Vitamin E attenuates alcohol-induced aortic wall damage in rats
- DOI
- 10.1016/j.artres.2015.01.003How to use a DOI?
- Keywords
- Ethanol; Rat; Oxidative stress; Vascular; Vitamin E
- Abstract
Background: In this study the effect of chronic ethanol consumption on vascular wall abnormality via oxidative stress was examined. It was also intended to find out whether vitamin E inhibits the abnormality induced by ethanol in rat vascular wall.
Methods: Twenty-four male wistar rats were divided into three groups, namely, control, ethanol (4.5 g/kgBW intragastrically), and vitamin E treated ethanolic groups(VETE) (300 mg interagastrically).
Results: After 6 weeks treatment of rats, the results revealed that along with a significant increase VSMC proliferation and aorta wall thickness with the increase in the level of Ox-LDL, protein carbonyl, as well as decrease total antioxidant capacity in animal that received ethanol compared to the control group. Significant amelioration of aorta wall changes, along restoration of the elevated level of Ox-LDL, protein carbonyl, lipid profile, and decreased level of total antioxidant capacity to that of controls were found in vitamin E-treated animals.
Conclusions: These findings strongly support the idea that heavy and chronic ethanol consumption initiate atherosclerosis by oxidative stress, and that these effects can be alleviated by vitamin E as an antioxidant.
- Copyright
- © 2015 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The association between chronic ethanol consumption and the risk of cardiovascular morbidity is intricate. On one hand, it has been reported that light-to-moderate ethanol consumption reduces cardiovascular mortality as well as the prevalence of ischemic stroke.1 In addition, it has been reported that light-to-moderate ethanol consumption has also been implicated with lower incidence of heart attacks and angina pectoris.2,3 It has also been shown that light-to-moderate ethanol consumption inhibits the synthesis of prostacyclin, aggregation of platelet, endogenous plasminogen activator.4 Thus, the protective effect of moderate alcohol intake is largely due to with improved homeostasis and attenuated thrombosis.
Long-term alcohol consumption, on the other hand, has been linked with adverse vascular effects.5 A growing body of evidence suggest that long term ethanol consumption impairs antioxidant balance through oxidative modification of biomolecules such as polyunsaturated fatty acid, proteins and DNA.6,7
We have recently demonstrated that long term ethanol administration (4.5 g/kg; 20% w/v in saline) once a day for six weeks induces the development of pro-inflammatory and pro-atherosclerotic processes including increased blood pressure, serum levels of C-reactive protein(CRP), intercellular adhesion molecule-1, endothelial leukocyte adhesion molecule-1 (E-selectin), and the appearance of infiltrated monocytes in the aorta, as well as foam-cell formation in early plaques.6 Based on the above-mentioned observations, we propose that the atherogenic effect of heavy long ethanol consumption is entirely or partially mediated by oxidative stress. To test this hypothesis, we attempted to investigate the role of oxidative stress in aorta VSMC proliferation in ethanol treated rats as well as the protective effect of vitamin E supplementation.
Materials and methods
All experiments were performed between 12:00 h and 17: 00 h. All research and animal care procedures were approved by the Ethics Committee of the Faculty of Medicine of Urmia Medical Science University and were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. The rats were housed in clean polyprophylene cages, maintained in temperature-controlled room (22 ± 0.5 °C) with a photoperiod of 12 h light and 12h dark cycle. Twenty-four male Wistar rats (6 months old, 240 ± 10 g) were randomly assigned to 3 groups (N = 8 for each group) including control group ©, ethanol (ET) group, and ethanol vitamin E treatment (VETE) group. Ethanol (Merck KGaA, Darmstadt, Germany) was intragastrically administered by gavage at 4.5 g/kg BW in normal saline (20%w/v) (once per day; for 6 weeks). According to our preliminary studies, rats in the VETE group received a non-toxic dose of 300 mg of vitamin E (Merck GmbH, Germany) intragastrically by gavage in addition to their regular daily diet and the same amount of ethanol. C group received tap water only. Food and water were supplied ad libitum throughout the experiment. After 6 weeks, the animals were intraperitoneally anesthetized using ethyl carbamate (1 g/kg). The thoracic cavities were opened and blood samples were collected from the heart by syringe. Ethylenediaminetetraacetic acid (EDTA) was added as an anticoagulant. The samples centrifuged at 4000 × g for 20 min within 30 min of collection. The plasma was transferred into Eppendorf tubes and stored at −80 °C. After blood collections, abdomens were opened. Aortic tissues were dissected from the root to the abdominal descending part. For the purpose of histopathology investigations, a section of the aorta was fixed in buffered formalin and it was embedded in paraffin after standard dehydration steps.
