Altered Central Hemodynamics in Individuals with Down Syndrome
- DOI
- 10.2991/artres.k.191204.001How to use a DOI?
- Keywords
- Down syndrome; vascular function; sympathetic stimulus; autonomic nervous system
- Abstract
Background: Individuals with Down Syndrome (DS) have autonomic dysfunction impacting regulation of heart rate, Blood Pressure (BP), and peripheral vasoconstriction. This may alter central hemodynamics through different wave reflections. We investigated central hemodynamics including wave reflection during rest and a sympathoexcitatory stimulus [Lower Body Negative Pressure (LBNP)] in individuals with DS and controls.
Methods: Radial applanation tonometry was performed on participants with and without DS before and during 5-min LBNP stimulus of −20 mmHg. Waveforms were calibrated to mean and diastolic BP. Generalized transfer function was used to estimate aortic pressures [Systolic Blood Pressure (aSBP), Diastolic Blood Pressure (aDBP), mean pressure (aMAP), pulse pressure (aPP)], Augmentation Index (AIx), augmentation index normalized for HR (AIx@75), Augmentation Pressure (AP), Reflection Index (RIx), Time to Reflection (Tr), forward and reflected wave magnitude (Pf and Pb).
Results: Fifteen individuals with DS (male n = 12, age 24 ± 4 years, BMI 28 ± 5 kg/m2) and 16 control participants (male n = 12, age 24 ± 4 years, BMI 25 ± 5 kg/m2) participated. Baseline differences showed greater AP, higher AIx and AIx@75, a greater RIx, shorter Tr and larger Pb in individuals with DS (p < 0.05). In response to LBNP, interaction effects were observed for AIx, AIx@75, AP, RIx and Pb, due to reductions in the outcomes in response to LBNP for individuals with DS with no change in the controls.
Conclusion: These results show that central hemodynamics and wave reflections are different in individuals with DS at rest and in response to LBNP, probably as a result of anatomical differences and reduced peripheral vasoconstrictive control during LBNP.
HIGHLIGHTS
- •
Individuals with DS exhibit greater central hemodynamic load at rest.
- •
Individuals with DS have greater indices of wave reflections at rest.
- •
Individuals with DS maintained blood pressure during LBNP with reduced wave reflection.
- •
- Copyright
- © 2019 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Individuals with Down Syndrome (DS) experience syndrome-specific early aging, however, improvements in medical care and living circumstances are continuing to increase their life expectancy [1]. Cardiovascular Disease (CVD) has not been a major cause of mortality in individuals with DS [2], to the extent they have even been suggested to be ‘protected’ from CVD because of their syndrome-specific physiology [3]. As the lower prevalence could have also been related to their shorter life span, the more recent increase in the aging population of individuals with DS thus requires a better understanding of CVD risk [4,5] as they exhibit low physical activity levels and low work capacity [6–9], more obesity [10,11] and high cholesterol levels [12], but lower blood pressure [12–14] and less atherosclerosis [3,4,12]. One contributor to cardiovascular morbiditiy and mortality [15] that has yet to be investigated in individuals with DS is the central hemodynamic load on the heart.
Left ventricular contraction initiates a forward traveling pressure wave that becomes partially reflected when encountering downstream bifurcations or changes in vasomotor tone [16]. A larger or faster travelling reflected wave will return to the heart and can combine with subsequent forward travelling waves, increasing aortic systolic pressure [17,18]. Individuals with DS are known to have lower peripheral blood pressure [12–14], which may suggest individuals with DS have a more favorable central hemodynamic profile. However, individuals with DS also have a shorter stature, which could cause the reflected wave to return faster to the heart and thus increasing central pressure and altering the expected risk profile. The first aim of this study was therefore to compare resting central hemodynamics of individuals with DS to healthy controls.
