Dihydromyricetin Attenuates Streptozotocin-induced Liver Injury and Inflammation in Rats via Regulation of NF-κB and AMPK Signaling Pathway
- DOI
- 10.2991/efood.k.200207.001How to use a DOI?
- Keywords
- Ampelopsin; AMPK; diabetes; dihydromyricetin; inflammation; liver-protective effects; NF-κB
- Abstract
Dihydromyricetin (DHM) dramatically improved the quality of life for Streptozotocin (STZ)-induced diabetic rats and significantly increased the activity of antioxidant enzymes in the liver. Moreover, DHM successfully ameliorated diabetes-induced liver damage by suppression of apoptosis in the liver, as indicated by the decreased levels of Bax and cleaved caspase-3. In diabetic rats, the levels of tumor necrosis factor-α and interleukin-1β in the liver were significantly increased. However, the administration of DHM (100–400 mg/kg/day) for 6 weeks restored the cytokine levels to their normal values in a dose-dependent manner in diabetic rats by the regulation of nuclear factor-kappa B signaling pathway. In addition, DHM significantly induced 5′ AMP-activated protein kinase (AMPK) phosphorylation and decreased MyD88, TLR4, p38, GSK-3β protein expression levels in the liver of diabetic rats. In conclusion, DHM could improve STZ-induced liver impairment by preventing oxidative stress, apoptosis, and inflammation.
- Copyright
- © 2020 International Association of Dietetic Nutrition and Safety. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Dihydromyricetin (DHM), or ampelopsin, is a flavonoid abundantly present in the leaves of Ampelopsis grossedentata (Hand.-Mazz.) W.T. Wang (Vitaceae), which is known as Tengcha in the south of China and is used to prepare vine tea [1]. DHM has been reported to exert a number of biological and pharmacological actions, including anti-inflammatory activity, antioxidant activity, antibacterial activity, hypoglycemic effect, hypolipidemic effect, and antitumor activity [2,3]. DHM is also traditionally used for the prevention and treatment of nephritis, hepatitis, halitosis. Studies have demonstrated that DHM (20 mg/kg/day) could improve behavioral disorders and ameliorate diabetes-related cognitive dysfunction in mice by regulation of oxidative stress [4]. For instance, oral administration of 600 mg/kg/day DHM for 4 weeks improved glucose and lipid metabolism in patients with nonalcoholic fatty liver disease [5]; in a hyperlipidemia mice model, DHM ameliorated harmful effects of hyperlipidemia on the liver [4]; DHM treatment protected Streptozotocin (STZ)-induced diabetic mice against cardiomyopathy [6]. However, detailed pharmacological analyses of the mechanism of action of DHM are scarce. Therefore, in this study, we sought to demonstrate the potential effects of DHM on liver dysfunction and possible mechanisms in STZ-induced diabetic rats. A randomized controlled trial revealed that flavonoids, including DHM, present in a food matrix triggered antioxidant activity and anti-inflammatory effects [5].
The inflammatory cytokines including Tumor Necrosis Factor-α (TNF-α), Interleukin-6 (IL-6), and IL-8 play important role in the development of diabetes-related liver inflammation [7]. Thus, all treatments that aimed to decrease the levels of these inflammatory cytokines in vivo might represent important strategies for prevention of pathophysiological complications related to diabetes. It has also been well documented that increased oxidative stress can promote the activation of Nuclear Factor-Kappa B (NF-κB), which, in turn, enhances the expression of inflammatory cytokines such as TNF-α and IL-6 [8], therefore modulating the insulin response in the liver. In view of these facts, therapeutic agents that can strictly control blood glucose, reduce oxidative stress, and modulate proinflammatory cytokines are expected to be beneficial for controlling diabetic complications. Moreover, the components of MAPK signaling pathway, which include p38 MAPK, participate in inflammation and are considered to be upstream factors of NF-κB [9]. The 5′ AMP-Activated Protein Kinase (AMPK) signaling pathway could modulate the inflammatory response in diabetic rats through regulation of the NF-κB signaling pathway.
2. MATERIALS AND METHODS
2.1. Chemicals
Dihydromyricetin and STZ were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). UNIQ-10 Column TRIzol Total RNA Isolation Kit was purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The IL-1β, TNF-α, phospho-p38, p38, phospho-AMPK, AMPK, phospho-GSK-3β (Tyr216), NF-κB p65, TLR4, and MyD88 antibodies were obtained from Ruiying Biological Co., Ltd. (Suzhou, China). Secondary antibodies were bought from Sangon Biotech Co., Ltd. (Shanghai, China). Water, methanol, acetonitrile, and formic acid were purchased from CNW Technologies GmbH (Düsseldorf, Germany).
2.2. Animals
Eight-week-old male Sprague-Dawley rats were supplied by Fuzhou General Hospital of Nanjing Military Command Animal Center (Fuzhou, China), and maintained in a controlled room under specific-pathogen-free conditions, at constant temperature (23 ± 2°C), humidity (50 ± 10%), and 12-h light/dark cycle (lights on at 8 AM) at the Animal Experiment Center of Fuzhou General Hospital of Nanjing Military Command. All rats were fed normal chow with ad libitum access to water and food during the experiment. All procedures were accomplished in strict accordance with the standard approved by the Ethics Committee of Fuzhou General Hospital.