For biochemical analysis, aorta tissue samples were washed with ice-cold physiological saline and then dried on filter papers. Ice-cold extraction buffer (10% wt/vol), containing a 50 mM phosphate buffer (pH 7.4) were added and subsequently homogenized using Ultra Turrax (T10B, IKA, Germany). The homogenates were then centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant sample was collected and stored at −80 °C until the time of analysis.
Ox-LDL measurement: The levels were measured using a capture ELISA coated with the capture antibody mAb-4E6 (Mercodia, Sweden). Diluted supernatant samples (1:6561) were used for the ELISA measurements. The optical density of the wells was read at 450 nm.
Lipid profile: Serum triglyceride (TG) and total cholesterol were measured by colorimetric and enzymatic methods. Serum LDL-C and HDL-C were assayed by direct methods (Biosystem, Barcelona, Spain).
Homocysteine (Hcy): The analysis was carried out by high-pressure liquid chromatography method (Recipe Chemical and Instruments GmbH, Munich, Germany), according to the manufacturer’s instructions.
Protein carbonyl: Protein carbonyl content was measured using protein carbonyl assay kit (Cayman chemical, Ann Arbor, USA) according to the manufacturer’s instructions.
Total protein: Protein concentration was determined by the method of Bradford using bovine serum albumin as the standard.
Proliferating cell nuclear antigen (PCNA) staining: After tissue processing steps, the monoclonal anti-PCNA antibody (Dako Denmark A/S, Denmark) was used to stain the slides after the appropriate Ag retrieval step, and optimal results were achieved with the EnVision™ visualization system (Dako Denmark A/S, Denmark). PCNA-positive indices were considered as indicators of proliferation of muscle cells. Scoring was performed in the following fashion:
One hundred cells were scored from each tissue section for assessing the percentage of PCNA-positive indices. The criteria for quality scoring of PCNA-positive indices were as follows: normal, PCNA-positive indices less than 5%; mild, PCNA-positive indices present in less than 25% of muscle cells; mild to moderate, PCNA-positive indices present in 25%–50% of muscle cells; moderate to severe, PCNA-positive indices present in 50%–75% of muscle cells; severe, PCNA-positive indices present in 75%–100% of muscle cells. The sections were examined under light microscope, and photomicrographs were taken.
Histopathologicalogical examinations: After tissue processing steps, the presence of elastin was evaluated by verhoff-van Gieson’s and Periodic Acid-Schiff (PAS) staining. The sections were examined with a digital camera equipped research microscope (Olympus, CX 31, Japan). Histological parameters were examined under 400 × magnification and as it shown in Fig. 1, quantification of microscopic components such as thickness of the total aorta, tunica media, tunica adventitia, smooth muscle cell (SMC) layers width and elastic fiber thickness (EFT) were carried out using a linear scale-ocular micrometer providing a 2.5 μm interval between divisions under 400 × magnification. Eight sections of the aorta from different groups were assessed and the results were expressed as mean ± SE. The following equations were used to calculate the different parameters:
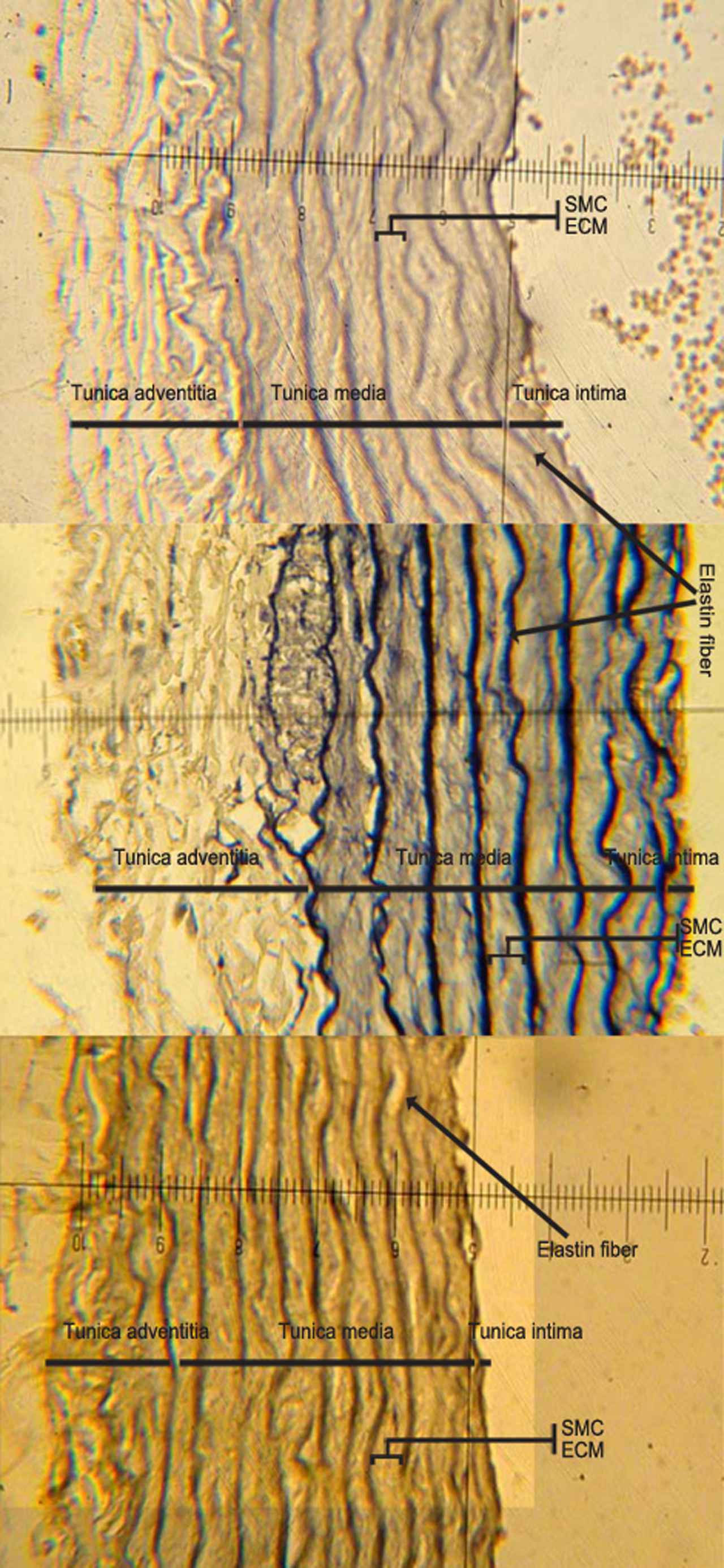
Morphological changes which are shown in the aorta of different groups. Abbreviations: SMC, smooth muscle cell; ECM, exteracellular matrix. (Magnification ×400).
Total aorta thickness = Tunica media thickness + Tunica adventitia + Tunica intima.
Total thickness of Media (μm) = ∑elastic fiber thickness (EFT) + ∑ smooth muscle cell layer (SMC), Entire elastin thickness (μm) = ∑elastic fiber thickness (EFT), Entire SMC thickness (μm) = ∑smooth muscle cell layers (SMC), Fiber interval (μm) = distance between two consecutive elastic fiber.
Statistical analysis
Statistical differences between the groups were tested by one-way ANOVA SPSS 16 software, followed by Tukey’s post hoc test. In each test, the data are expressed as the mean ± S.D, and p < 0.05 was considered statistically significant.