Additionally, individuals with DS exhibit autonomic dysfunction impacting the regulation of heart rate and blood pressure [14]. Individuals with DS show less vagal withdrawal [19–21] and reduced sympathetic responses to most sympatho-excitatory tasks, with smaller increases in heart rate and blood pressure in response to exercise, active and passive orthostasis and cold pressor testing [22–25]. The systemic response to sympatho-excitatory tasks has been thoroughly characterized; however the peripheral consequences of decreased sympathetic activation have only recently become the focus of investigation [26]. In response to a mild sympatho-excitatory stimulus, −20 mmHg of Lower Body Negative Pressure (LBNP), individuals with DS did not decrease brachial blood flow and forearm vascular conductance as expected, indicative of less vasoconstrictive control of peripheral blood flow [26]. Vasoconstriction during LBNP would increase wave reflection back to the heart and augment systolic pressure. Investigating wave reflection during LBNP could provide further support of impaired vasoconstriction during a sympatho-excitatory task, and can provide insight in the contribution of autonomic dysfunction to wave reflection and hemodynamic load in individuals with DS. Therefore, the second aim of this study was to compare central hemodynamics during −20 mmHg LBNP in individuals with and without DS. In this study we therefore compared central hemodynamics (aortic pressure and wave reflections) (1) during rest and (2) during a sympatho-excitatory task in individuals with and without DS. Individuals with DS are hypothesized to show lower central arterial pressure at rest and a larger reduction in wave reflection and aortic pressures during LBNP compared with control participants.
2. MATERIALS AND METHODS
2.1. Participants
Participants were recruited from the campus of the University of Illinois at Chicago and the Chicago community via support groups and organizations for individuals with DS, word of mouth, as well as online postings. Potential participants were invited for an on-site screening visit in order to determine eligibility for the study. Inclusion criteria were: 18–40 years, non-athletic, in general good health, and diagnosed with DS (only for the participants in the group with DS). Individuals were excluded if they had uncorrected congenital heart disease, cardiovascular disease, a BMI over 40 kg/m2, any conditions listed as absolute or relative contraindications to exercise according to The American College of Sports Medicine, blood pressure over 140/90 mmHg and self-reported fasting glucose or diabetes (fasting glucose > 100 mg/dl). The Institutional Review Board of the University of Illinois at Chicago approved this protocol and all participants and their parent or caregiver provided written informed consent.
2.2. Study Protocol
Participants were instructed to abstain from caffeine, alcohol, multivitamins and exercise for at least 12 h and a minimum 4 h fast (only water allowed) prior to the experimental study visit. For participants with DS, a familiarization visit was completed prior to the experimental visit to allow the participant to become comfortable with research personnel, the laboratory environment and the research protocol. Upon arrival for the experimental visit, height, weight, and waist circumference were measured and all participants completed a health history and physical activity questionnaire. BMI was determined using the standard calculation (kg/m2). Participants were then instrumented with a 3-lead Electrocardiogram (ECG). They assumed a supine position in the LBNP chamber and were sealed at the waist. Blood pressure was continuously monitored non-invasively using finger photoplethysmography (Finometer Pro, Finapres Medical System, The Netherlands). Analog signals from ECG, Finometer and the LBNP chamber were continuously recorded during the protocol using Acknowledge software (BIOPAC System, Inc., CA, USA) for storage and offline analysis. Measures were obtained in the last minute of 10 min supine resting and in the last minute of 5 min of −20 mmHg LBNP application.