2.3. Induction of Diabetes Mellitus
Rats were fasted overnight prior to STZ injection. Then, diabetes was induced using a single intraperitoneal injection of STZ (40 mg/kg body weight) dissolved in ice-cold saline as reported in a previous investigation [6]. Forty eight hours after STZ injection, the diabetic state of these rats was confirmed by measuring fasting blood glucose levels (>11.1 mmol/L; URight TD-4279 Blood Glucose Monitoring System, Germany). The metformin and different doses of DHM were administered by oral gavage of their aqueous solution (3% v/v Tween-80 in water) once a day for 6 weeks.
2.4. Experimental Procedure
Forty-two male Sprague-Dawley rats were allowed to adapt to the new environment for 7 days, and then were divided into six groups (Figure 1): normal group (normal), diabetes group (diabetic), positive group (metformin, 55 mg/kg/day), and DHM (high dose: 400 mg/kg/day; medium dose: 200 mg/kg/day; low dose: 100 mg/kg/day). All groups comprised six rats, selected randomly. Rats in the normal group were injected with 100 mM citrate buffer (pH 4.5) and the others received 55 mg/kg of STZ.
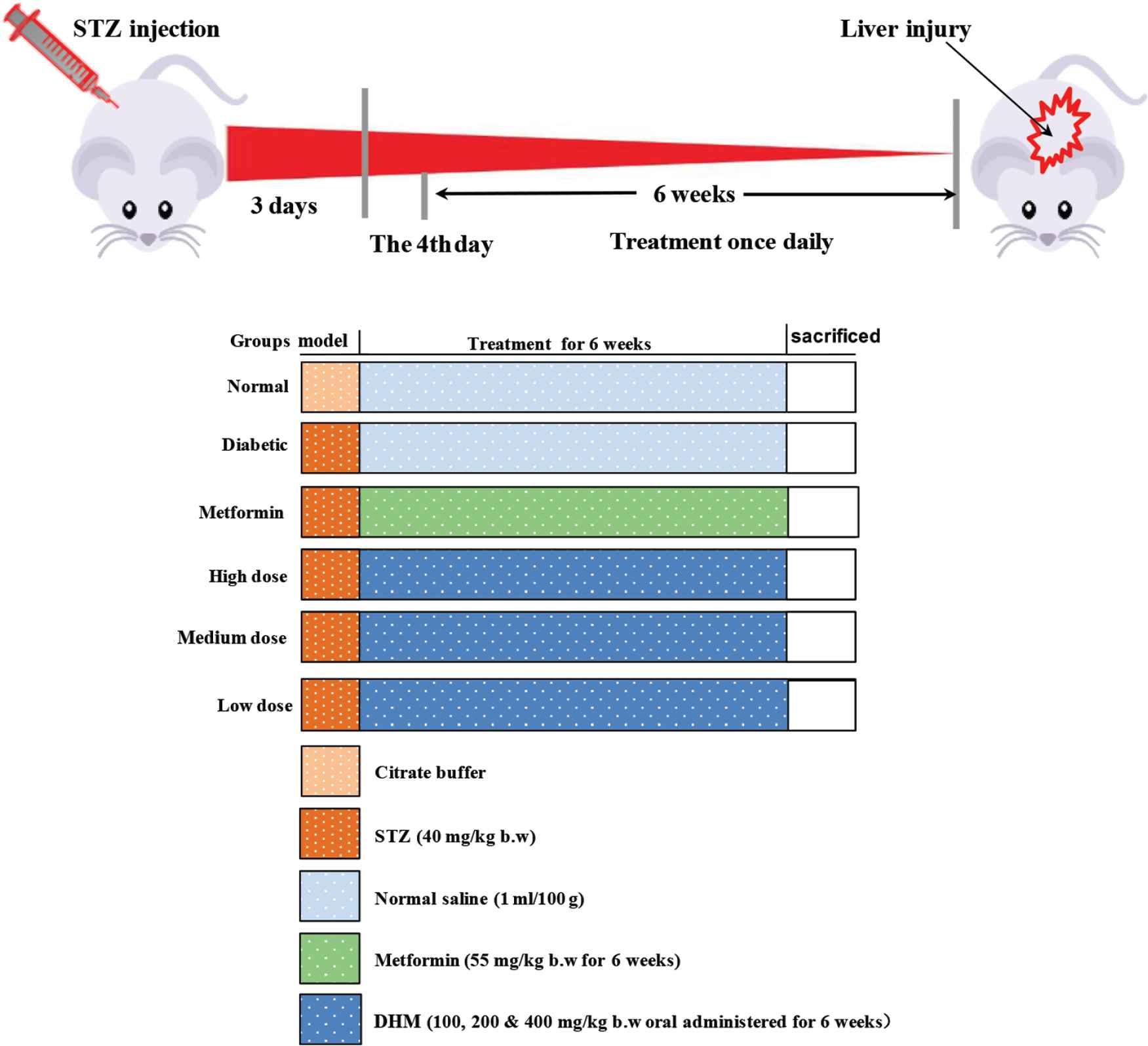
Experimental protocol. Group I: normal control (vehicle treated); Group II: diabetic control (vehicle treated); Group III: diabetic rats supplemented with metformin; Groups IV, V, and VI: STZ hyperglycemic rats supplemented with DHM (100, 200, and 400 mg/kg body weight, respectively). b.w., body weight; DHM, dihydromyricetin; STZ, streptozotocin.