Results
The levels of LDL, TG, as well as cholesterol showed a significant increase in the ethanol group as compared to the control group (p = 0.05). However, there were no significant differences between VETE rats and the control rats (P = 0.8). The levels of serum HDL and the ratio of HDL/TG remained unchanged in all the experimental groups after 6 weeks of treatment. (Table 1).
| Control | Ethanol | VETE | |
|---|---|---|---|
| Triglyceride(mg/dl) | 32.85 ± 1.42 | 53.57 ± 3.6a | 35.71 ± 1.4b |
| Cholesterol(mg/dl) | 48.85 ± 1.98 | 64.71 ± 2.11a | 50.14 ± 0.55b |
| LDL(mg/dl) | 27.71 ± 1.47 | 34.85 ± 2.13a | 19 ± 0.81a,b |
| HDL(mg/dl) | 31.57 ± 1.68 | 32.57 ± 1.5 | 30 ± 0.92 |
| HDL/T.G | 0.9 ± 0.07 | 0.79 ± 0.07 | 0.84 ± 0.02 |
| Hcy(mg/l) | 4.28 ± 0.1 | 6.1 ± 0.4a | 4.92 ± 0.24b |
| Ox-LDL(1U/I) | 32.71 ± 0.91 | 41.85 ± 1.8a | 34.57 ± 1.81b |
| Protein carbonyl(nmol/mg pr) | 1 ± 0.078 | 7.73 ± 0.65a | 4.55 ± 0.44a,b |
| Total antioxidant capacity(mM) | 0.061 ± 0.0019 | 0.0361 ± 0.0035a | 0.066 ± 0.006b |
| Total protein(mg/ml) | 7.78 ± 0.17 | 5.95 ± 0.19a | 6.12 ± 0.34 |
Values expressed as mean ± SEM.
Significant difference compared to the control.
Denotes significant difference (p < 0.05) compared to the ethanol group.
Lipids and oxidative stress parameters in different groups of rats.
Aorta tissue Hcy concentrations in the ethanol-treated rats were significantly higher than in controls (p = 0.005). In addition, administration of vitamin E decreased the elevated levels compared with those exhibited by the control group (p = 0.2) (Table 1).
As shown in Table 1, total antioxidant capacity was significantly decreased in aorta of ethanol group compared to that of the controls (p = 0.005), however, it was restored to normal status in VETE group (p = 0.6).
The amount of protein carbonyl and Ox-LDL in the aorta tissue of the ethanol group were increased significantly, as compared with that in the controls (p = 0.001). In comparison to the ethanol group, the amount of protein carbonyl and Ox-LDL were decreased significantly in VETE rats (p = 0.005), (Table 1).
The width of the aortic media wall, elastin fiber thickness, and smooth muscle cell layers and the entire aorta thickness were statistically greater in the ethanol rats compared to the control animals (p = 0.005) (Fig. 1, and Table 2). Treatment with vitamin E alleviated the increased media width, fiber thickness, smooth muscle cell layers and the entire aorta thickness compared to the ethanol group. There were no significant differences between SMC thickness/fiber thickness ratios in different groups. Significant increases were observed in the entire SMC layers and entire elastin fiber thickness in the ethanol group compared to the control (p = 0.005). In the ethanol group, the ratio of SMC thickness/media thickness and elastin finers thickness/media thickness were significantly higher than the samples taken from the control animals (p = 0.005); while it remained unchanged in the VETE rats compared to the control rats.