2.3. Aortic Blood Pressure and Pressure Wave Reflection
Pressure waveforms were collected in duplicate via applanation tonometry (SphygmoCor Model EM3, AtCor Medical, Sydney, Australia) from the radial artery in 10 s-epochs and ensemble averaged to create representative waveforms (SphygmoCor Software Version 9). Brachial Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) were obtained from the Finometer at the time of the radial Sphygmocor measurements and averaged. Mean Aortic Pressure (MAP) was then calculated as 1/3 SBP + 2/3 DBP. Post analyses, all waveforms were calibrated to brachial mean (MAP) and DBP, and Aortic SBP (aSBP) was estimated using a validated generalized transfer function applied to the radial waveform [27,28]. Aortic Pulse Pressure (aPP) was calculated as aSBP–aDBP. Based on the aortic waveforms, additional variables were calculated. Augmentation Index (AIx), a measure of global wave reflections, was calculated as the percent of Augmented Pressure (AP) (difference between late and early systolic peaks of the waveform) to total aPP: ([P2_P1]/aPP * 100). Due to the influence of heart rate on AIx, it was also normalized to a heart rate of 75 bpm (AIx@75). Time to Reflection (Tr) is the round trip travel time of the forward wave from the ascending aorta to the major “effective” reflection site and back. Tr is measured as the time between the start of the waveform (T0) and Pi, which is the inflection point or upstroke of the reflected wave [29]. Wave separation analyses were performed on the aortic waveforms to determine the forward (Pf) and reflected (Pb) wave magnitude. This is based on the flow triangulation method of Westerhof et al. [30] and uses a modified average-flow waveform. Reflection index was calculated by dividing Pb by Pf and is considered a measure of reflection magnitude not dependent on heart rate [31].
2.4. Statistical Analysis
Data were checked for normality and outliers and log transformed when necessary. Differences between groups in descriptive and resting hemodynamic measures were tested with Chi-square (for sex) and independent t-tests (for all other variables). A secondary analysis with analysis of covariance (ANCOVA) was run on indices of wave reflection (AIx, AIx@75, and RIx) with height as a covariate to account for its potential influence. The effects of LBNP on central hemodynamics were tested using a mixed analysis of variance (ANOVA) with Group (DS vs. control), Condition (baseline vs. LBNP) and Group × Condition interaction effects. When significant interaction effects were detected, post-hoc pair wise comparisons with the Bonferroni correction for multiple comparisons were applied. Statistical analyses were performed with SPSS 25.0 (IBM, Armonk, NY, USA) and all p-values are two-sided, with an a priori α-level of <0.05 determined to be significant.
3. RESULTS
The descriptive characteristics of the DS group (n = 15) and the control group (n = 16) are presented in Table 1. Groups were well-matched on sex, age, and BMI but the control group was taller (p < 0.01).
| DS | Control | |
|---|---|---|
| Sex (male/female) | 12/3 | 12/4 |
| Age (years) | 24 ± 4 | 24 ± 4 |
| Height (m) | 1.59 ± 0.07 | 1.72 ± 0.09* |
| Weight (kg) | 71.5 ± 14.4 | 75.4 ± 18.3 |
| BMI (kg/m2) | 28.1 ± 4.8 | 25.3 ± 4.6 |
p < 0.05.
BMI, body mass index.
Descriptive characteristics
3.1. Resting Hemodynamics
Resting hemodynamics and wave reflections are presented in Table 2. Individuals with DS had similar peripheral and aortic blood pressure in comparison to controls. Individuals with DS had greater augmented pressure, higher AIx and AIx@75, a greater reflection index, shorter time to reflected wave and larger reflected wave magnitude. After controlling for height, higher AIx, AIx@75 and RIx were still observed in individuals with DS.
| DS (n = 15) | Control (n = 16) | p-value | |
|---|---|---|---|
| bSBP (mmHg) | 128 ± 14 | 129 ± 11 | 0.80 |
| bDBP (mmHg) | 69 ± 9 | 72 ± 5 | 0.44 |
| bMAP (mmHg) | 86 ± 10 | 88 ± 5 | 0.61 |
| bPP (mmHg) | 59 ± 9 | 58 ± 12 | 0.80 |
| aSBP (mmHg) | 107 ± 11 | 108 ± 7 | 0.90 |
| HR (bpm) | 60 ± 10 | 59 ± 11 | 0.87 |
| AIx (%) | 9 ± 11 | −3 ± 14 | 0.01* |
| controlled for height | <0.01* | ||
| AIx@75 (%) | 2 ± 11 | −11 ± 13 | 0.01* |
| controlled for height | <0.01* | ||
| AP (mmHg) | 3 ± 4 | −1 ± 5 | 0.01* |
| RIxa (%) | 53 ± 10 | 41 ± 11 | 0.01* |
| controlled for height | <0.01* | ||
| Tr (ms) | 143 ± 19 | 167 ± 25 | 0.01* |
| Pfa (mmHg) | 32 ± 5 | 33 ± 7 | 0.88 |
| Pba (mmHg) | 17 ± 4 | 13 ± 3 | 0.01* |
p < 0.05.