2.5. Tissue Sample Collection and Hematoxylin and Eosin Examination
At the end of the 6-week experiment period, the 12-h fasted rats were anesthetized and killed. The liver was quickly removed and rinsed in saline solution to remove blood, and then immediately frozen and stored at −80°C for various analyses. The fixed livers were dehydrated through graded alcohol and embedded in paraffin. Then, the embedded tissues were cut into 4-μm sections and stained with hematoxylin and eosin (BBI, China). Histological images were recorded by a QCapture Pro 6.0 imaging system.
2.6. Evaluation of Antioxidant Activity
Liver tissue (1 g) was homogenized with 2.0 mL of 20 mM HEPES buffer. Then, 70 mM sucrose and 210 mM mannitol were fully mixed and centrifuged at 10,000 × g at 4°C for 10 min. The Superoxide Dismutase (SOD) activity of the supernatant was measured using the SOD Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s instructions. For the determination of Glutathione (GSH) activity, 1 g of liver was homogenized with 2.0 mL of 50 mM Tris–HCl (containing 5 mM EDTA and 1 mM dithiothreitol) and fully mixed. The mixture was then centrifuged at 10,000 × g at 4°C for 20 min. The total GSH activity was measured using a commercial kit (Cayman Chemical Company, Ann Arbor, MI, USA). Lipid peroxidation was determined as the amount of malondialdehyde (MDA). The method was performed using the MDA assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.7. Western Blot Analysis
Proteins in liver tissue were extracted with radioimmunoprecipitation assay buffer and then centrifuged at 12,000 × g for 10 min at 4°C. The total protein was determined by the bicinchoninic acid protein assay kit (Sangon Biotech, Shanghai, China). Subsequently, the protein extract was mixed with buffer and then boiled for 10 min. Equal amounts of each protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 0.5 h at 80 V, and then for 2.5 h at 100 V. The protein samples were subsequently transferred to a Merk Millipore polyvinylidene difluoride membrane (Darmstadt, Germany) and then blocked with 5% skimmed powdered milk in Tris-buffered saline and Tween-20 (Sangon Biotech, Shanghai, China) for 2 h at room temperature in a shaker. Incubation of the primary antibodies was carried out at 4°C overnight for NF-κB, AMPK, TNF-α, and IL-1β, followed by incubation with the corresponding secondary antibodies. The membrane was exposed to enhanced chemiluminescent reagents according to the manufacturer’s instructions. The Tannon imaging system was used to detect protein bands and the intensity of bands was quantified by ImageJ software (https://imagej.net/ImageJ).
2.8. Statistical Analysis
All analyses were performed in triplicate and statistical analyses were carried out using the Statistical Package for DPS 16.05 system (Zhejiang University, Hangzhou, China). The analysis of variance was performed at the 5% level of significance.
3. RESULTS
3.1. Protective Effects of DHM on the Liver Injury
Histopathological examination of the normal liver tissue demonstrated the healthy hepatic structure in which the lobule is made up of radiating plates, strands of cells forming a network around a central vein (Figure 2A, a and b). Liver biopsy of diabetes-induced rats showed moderate fibrosis, leucocyte infiltration around central vein indicating inflammation, dilation in central vein (Figure 2A, c and d), and the loss of normal architecture indicating liver (hepatocellular) injury. These structural characteristics were less pronounced in diabetic animals administered either metformin (Figure 2A, e and f) or DHM (Figure 2A, g and h). By contrast, as shown in Figure 2B, hepatic cholesterol (total cholesterol) and hepatic triglyceride (total triglyceride) levels were significantly (p < 0.05) decreased at the DHM dose of 400 mg/kg, compared with the diabetes group. DHM also significantly (p < 0.01) decreased the low-density lipoprotein-cholesterol level and increased the high-density lipoprotein-cholesterol level (Figure 2C). In addition, as shown in Figure 2D, both TNF-α and IL-1β levels were significantly increased in the diabetes group in comparison with the normal group. Both DHM and metformin obviously decreased TNF-α and IL-1β levels in serum (p < 0.01). Values of TNF-α and IL-1β expression obtained after treatment with metformin or DHM were not statistically different.
