | Ref |
| • >/= 65 | 27 | 5 (19) | 2.0 (0.8, 4.8) |
| Gender | |||
| • Male | 520 | 57 (11) | Ref |
| • Female | 108 | 17 (16) | 1.4 (0.9, 2.4) |
| Health facility | |||
| • Primary/Secondary level | 459 | 47 (10) | Ref |
| • District level | 151 | 23 (15) | 1.5 (0.9, 2.4) |
| • Medical college/others | 18 | 4 (22) | 2.2 (0.9, 5.4) |
| Presumptive MDR-TB criteria | |||
| • Retreatment-relapse | 198 | 23 (12) | 1.2 (0.7, 2.2) |
| • Retreatment-loss to follow up | 215 | 20 (9) | Ref |
| • Retreatment-failure | 29 | 2 (7) | 0.7 (0.2, 3.0) |
| • Retreatment-others | 55 | 14 (26) | 2.7 (1.5, 5.1)* |
| • Follow up smear + | 73 | 7 (10) | 1.0 (0.5, 2.3) |
| • New patient with TB/HIV | 58 | 8 (14) | 1.5 (0.7, 3.2) |
| Site of Tuberculosis | |||
| • Extra pulmonary | 32 | 9 (28) | 2.9 (1.6, 5.4)* |
| • Pulmonary-smear negative | 77 | 16 (21) | 2.2 (1.3, 3.6)* |
| • Pulmonary-smear positive | 512 | 49 (10) | Ref |
| • Pulmonary-smear missing | 7 | 0 (0) | – |
| Quarter | |||
| • Jan – Mar 2014 | 179 | 32 (18) | 2.4 (1.3, 4.3)* |
| • Apr – Jun 2014 | 186 | 14 (8) | Ref |
| • Jul – Sep 2014 | 142 | 11 (8) | 1.0 (0.5, 2.2) |
| • Oct – Dec 2014 | 121 | 17 (14) | 1.9 (0.96, 3.7) |
MDR-TB: Multi drug-resistant tuberculosis, DST: Drug susceptibility testing.
p < 0.05.
Clinical and socio-demographic factors and their association with not getting DST among patients with presumptive MDR-TB, North and Central Chennai, India (2014).
| Variable | Total [N] | Not initiated on treatment [n (%)] | RR (0.95 CI) |
|---|---|---|---|
| Total | 74 | 28 (38) | |
| Age (years) | |||
| • <14 | 2 | 0 (0) | – |
| • 14–44 | 38 | 17 (54) | 1.3 (0.7, 2.4) |
| • 45–64 | 33 | 11 (33) | Ref |
| • >/= 65 | 1 | 0 (0) | – |
| Gender | |||
| • Male | 57 | 23 (40) | 1.4 (0.6, 3.1) |
| • Female | 17 | 5 (29) | Ref |
| Health facility | |||
| • Primary/Secondary level | 59 | 23 (39) | 1.2 (0.5, 2.6) |
| • District level | 15 | 5 (33) | Ref |
| • Medical college/others | 0 | 0 (0) | – |
| Presumptive MDR-TB criteria | |||
| • Retreatment | 61 | 24 (39) | 2.0 (0.5, 7.1) |
| • Follow up smear + | 10 | 2 (20) | Ref |
| • New patient with TB/HIV | 3 | 2 (67) | – |
| Site of Tuberculosis | |||
| • Extra pulmonary | 4 | (75) | – |
| • Pulmonary-smear negative | 5 | (40) | – |
| • Pulmonary-smear positive | 62 | 23 (37) | Ref |
| • Pulmonary-smear missing | 3 | 0 (0) | – |
| Quarter | |||
| • Jan – Mar 2014 | 29 | 1 (3) | Ref |
| • Apr – Jun 2014 | 12 | 6 (50) | 14.5 (1.9, 107.9)* |
| • Jul – Sep 2014 | 22 | 14 (64) | 18.5 (2.6, 129.9)* |
| • Oct – Dec 2014 | 11 | 7 (64) | 18.5 (2.6, 133.3)* |
MDR-TB: Multi drug-resistant tuberculosis, DST: Drug susceptibility testing.
p < 0.05.