Down syndrome n = 14 due to missing data.
bSBP, brachial systolic blood pressure; bDBP, brachial diastolic blood pressure; bMAP, brachial mean aortic pressure; bPP, brachial pulse pressure; aSBP, aortic SBP; HR, heart rate; AIx, augmentation index; AIx@75, augmentation index normalized for HR; AP, augmented pressure; RIx, reflection index; Tr, time to reflection; Pf and Pb, forward and reflected wave magnitude.
Resting central hemodynamics
3.2. Central Hemodynamic Response to LBNP
Both groups had similar blood pressure and heart rates responses to LBNP, with decreases in bSBP, bMAP, bPP and aSBP and increases in HR (condition effects, see Table 3). Despite a similar blood pressure response, the components of wave reflection only changed in individuals with DS. Individuals with DS reduced AIx, AIx@75, AP, RIx, and Pb during LBNP whereas controls had no significant changes (p for interactions <0.05, see Table 3 and Figure 1A–F). Further post-hoc analyses showed that individuals with DS had significantly higher AIx, AIx@75, AP, RIx and Pb compared with controls at baseline, but these differences were not present during LBNP. After controlling AIx, AIx@75 and RIx for height, only AIx@75 still showed a significant interaction effect, with a decrease in response to LBNP in individuals with DS compared with no significant change in controls. Tr is only showing a group effect, indicative of shorter time to reflected wave for individuals with DS throughout the protocol.
| DS (n = 15) | Control (n = 16) | Effects (p-values) | |||||
|---|---|---|---|---|---|---|---|
| Resting | LBNP | Resting | LBNP | Interaction | Condition | Group | |
| bSBP (mmHg) | 128 ± 14 | 122 ± 15 | 129 ± 11 | 126 ± 10 | 0.54 | 0.03* | 0.01* |
| bDBP (mmHg) | 69 ± 9 | 68 ± 8 | 72 ± 5 | 71 ± 7 | 0.59 | 0.53 | 0.32 |
| bMAP (mmHg) | 86 ± 10 | 84 ± 9 | 88 ± 5 | 87 ± 7 | 0.41 | 0.04* | 0.41 |
| bPP (mmHg) | 59 ± 9 | 53 ± 11 | 58 ± 12 | 54 ± 8 | 0.65 | 0.03* | 0.97 |
| aSBP (mmHg) | 107 ± 11 | 101 ± 11 | 108 ± 7 | 105 ± 8 | 0.21 | <0.01* | 0.48 |
| HR (bpm) | 60 ± 10 | 64 ± 10 | 59 ± 11 | 64 ± 12 | 0.37 | <0.01* | 0.97 |
| AIxb (%) | 9 ± 11†,‡ | −0.1 ± 12† | −3 ± 14‡ | −5 ± 15 | 0.047* | <0.01* | 0.08 |
| AIx@75b (%) | 2 ± 11†,‡ | −6 ± 11† | −10 ± 14‡ | −10 ± 13 | 0.02* | 0.01* | 0.06 |
| AP (mmHg) | 3 ± 4†,‡ | −0.1 ± 3† | −1 ± 6‡ | −2 ± 5 | 0.02* | <0.01* | 0.07 |
| RIxa,b (%) | 53 ± 10†,‡ | 46 ± 12† | 41 ± 11‡ | 41 ± 13 | 0.03* | 0.01* | 0.04* |
| Tr (ms) | 143 ± 19 | 140 ± 19 | 167 ± 25 | 164 ± 19 | 0.99 | 0.10 | <0.01* |
| Pfa (mmHg) | 32 ± 5 | 30 ± 6 | 33 ± 7 | 31 ± 5 | 0.70 | 0.050 | 0.69 |
| Pba (mmHg) | 17 ± 4†,‡ | 14 ± 3† | 13 ± 3‡ | 12 ± 4 | <0.01* | <0.01* | 0.051 |
Down syndrome n = 14 due to missing data.