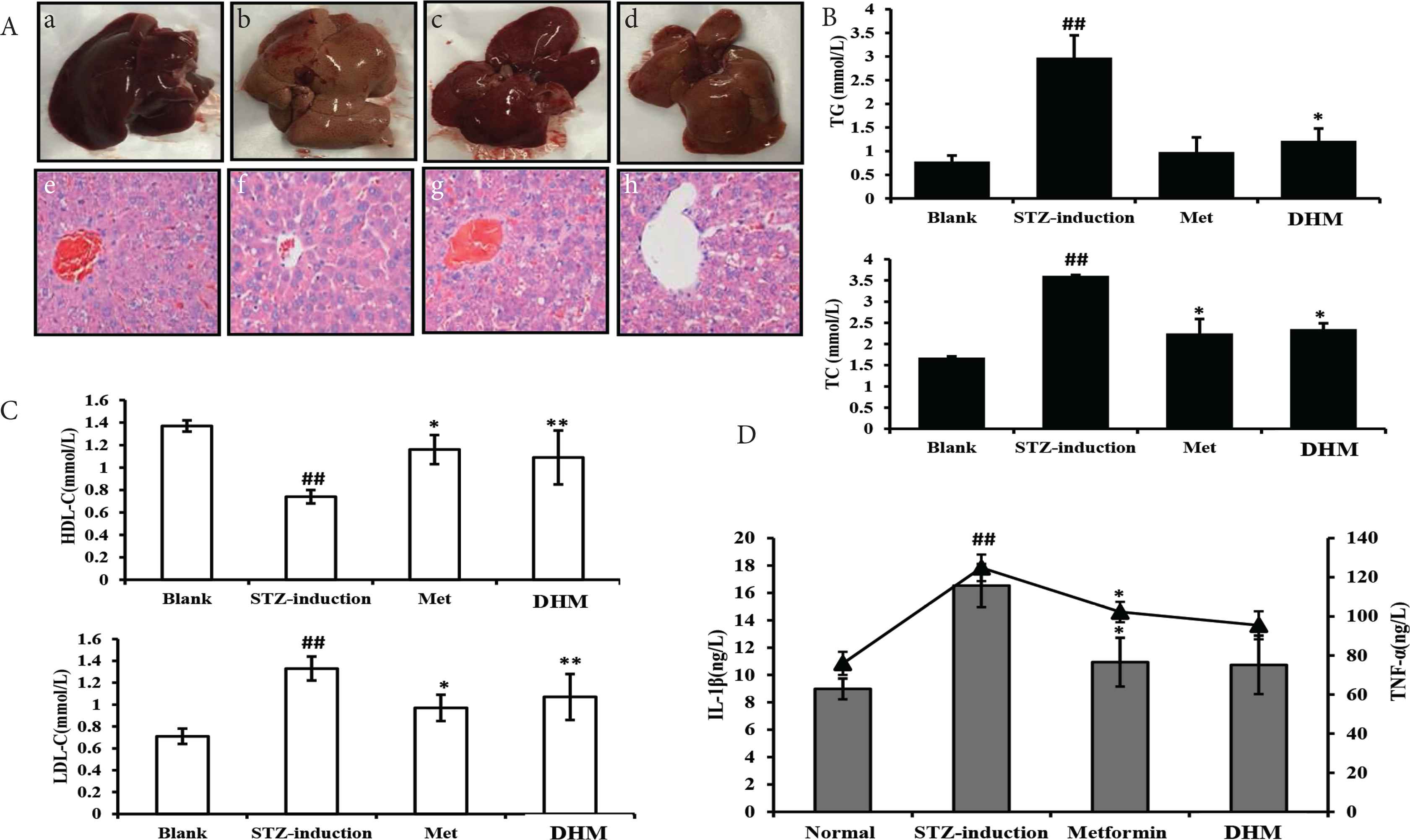
Representative photomicrographs showing the effect of DHM and inhibition of STZ-induced liver change in diabetic rats: (A) liver of diabetic rat showing the loss of normal architecture with the distended portal vein, fibrosis, and leucocyte infiltration (hematoxylin and eosin-stained section); (B) effects of DHM on serum glycolipid metabolism (TG and TC); (C) effects of DHM on serum glycolipid metabolism (HDL-c and LDL-c); (D) effects of DHM on serum levels of IL-1β and TNF-α. Data are shown as mean ± standard deviation; ## p < 0.01, compared with the control (normal) group; *p < 0.05, **p < 0.01, compared with the vehicle group (diabetic vs. normal group). N = 6 in each group. DHM, dihydromyricetin; HDL-c, high-density lipoprotein-cholesterol; IL, interleukin; LDL-c, low-density lipoprotein-cholesterol; Met, metformin; STZ, streptozotocin; TC, total cholesterol; TG, total triglyceride; TNF-α, tumor necrosis factor-α.
3.2. DHM Protected the Liver of Diabetic Rats from Oxidative Stress
To gain insights into the beneficial effects of DHM on the liver of diabetic rats, we evaluated the ability of DHM to increase both enzymatic SOD activity and GSH levels, as well as to decrease MDA levels. Data obtained are presented in Table 1. The diabetic group showed a significant decrease in SOD activity (175.78 ± 27.00 U/mg prot, p < 0.001) and GSH levels (25.42 ± 5.41 mg/g prot, p < 0.001) compared with the control group. Meanwhile, an elevated MDA level (7.68 ± 1.71 nmol/g prot, p < 0.001) in the diabetic liver tissue was confirmed. However, DHM supplementation significantly restored SOD activity (251.35 ± 69.03 U/mg prot at a dose of 100 mg/kg) (p < 0.01) and GSH levels (28.19 ± 9.22 mg/g prot, p < 0.01) compared with nontreated diabetic rats. Furthermore, DHM significantly reversed the increase of lipid peroxidation in STZ-induced diabetic rats, as indicated by the reduction in MDA levels (from 7.68 to 4.72 nmol/g prot at a dose of 400 mg/kg).