| Control | Ethanol | Ethanol + Vitamin E | |
|---|---|---|---|
| Total aorta thickness(μm) | 154/58 ± 1.98 | 210 ± 7.8a | 152 ± 3.8b |
| Tunica media(μm) | 93.6 ± 2.94 | 147 ± 4.31a | 119 ± 6.69a,b |
| Tunica adventitia(μm) | 51.25 ± 2.39 | 67.75 ± 2.47a | 50 ± 5.5b |
| EFT (μm) | 2.36 ± 0.13 | 4.3 ± 0.21a | 2.5 ± .01 |
| Fiber interwall (μm) | 9.38 ± 0.25 | 15.34 ± 0.98a | 9.25 ± 0.64b |
| Entire elastin thickness(μm) | 18.9 ± 1.28 | 32.6 ± 1.81a | 25.55 ± 1.34a,b |
| Elastin/media | 20 ± 1 | 22 ± 0.8 | 21.6 ± 1 |
| Entire SMC thickness(μm) | 74.7 ± 1.9 | 114.6 ± 3.2a | 93.7 ± 6.1a,b |
| Media/aorta thickness | 66.87 ± 1.29 | 68.69 ± 3.1 | 67.1 ± 3.6 |
| Advantia/aorta thickness | 33.1 ± 1.29 | 33.1 ± 2.39 | 32.85 ± 3.6 |
| SMC/EFT | 3.78 ± 0.11 | 3.58 ± 0.16 | 3.67 ± 0.09 |
| SMC/media thickness(%) | 79.9 ± 1% | 77.9 ± 9% | 78.4 ± 1% |
Values expressed as mean ± SEM.
Significant difference compared to the control.
Denotes significant difference (p < 0.05) compared to the ethanol group.
One-way ANOVA considering the effect of the ethanol and vitamin E treatment on data related to aorta wall. Abbreviations: SMC, smooth muscle cell; EFT, elastin fiber thickness.
The ratio of tunica media/entire aorta wall thickness and tunica adventitia/entire aorta wall thickness remained unchanged in all experimental groups at the end of the experiment (Fig. 1, and Table 2).
The PCNA-positive indices (as indicators of proliferation) were increased (35%, mild to moderate) in the Et group compared with the indices in the control group. The ratio of proliferated cells (PCNA-positive indices) in the aorta’s of the control and VETE groups were under 5%, and were considered as having normal status (Fig. 2).
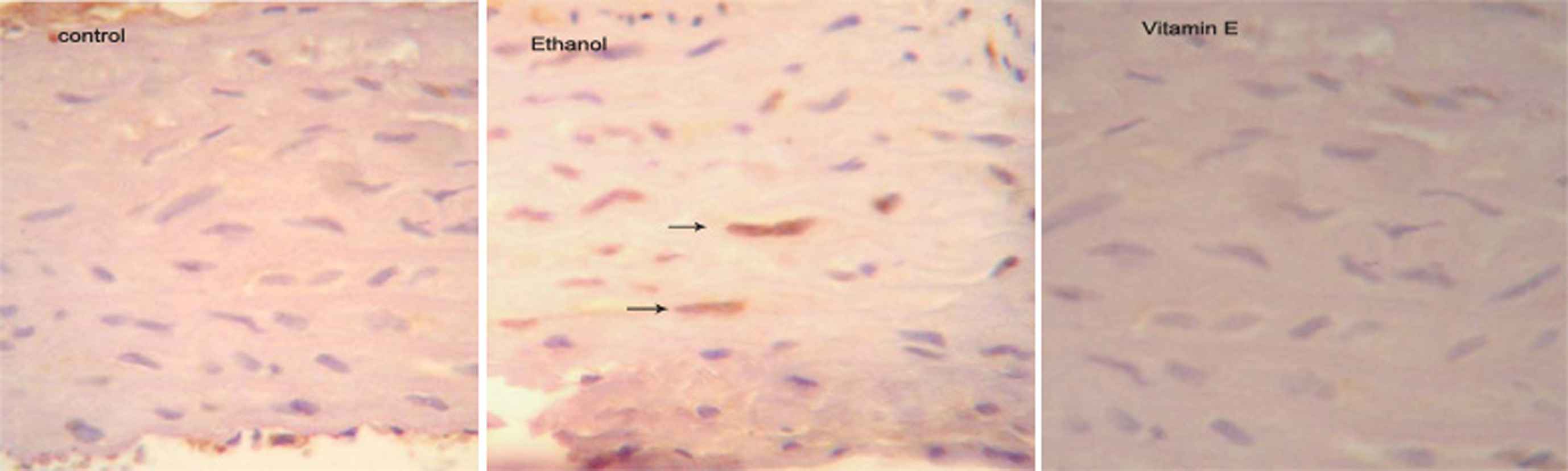
Photomicrographs which shows PCNA positive cells expression in aorta of different groups (Magnification ×400). (➙) PCNA-positive indices.