Clinical and socio-demographic factors and their association with pre-treatment attrition among patients with MDR-TB, North and Central Chennai, India (2014).
4. Discussion
4.1. Summary of key findings
In Chennai, India, the programme tested most of the patients eligible for DST. However, one-third of the diagnosed MDR-TB patients were not started on treatment. TAT to test from eligibility for DST and testing among patients with smear negative TB needs special attention.
4.2. Interpretation of key findings
Around 7% of eligible patients were not identified and referred for testing. This was lower than 10% in Puducherry and 50% in Bhopal, India [12,15]. This aspect has been missed in many studies previously where they started the cohort with programme identified/referred patients with MDR-TB [2,3,5,6].
Around 90% patients with presumptive MDR-TB got tested. This is commendable when compared to 40–74% reported in previous reports from India [3,12,13,15] and 10% in Tanzania, 39% in China, 40% in Malawi, 50% in Cambodia and 79% in Sri Lanka [2,4–6,8]. This could be partly attributed to improved patient referral/sample transport system and partly to availability of rapid diagnostics like LPA. Since non-referral of the eligible patients contributed to two-thirds of pre-diagnosis attrition and half of the overall delay in testing was contributed by delays in identification and referral, this can be easily addressed by refresher training of health workers. There was some attrition from referral to sample receipt at SNRL, which can be addressed by improved mechanisms of sample transport and enhanced tracking using culture and DST register.
Pre-diagnosis attrition was higher among patients with smear negative pulmonary TB and extra pulmonary TB. The former was not explored as a risk factor in previous studies from India [12,15]. With regards to extra-pulmonary TB, there was lack of clarity in the national PMDT guidelines during study period regarding specimens to be collected and the methods for storage and processing before sending to the laboratory [11]. The recently released RNTCP technical and operational guidelines for TB (2016) have clarified this point [16]. Pre-diagnosis attrition, similar to Bhopal, was highest in quarter I of 2014 which improved with time, though there was a marginal rise in the fourth quarter [15]. Reasons for these differences are not known.
DMCs in primary/secondary level facilities made significant contribution (around three fourths) to the cohort of patients with presumptive MDR-TB. (Table 2) These facilities served as a major referral point for patients eligible for DST which is expected as per programme [11]. In contrast, this was only 17% and 47% in Puducherry and Bhopal, India [12,15].
Around 62% of patients with MDR-TB were put on DR-TB treatment and time to treatment after diagnosis was relatively better than previous reports [12]. Reasons for low pre-treatment attrition in first quarter of 2014 were not known. We did not have sufficient number of patients in many sub-group to study factors associated with pre-treatment attrition.
4.3. Implications for policy and practice
There is a call for universal access to DST and appropriate treatment in the post-2015 End TB Strategy [17]. India should aim to make DST accessible to all notified TB patients in coming few years [18]. This requires strengthening of laboratories and accelerated uptake of rapid diagnostics like LPA and Xpert MTB/Rif, as well as use of information and communication technology to improve completeness of reporting including to RR/MDR TB cases notified from the private sector [1,17]. A study conducted by Central TB Division revealed that universal DST using Xpert MTB/Rif, up front to all patients with presumptive TB increased MDR-TB case notification five folds [19].
Chennai with high uptake for DST among patients with presumptive MDR-TB is an ideal site for implementation of universal access to DST. Central TB Division also plans to implement DST-guided treatment for mono and poly resistance (non MDR resistance) for all presumptive MDR-TB in select districts in a phased manner [20]. The pre-treatment attrition might be due to death, refusal to take treatment, treatment in private sector, inability to trace the patient at the given address etc. A systematic qualitative enquiry is required to understand i) what RNTCP in Chennai is doing to ensure high levels of testing; ii) reasons for delays in referral and for not reaching SNRL after referral; and iii) the reasons for non-initiation of treatment from patients’ perspective. This would help programme managers in other parts of the country to learn and implement corrective measures. Addressing non-retrieval of patients would facilitate initiation of treatment and thus the reduction in transmission of MDR-TB.