| Groups | Superoxide dismutase (U/mg prot) | Glutathione (mg/g prot) | MDA (nmol/g prot) |
|---|---|---|---|
| Normal | 230.10 ± 33.03# | 34.27 ± 6.09# | 4.79 ± 0.81# |
| Diabetic | 175.78 ± 27.00* | 25.42 ± 5.41* | 7.68 ± 1.71* |
| Diabetic + metformin | 201.27 ± 29.62 | 30.40 ± 3.37# | 5.50 ± 1.06# |
| Diabetic + high dose | 222.87 ± 29.20# | 31.18 ± 2.11# | 4.72 ± 0.87# |
| Diabetic + medium dose | 195.58 ± 15.51 | 29.66 ± 7.161 | 7.60 ± 1.13# |
| Diabetic + low dose | 251.35 ± 69.03# | 28.19 ± 9.22## | 6.10 ± 0.85** |
Data are reported as mean ± standard error. Statistical significance was analyzed by the generalized linear model followed by analysis of variance.
p < 0.05,
p < 0.01, compared with normal control;
p < 0.05,
p < 0.01, compared with the vehicle group (diabetic vs. normal group).
N = 6 for each group. MDA, malondialdehyde.
Dihydromyricetin suppresses streptozotocin-induced oxidative stress in the liver of rats
3.3. DHM Inhibited STZ-induced Apoptosis in Liver
Streptozotocin is known to induce apoptosis of β-cells [10,11]. To reveal whether DHM could inhibit STZ-induced apoptosis in the liver of rat, we measured the protein expressions of Bcl-2, Bax, and caspase-3. As shown in Figure 3, STZ injection significantly increased the expression levels of Bax and caspase-3, but decreased the expression level of Bcl-2, compared with the control group. However, treatment with DHM effectively blocked STZ-induced changes in these apoptotic proteins.
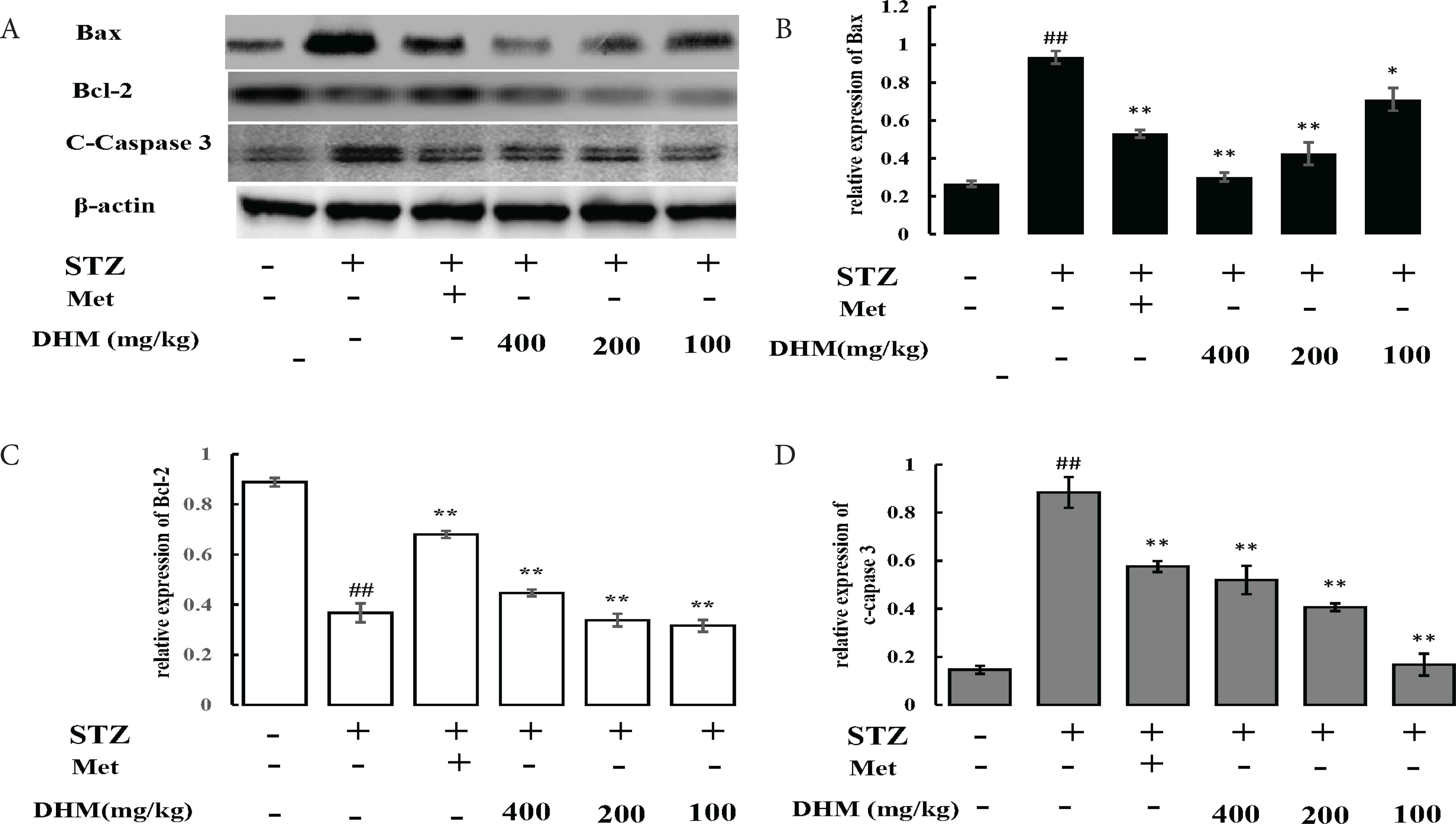
DHM inhibited STZ-induced apoptosis in the liver of rats. (A) Western blot analysis of the Bax, Bcl-2, and caspase-3 proteins in the liver. Relative density analysis of the (B) Bax protein bands, (C) Bcl-2 protein bands, and (D) cleaved caspase-3 protein bands. β-Actin was probed as an internal control in the relative density analysis. The vehicle control is set as 1.0. Values are averages from three independent experiments. Data are shown as mean ± standard deviation; ##p < 0.01, compared with the control (normal) group; *p < 0.05, **p < 0.01, compared with the vehicle group (diabetic vs. normal group). N = 6 in each group. DHM, dihydromyricetin; Met, metformin; STZ, streptozotocin.