Discussion
The salient findings of the present study are as follows: (1) After intake of chronic ethanol, an increase in the level of different serum lipids such as cholesterol, TG, LDL, and aorta tissue Ox-LDL with no change in HDL and HDL/TG ratio was observed in the ethanol group; (2) Ethanol consumption in our study markedly increased the level of protein carbonyl and Hcy in the aorta tissue, and it decreased total antioxidant capacity in the ethanol group as compared to that in the control; (3) Structural changes in the aorta such as significant increases in width of the media tunica, adventitia and intima layers of the aorta wall, along with increasing VSMC proliferation with PCNA-positive indices, were found in the ethanol group; (4) Significant amelioration of aorta tissue, as well as restoration of oxidative stress and lipid profile levels, compared to those of the controls, were observed in vitamin E-treated rats.
Artery intima-media thickness is generally accepted as an indicator of generalized atherosclerosis.8 In terms of the media layer, both elastin fibers thickness and fiber intervals or SMS layers showed a significant increase in Et group, compared to the controls. However, there were no significant differences were found between the ratios of SMC thickness/media thickness and elastin thickness/media thickness, as well as the entire media thickness/total aorta wall thickness among the different groups. This indicates that ethanol induced aorta wall thickness is parallel in all parts and content of the aorta wall.
Although a limited number of studies have shown that chronic ethanol intake induces alterations in some measures of structural and physiological changes in the cardiovascular system, the mechanism underlying the deleterious effects of heavy ethanol consumption on the developing atherosclerosis remains largely unknown and no efficient treatment is currently available.6,9 Several factors are important in predicting the advancement of artery atherosclerosis in human and animal models. Hcy is one of the most important factors, as it is an independent risk factor for atherosclerosis and artery stiffness.10 A Growing body of evidence indicates that Hcy is an atherogenic determinant that promotes endothelial dysfunction, oxidant stress, VSMCs proliferation, and thrombosis.11 The adverse effect of Hcy on vascular abnormalities is induced through multiple factors. Hcy increases the expression of proteins known as VSMCs proliferation promoter proteins.12 Growth and migration of VSMCs are considered to be the key events in the pathogenesis of atherosclerosis.13 In addition, Hcy increases free radical generation, not only by oxidation of its sulfhydryl group, but also by increasing lipid peroxidation, protein oxidation, and decreasing the antioxidant defense system.14 Moreover, Hcy increases triglyceride and cholesterol concentration in the serum of rats.15 Interestingly, in the present study, ethanol consumption increased Hcy concentration in aorta tissue which was along with significant VSMCs proliferation, protein oxidation, and elevated levels of lipid profiles such as cholesterol, triglyceride, LDL, and Ox-LDL as well. Recently, lipid peroxidation and protein oxidation have become the subject of interest and have been investigated as markers of oxidative damage in vitro, in vivo, and in human studies.16 It has been suggested that ethanol may cause tissue damage through lipid peroxidation.17 In the current study, we showed that ethanol significantly stimulates protein carbonyl and Ox-LDL levels in the aorta tissue of rats. We would like to point out that the marker of oxidation modification of proteins assessed in the current study, namely protein carbonyls, is preferentially formed by a different neurochemical mechanism. Proteins or peptides containing reactive carbonyls groups can be generated by: (1) direct reactions of proteins with ROS; (2) secondary reactions of primary amino groups of lysine residues to proteins with reducing sugars or their oxidation products and Michael-addition reactions of histidine, lysine, or cysteine residues with α, β-unsaturated aldehydes formed during the peroxidation of polyunsaturated fatty acids. In view of the fact that the formation of protein carbonyl groups is the order of magnitude greater than other oxidative modifications, the level of protein carbonyl groups has become the most widely used marker of protein oxidation during oxidative stress and disease.18 This is why we identified a selective increase in protein carbonyls due to ethanol exposure in this study. Oxidative modification of proteins may lead to the structural alteration and functional inactivation of many enzymes.19