4.4. Strengths and limitations
The study had several strengths. This was an operational research study under the programme conditions using programme staff. Methodology used was robust with pre-defined operational definitions and a clear and uniform follow-up period defined for record review. Data was quality assured and robust as double data entry and validation was done. Since we studied the entire population of patients with presumptive MDR-TB in north and central Chennai without any sampling, the results are likely to be representative and reflect realities on the ground and have implication for policy. STROBE guidelines were followed for the conduct and reporting of this OR [21].
There were no patients under the criterion “patients with close contact of known MDR-TB”. Currently, this information is not systematically captured in any of the programme records. Since our study relied on record reviews, it was challenging to obtain this information. Barriers related to access including distance of patient’s residence to DMC, SNRL and DR-TB centre and travel costs were not collected as this information is not routinely collected and thus was beyond the scope of this OR. There are inherent limitations of a record review study, although records in RNTCP are monitored and supervised which includes periodic data validation.
5. Conclusions
This OR assessed the gaps and operational challenges in DTP of patients with presumptive/confirmed MDR-TB from eligibility for DST to treatment initiation. Uptake for DST is high and Chennai RNTCP may now aim for universal DST for all notified TB patients and invest in interventions for prevention of emergence of DR-TB. These are crucial if we are to attain the target of ending the epidemic of TB by 2030 in line with the recently launched Sustainable Development Goals [22]. The programme need to intensify monitoring and undertake evidence based course corrections to address issues related to non-initiation of treatment among MDR-TB patients.
Abbreviations
- DR-TB
drug resistant tuberculosis;
- MDR-TB
multidrug resistant tuberculosis;
- DST
drug susceptibility testing;
- DTP
diagnosis and treatment pathway;
- RNTCP
revised national tuberculosis programme;
- PMDT
programmatic management of drug resistant tuberculosis;
- OR
operational research;
- TAT
turn-around time;
- DTC
district tuberculosis centre;
- TU
tuberculosis unit;
- DMC
designated microscopy centre;
- SNRL
supranational reference laboratory;
- LPA
line probe assay;
- FUS+
follow up smear positive;
- IQR
interquartile range;
- RR
relative risk;
- CI
confidence interval;
- DOTS
directly observed treatment short course.
Funding
The study was conducted as an operational research under the programme conditions using programme staff. Therefore, no separate budget was required.
Availability of data and materials
The dataset(s) supporting the conclusions of this article are available on request from the corresponding author.
Authors’ contribution
HDS was the principal investigator; DN was the site principal investigator; AMVK was the senior author; HDS, MP, VG, JPT, and AMVK conceived and designed the protocol; HDS developed the data collection tool and plan of analysis; HDS, DN, JSK, LJ and LM collected and entered the data; HDS, DN, JSK and SS analysed and interpreted the data; HDS prepared the first draft; all authors were involved in critically reviewing the paper and giving approval for the final version to be published.
Competing interests
The authors declare that they have no competing interests.
References
Cite this article
TY - JOUR AU - Hemant Deepak Shewade AU - Dina Nair AU - Joel S. Klinton AU - Malik Parmar AU - J. Lavanya AU - Lakshmi Murali AU - Vivek Gupta AU - Jaya Prasad Tripathy AU - Soumya Swaminathan AU - Ajay M.V. Kumar PY - 2017 DA - 2017/07/06 TI - Low pre-diagnosis attrition but high pre-treatment attrition among patients with MDR-TB: An operational research from Chennai, India JO - Journal of Epidemiology and Global Health SP - 227 EP - 233 VL - 7 IS - 4 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2017.07.001 DO - 10.1016/j.jegh.2017.07.001 ID - Shewade2017 ER -