3.4. Effect of DHM on the Levels of TLR4 and MyD88 in Liver
Activation of the TLR4 and MyD88 pathways could induce excessive expression of inflammatory cytokines [12]. In this study, the protein expression of TLR4 (Figure 4A) and MyD88 (Figure 4B) was remarkably upregulated in the livers of STZ-induced diabetic rats. However, DHM significantly decreased these protein expression levels in the diabetic group (p < 0.01).
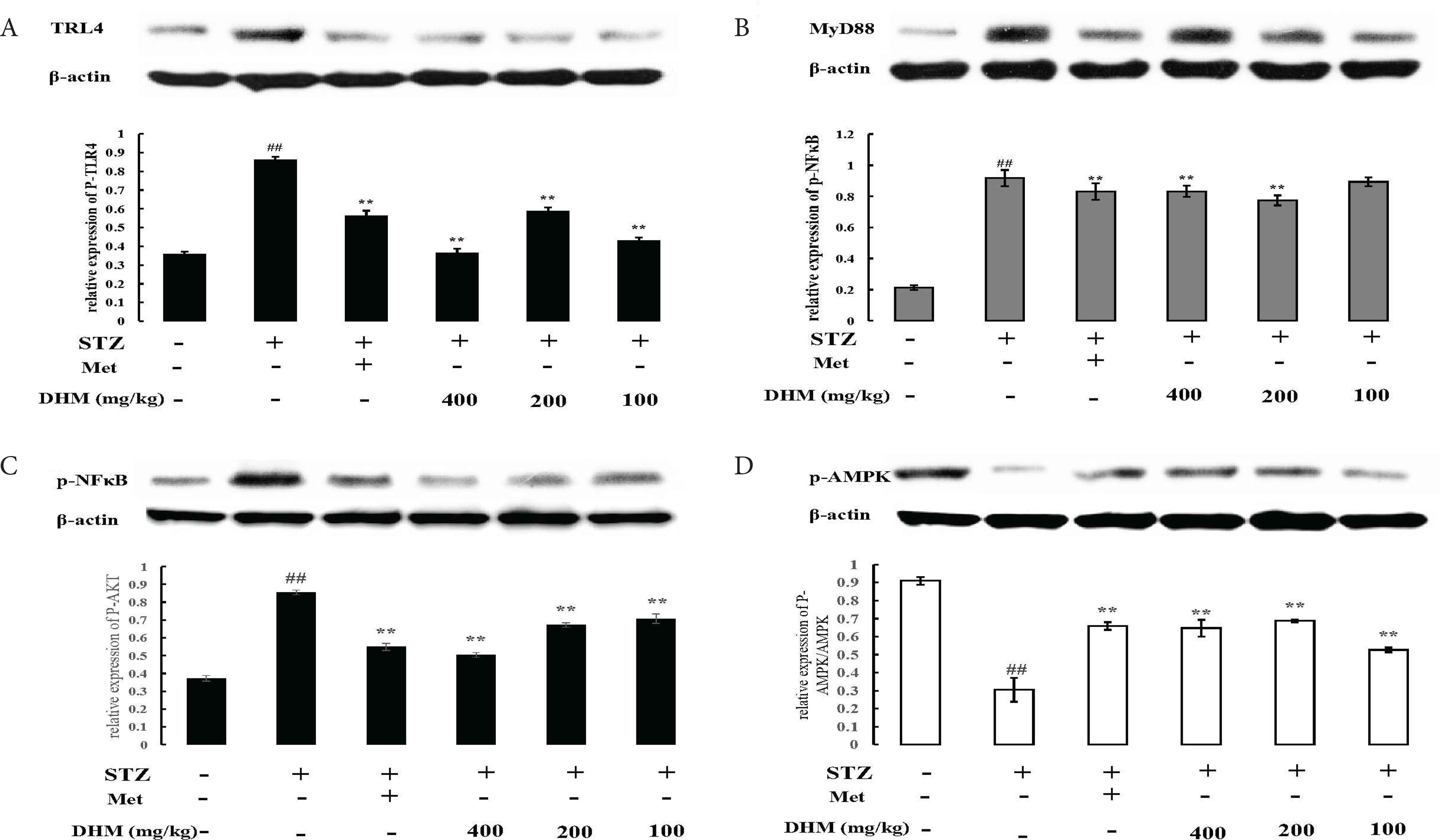
DHM decreased the levels of TLR4, MyD88, NF-κB, and AMPK phosphorylation in the liver of rats. Relative density analysis of the (A) TLR4 protein bands, (B) MyD88 protein bands, (C) NF-κB protein bands, and (D) AMPK protein bands. β-Actin was probed as an internal control in the relative density analysis. The vehicle control is set as 1.0. Values are averages from three independent experiments. Data are shown as mean ± standard deviation; ## p < 0.01, compared with the control (normal) group; **p < 0.01, compared with the vehicle group (diabetic vs. normal group). N = 6 in each group. AMPK, 5′ AMP-activated protein kinase; DHM, dihydromyricetin; Met, metformin; NF-κB, nuclear factor-kappa B; STZ, streptozotocin; TLR4, toll-like receptor 4.