Control group n = 15 due to missing data.
Significant effect p < 0.05.
Post-hoc analyses difference between baseline and LBNP.
Post-hoc analyses difference between DS and control group at baseline.
LBNP, Lower body negative pressure; bSBP, brachial systolic blood pressure; bDBP, brachial diastolic blood pressure; bMAP, brachial mean aortic pressure; bPP, brachial pulse pressure; aSBP, aortic SBP; HR, heart rate; AIx, augmentation index; AIx@75, augmentation index normalized for HR; AP, augmented pressure; RIx, reflection index; Tr, time to reflection; Pf and Pb, forward and reflected wave magnitude.
Central hemodynamic response to LBNP
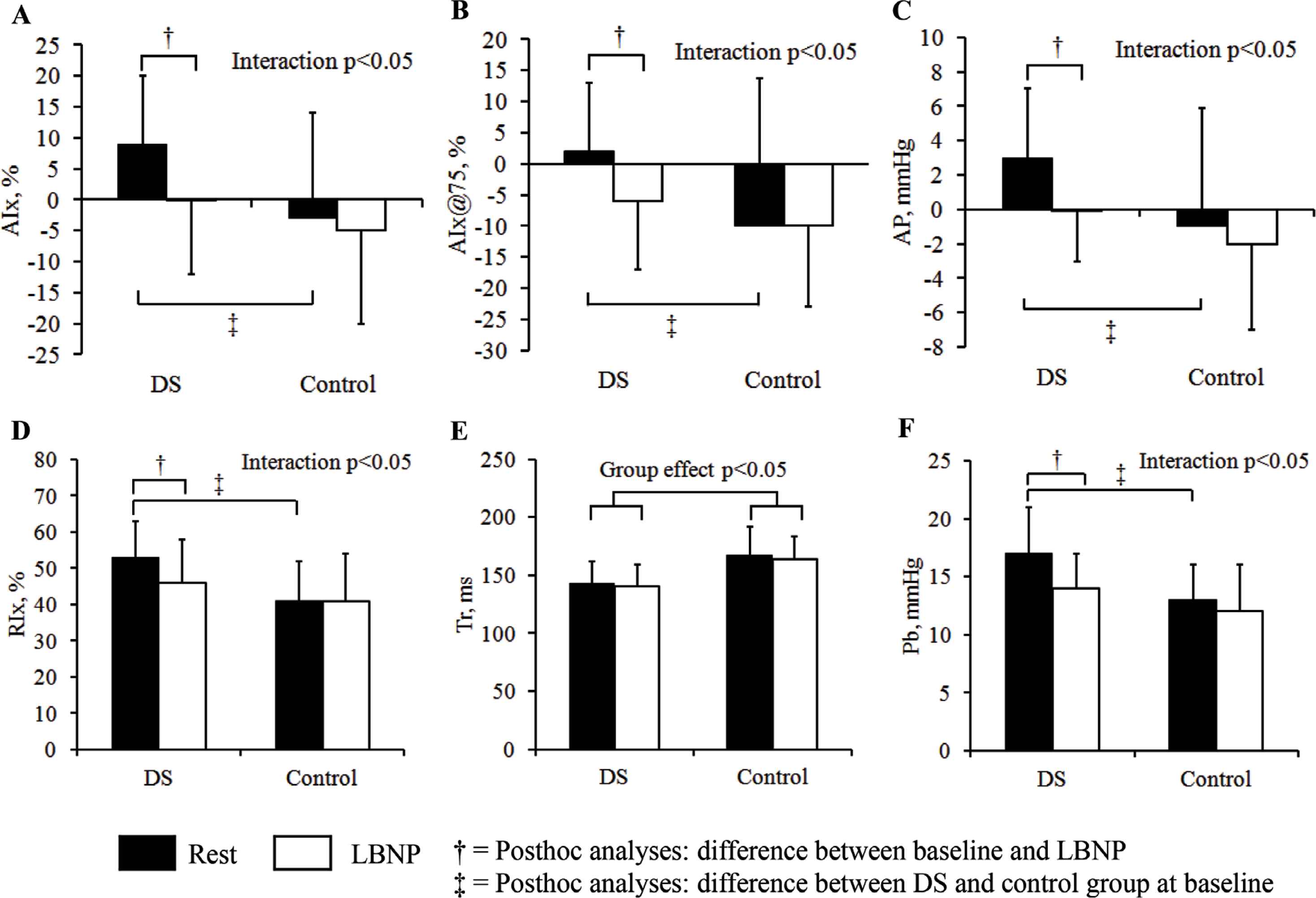
Central hemodynamics at rest and in response to 5 min −20 mmHg of LBNP in individuals with DS and without DS. (A) Augmentation index, (B) augmentation index standardized for heart rate, (C) augmentation pressure, (D) reflection index, (E) time to reflection, (F) reflected wave magnitude.
4. DISCUSSION
To the best of our knowledge, this is the first study examining resting central hemodynamics and wave reflections in individuals with DS and the response to a mild sympatho-excitatory stimulus in comparison to a sex- and age-matched control group without DS. Contrary to our hypothesis, individuals with DS demonstrated similar central blood pressure as controls however with greater indices of wave reflection. During LBNP, individuals with DS showed reductions in wave reflection while no reductions were observed in the control participants. These differences in wave reflection indices occurred despite similar changes in blood pressure between groups.