Oxidative stress and the production of intracellular ROS have been implicated in the pathogenesis of cardiovascular disease, partly by promoting vascular smooth muscle proliferation.20 In addition, we have shown that ethanol exposure causes an increase in lipid and Ox-LDL levels in aorta tissue. The earliest event in the development of atherosclerotic lesion is the transport of LDL into and its retention in the aorta.21 LDL then becomes subject to oxidation and is taken up by macrophages. Subsequently, foam cells are formed and oxidatized lipid is accumulated in the artery wall.21 It has been shown that VSMC growth-inducing proliferation or apoptosis is affected by Ox-LDL, depending on its concentration and level of oxidation.22 Ox-LDL stimulates growth via an oxidative mechanism that leads to the release of fibroblast growth factor-2(FGF-2), enhances the mitogenic effect of angiotensin II, and invigorates MAPK activation.23,24 Furthermore, it has been shown that OX-LDL induces an increase in the cell cycle regulatory proteins expression.24
In the current study, we found that vitamin E treatment alleviated the increase in protein carbonyl and Ox-LDL formation associated with ethanol exposure, and restored structural changes in the aorta wall. Although a few studies exist on the effect of antioxidant treatment on alcohol-induced protein carbonyl formation, our previous study and some others have shown that vitamin E treatment promotes the production of protein carbonyls in hippocampus,25 liver,26 and plasma27 in response to various oxidative stressors. Thus, our results support the antioxidant role that has been attributed to vitamin E by previous studies. This effect of vitamin E may be related to its antioxidant property, which consequently holds back accumulation of free radicals or other toxic materials and induction of atherosclerosis. Though it is beyond the scope of this investigation to address the protective mechanism of vitamin E against atherosclerosis, it should be mentioned that a number of possibilities exist. One of the most likely antioxidant functions of vitamin E in this situation is its ability to scavenge free radicals. Vitamin E is a scavenger of peroxyl radicals and quenches other free radicals, such as singlet O2, superoxide and hydroxyl radicals. Indeed, previous studies have shown that ethanol exposure induces the production of free radicals in vitro. Ethanol exposure has been shown to increase the production of hydrogen peroxide in cultured fetal rat hepatocytes28 and to induce the generation of superoxide, hydrogen peroxide and hydroxyl anions in cultured neural crests.29 Therefore, if alcohol exposure increases the production of free radicals and ROS in vivo, as it has been demonstrated in vitro, then vitamin E protest effects may result from its ability to sequester these species, thereby preventing cellular damage. In addition to its ability to scavenge free radicals, vitamin E treatment can also influence levels of other endogenous antioxidants, perhaps contributing to its atherosclerosis protective effects.30,31 Drawing from the above results, we suggest that increase of oxidative state of the rat aortic smooth muscle cell may trigger atherogenic signaling pathways, and contribute to the proliferation of VSMCs. Moreover, aorta wall thickness in all parts seemed to be mediated in part by oxidative stress in aorta tissue.
Conclusion
Our results clearly demonstrate that chronic ethanol consumption induce VSMCs proliferation and aorta wall thickness via oxidative stress and administration of vitamin E improve ethanol-induced oxidative stress by decreasing protein oxidation, Ox-LDL, and Hcy. Furthermore, vitamin E alleviated the ethanol –induced vascular structural change in aorta of ethanolic rats. Further research is, however, required to elucidate the mechanisms by which ethanol produces these vascular deficits in order to develop practicable prevention or treatment strategies for alcohol-induce adverse effects in cardiovascular system.
Conflict of interest
None declared.
Acknowledgments
This work derived from a Master of Science thesis in physiology and supported by Urmia University of Medical Sciences, Urmia, Iran.
References
Cite this article
TY - JOUR AU - Leila Norouzi AU - Alireza Shirpoor AU - Mohammad-Hasan Khadem Ansari AU - Behrouz Ilkhanizadeh PY - 2015 DA - 2015/02/14 TI - Vitamin E attenuates alcohol-induced aortic wall damage in rats JO - Artery Research SP - 20 EP - 26 VL - 10 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2015.01.003 DO - 10.1016/j.artres.2015.01.003 ID - Norouzi2015 ER -