3.5. DHM Affected the Level of NF-κB
As presented in Figure 4C, the expression level of p-NF-κB in the STZ-induced diabetes group was markedly higher than in the other groups. Figure 4A and 4B showed that the total content of TLR4 in liver tissues was significantly higher in the STZ-induced diabetes group compared with other groups. Our results further showed that DHM could inhibit the lipopolysaccharide-activated TLR4–NF-κB pathway.
3.6. DHM Impacted the Level of AMPK
5′ AMP-activated protein kinase was reported to be involved in oxidative stress and chronic inflammatory disorders. We next examined whether DHM could inhibit STZ-induced liver damage via AMPK activation. STZ exposure significantly suppressed AMPK phosphorylation in the rat liver (p < 0.01). However, treatment with DHM restored the decreased phosphorylation levels of AMPK to normal levels (Figure 4D).
3.7. DHM Mediated the Protective Effects through GSK-3β and p38 Pathways
GSK-3β and p38 MAPK play important roles in inflammation and apoptosis. Thus, we also measured the phosphorylation levels of GSK-3β and p38 (Figure 5). We observed that the protein phosphorylation of GSK-3β (Tyr216) and p38 was reduced as a result of STZ exposure, whereas oral administration of DHM markedly inhibited the phosphorylation levels of GSK-3β (Tyr216) and p38 (p < 0.01).

DHM decreased the phosphorylation of p38 and GSK-3β (Ty216) in the livers of rat. Relative density analysis of the (A) phospho-p38 and (B) phospho-GSK-3β (Ty216) protein bands. β-Actin was probed as an internal control in the relative density analysis. Data are shown as mean ± standard deviation; ## p < 0.01, compared with the control (normal) group; **p < 0.01, compared with the vehicle group (diabetic vs. normal group). N = 6 in each group. DHM, dihydromyricetin; Met, metformin.
4. DISCUSSION
In this study, DHM ameliorated impairments by regulating the expression of AMPK, NF-κB, TLR4, MyD88, p38, and GSK-3β in rats. Diabetes is characterized by insulin resistance, which is associated with hyperglycemia. To maintain glucose homeostasis, a complex mechanism is involved in glucose uptake and production. In this regard, liver always plays a key role in modulating glucose metabolism, including in glycolysis, gluconeogenesis, glycogenolysis, and glycogenesis. It is well known that secretion of inflammatory cytokines is associated with insulin sensitivity, thus, diabetic patients typically have comparatively high levels of TNF-α and IL-1β. To elucidate the underlying mechanism of the anti-inflammatory potential of DHM, genetic approaches were used in this study (Figure 2). As mentioned earlier, TNF-α and IL-6 are key factors that influence diabetic status, with increase in TNF-α negatively affecting insulin signal transduction. TNF-α activates signaling pathways associated with cell survival, apoptosis, inflammatory response, and cell differentiation. In addition, TNF-α is involved in the regulation of IL-1β expression, induction of acute phase response, and NF-κB p65 activation. We noted increases in the TNF-α and IL-1β expression in the liver of STZ-induced diabetic rats. The result presented here indicated that increased TNF and IL levels were elevated by DHM. Several lines of evidence show a close link between oxidative stress and inflammation [13,14]. STZ can induce extensive Reactive Oxygen Species (ROS) formation, which may cause damage to proteins, lipids, and DNA [15]. In the next stage of our experiment, we found that DHM treatment significantly improved glucose homeostasis through enhancement of insulin secretion in STZ-induced diabetic rats (data not shown). Six weeks of daily treatment with DHM dramatically improved the quality of life for diabetic rats by decreasing plasma glucose and increasing plasma insulin. In addition, DHM was found to significantly alleviate liver damage in diabetic rats. The underlying mechanism related to this antidiabetic action might be due to both liver protection and anti-inflammatory action. The first mechanism responsible for the protective action of DHM was the upregulation of SOD and GSH activities in the liver, resulting in lower levels of MDA (Table 1). By regulating the balance of defense enzymes that catalyze the transformation of singlet oxygen into H2O2, the cellular constituents are protected from oxidative damage. It is well known that oxidative stress plays an important role in the development of injury caused by diabetes and these injuries are exacerbated in diabetes. Several investigators have reported that SOD concentration is decreased in patients with type 2 diabetes [14]. Treatment with DHM improved the activities of SOD and therefore ameliorated tissue damage in diabetic rats. The activity of GSH in the liver of diabetic rats was also significantly inhibited (p < 0.01). GSH can reduce the scavenging rate of H2O2 and endorses hydroxyl radical formation which leads to more internal radicals. Treatment with DHM increased hepatic GSH level in diabetic rats. Thus, the improved SOD and GSH levels likely resulted in inhibition of oxidative stress. In addition, MDA levels in the diabetic group were significantly increased compared with the normal group, indicating that oxidative stress was stimulated in diabetic rats after STZ induction. These results are partially in agreement with a previous research in which investigators found that curcumin could inhibit lipid peroxidation and protect tissues from external damage in glycemic rats [15].