Individuals with DS exhibited greater wave reflection at baseline than controls. This is supported by both augmentation index and the reflection index. The effect of wave reflection from the periphery on central hemodynamics is highly influenced by several factors such as the timing of the reflected wave, stiffness of the arterial tree, and height. Previous studies in individuals with DS focused mainly on arterial stiffness as a marker for atherosclerotic burden. Rodrigues et al. [13] found lower carotid-femoral Pulse Wave Velocity (PWV) in individuals with DS compared to sex- and age-matched controls, but this difference disappeared after correcting for their lower systolic blood pressure. Parra et al. [4] confirmed that initial differences in cIMT and PWV between individuals with DS and controls disappeared after correcting for systolic blood pressure. Although our study is limited in that we did not measure arterial stiffness, based on these previous studies, arterial stiffness likely does not explain the differences we observed in wave reflection.
One potential explanation is their shorter stature, which imposes earlier sites of reflection and travel times. Irrespective of the cause of increased wave reflection, the increase in augmented pressure imposes a larger central hemodynamic load. The increased afterload and greater strain on the left ventricle is associated with higher cardiovascular risk in various populations [32,33]. As individuals with DS are often assumed to have lower blood pressure, their central hemodynamics have thus far been an overlooked aspect of their cardiovascular risk profile. Further research is necessary to determine the predictive ability of central hemodynamics and wave reflection on cardiovascular disease and risk in individuals with DS.
Individuals with DS showed a larger decrease in wave reflection indices in response to LBNP, resulting in a reduced hemodynamic load to the heart. LBNP elicits venous pooling and unloads the central aortic and carotid baroreceptors. To maintain systemic arterial blood pressure, sympathetic activity is enhanced and increases HR and total peripheral resistance to reduce venous pooling and maintain stroke volume [34,35]. Our control participants did not show any significant changes in AIx, AIx@75 or AP, likely because they were capable of combating the venous pooling resulting from this mild stimulus with an appropriate sympathetic response and an increase in heart rate. However, for the individuals with DS, this stimulus did result in decreased AIx, AIx@75, RIx, AP, and reflected wave magnitude, suggesting they were not able to increase vasoconstriction and peripheral vascular resistance enough to counteract the venous pooling caused by the LBNP. This lack of vasoconstriction is in line with previous research showing no decrease in brachial blood flow and forearm vascular conductance in individuals with DS in response to LBNP [26]. The hypothesized lack of vasoconstriction to counteract the venous pooling is likely indicative of reduced sympathetic control, although Muscle Sympathetic Nerve Activity (MSNA) would be necessary to confirm this hypothesis. As the individuals with DS were able to maintain systemic blood pressure during LBNP, they likely utilize other methods to increase cardiac output by not just increasing HR, but also stroke volume. Further research is required to investigate their cardiovascular responses.
The lack of more rigorous measures of sympathetic activity, like MSNA, is a limitation of this study, but these methods are not an option for most individuals with DS due to feasibility and ethical considerations. We observed similar resting brachial and aortic blood pressures in individuals with DS in comparison to our control group. Based on previous research, we would have expected the individuals with DS to have lower resting peripheral BP [4,12]. This may suggest individuals with DS had higher arousal throughout the protocol however this was not supported by verbal or non-verbal signs during the visits. Individuals with DS also participated in a familiarization visit in an attempt to rid of this effect and to get comfortable with the equipment and procedures. Furthermore, the sample consisted of 12 males and two (DS) or three (control) females for each group, which could be considered a limitation due to sex differences in autonomic regulation and blood pressure control, however, Hughes and Casey [35] did not find any sex differences in the hemodynamic response to LBNP in young adults. Finally, our sample was relatively young, and our findings have limited generalizability to the entire age range of individuals with DS.
4.1. Implications
Individuals with DS do not show a high prevalence of CVD [11,36]. Whether this is due to physiological ‘protective’ mechanisms or the fact that until recently their lifespan was too short to develop CVD is to be investigated with longitudinal epidemiological studies. Reviewing the few available studies on CVD risk factors in individuals with DS [4,12,29,37], what emerges is an incongruent cardiovascular risk factor profile, likely to be interpreted differently with regards to predictive value of single or combined sets of CVD risk factors. Further research is necessary to investigate the correlation between these risk factors, and potential explanations related to the genetic syndrome-specific physiology.
5. CONCLUSION
These results show that despite similar resting aortic pressure, individuals with DS have greater indices of wave reflection, indicative of a less favorable central hemodynamic profile. In response to a sympatho-excitatory stimulus, individuals with DS maintain central pressure however show reductions in wave reflection, unlike control participants. This suggests individuals with DS vasoconstrict less in the periphery during the sympatho-excitatory stimulus, however, maintain blood pressure by different means, likely by increasing cardiac output.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHOR’S CONTRIBUTION
SOW and BF study conceptualization and writing (review & editing) the manuscript, SOW, GG and AR data curation and writing (review & editing) the manuscript, ES data curation, formal analysis and writing (original draft) the manuscript, TH formal analysis and writing (original draft), BF and TH funding acquisition, TB and BF supervised the project and writing (review & editing).
FUNDING
This work has been supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH K99/R00 1 K99 HD092606-01). This funding body had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
ACKNOWLEDGMENTS
We would like to thank all the participants, their parents and caregivers for supporting this research by investing their time and energy in this research study.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Thessa Irena Maria Hilgenkamp AU - Elizabeth Cornellia Schroeder AU - Sang Ouk Wee AU - Georgios Grigoriadis AU - Alexander Jacob Rosenberg AU - Tracy Baynard AU - Bo Fernhall PY - 2019 DA - 2019/12/24 TI - Altered Central Hemodynamics in Individuals with Down Syndrome JO - Artery Research SP - 107 EP - 112 VL - 25 IS - 3-4 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.191204.001 DO - 10.2991/artres.k.191204.001 ID - Hilgenkamp2019 ER -