Further studies have indicated that STZ can elicit a nonspecific islet inflammation and apoptosis in animals [16]. Bcl-2 protein family including proapoptotic Bax and caspases family play key roles in development of apoptosis under oxidative stress condition [16]. These sequences of events were observed in this study by immunoblot assay (Figure 3). Interestingly, DHM treatment markedly inhibited protein expressions of Bax and cleavage caspase-3, suggesting that DHM could inhibit the activation of the apoptotic pathway (Figure 3). Moreover, DHM increased STZ-induced Bcl-2, further confirming that the protective effects of DHM are mediated by suppression of apoptosis.
Initiating the study in hepatocytes, we separated signaling effects of DHM on inflammatory signaling (Figure 4). Interestingly, we found that DHM as well as metformin significantly elevated protein expression of AMPK. This mechanism is fully consistent with our other observations that metformin directly suppressed ROS and allergic eosinophilic inflammation via AMPK activation in mouse lung tissue [16]. Because TLR4, MyD88, and NF-κB activation, as well as AMPK modulation could mediate proinflammatory cytokines expression, we next tested the expression of these proteins in liver tissues. As shown in Figure 4, diabetes was found to activate TLR4, MyD88, and NF-κB, and western blotting experiments have demonstrated that activation of expression of these proteins could be inhibited by administration of DHM at tested doses for 6 weeks. TLR4 could regulate inflammatory response through the MyD88 pathway. Previous evidence has found that STZ exposure could induce diabetic liver injury via the TLR4/MyD88/NF-κB signaling pathway [17]. The inhibition of TLR4, MyD88, and NF-κB might be a critical step for the prevention of cascading inflammatory responses in complications of diabetes [17]. DHM was previously found to markedly inhibit inflammatory injury by inhibiting TLR4 activation and subsequently suppressing the associated downstream signaling pathway of NF-κB [18]. Notably, metformin was proved to have the ability to inhibit liver NF-κB activation in diabetic rats under our experimental conditions. This result confirms the findings of Hou et al. [18], but is inconsistent with many other previous reports [1,4,7]. Overall, these results suggest that DHM exhibited protective effect against diabetes which may also occur partly by its action against diabetes-induced liver inflammation in the NF-κB-dependent pathway. TLR4 is another membrane-bound receptor that plays a critical role in the innate immune response.
In addition, p38 could regulate cellular properties in response to oxidative stress and inflammatory cytokines [19]. Both in vivo and in vitro evidence have suggested that p38 may be also involved in high-glucose-induced cellular hypertrophy [20]. Studies have also indicated that DHM reduces endotoxic inflammation via repressing ROS-mediated activation by suppressing p38 [21,22]. In the last section of this study, DHM was found to inhibit p38 expression and attenuate inflammatory-induced damage (Figure 5), suggesting that DHM administration may have a critical role in preventing the progression of liver inflammatory diseases. Activation of Akt leads to inactivation of GSK-3β, which in turn enhances the expression of GS protein, potentially, on glucose transport activity. Inhibition of GSK-3 by the AMPK signaling pathway is involved in the regulation of the NF-κB activation and inflammatory pathway [23]. Recently, DHM was proved to have protective effects on STZ-induced neurotoxicity by suppressing the regulation of GSK-3β/Nrf2 signaling [4]. DHM also elicits potent neuroprotective effects in a model of major depressive disorder by inhibiting GSK-3β activation [3]. In our animal model, phosphorylation of GSK-3β was stimulated after STZ induction, which might cause an inflammatory response in liver (Figure 5). However, DHM treatment inhibited inflammation and suppressing the GSK-3β activity. These results provide the first evidence that DHM exerts protective effects on STZ-induced liver damage via the GSK-3β pathway.
5. CONCLUSION
In conclusion, DHM reversed STZ-induced hepatic impairments by suppressing oxidative stress, inflammation, and apoptosis (Figure 6). In particular, DHM ameliorated diabetic oxidative damage in the liver. DHM inhibited hepatic apoptosis by triggering the levels of Bcl, Bax, and cleaved caspase-3. Finally, DHM inhibited inflammatory response by modulating the TLR4, MyD88, AMPK, p38, and GSK-3β pathways.
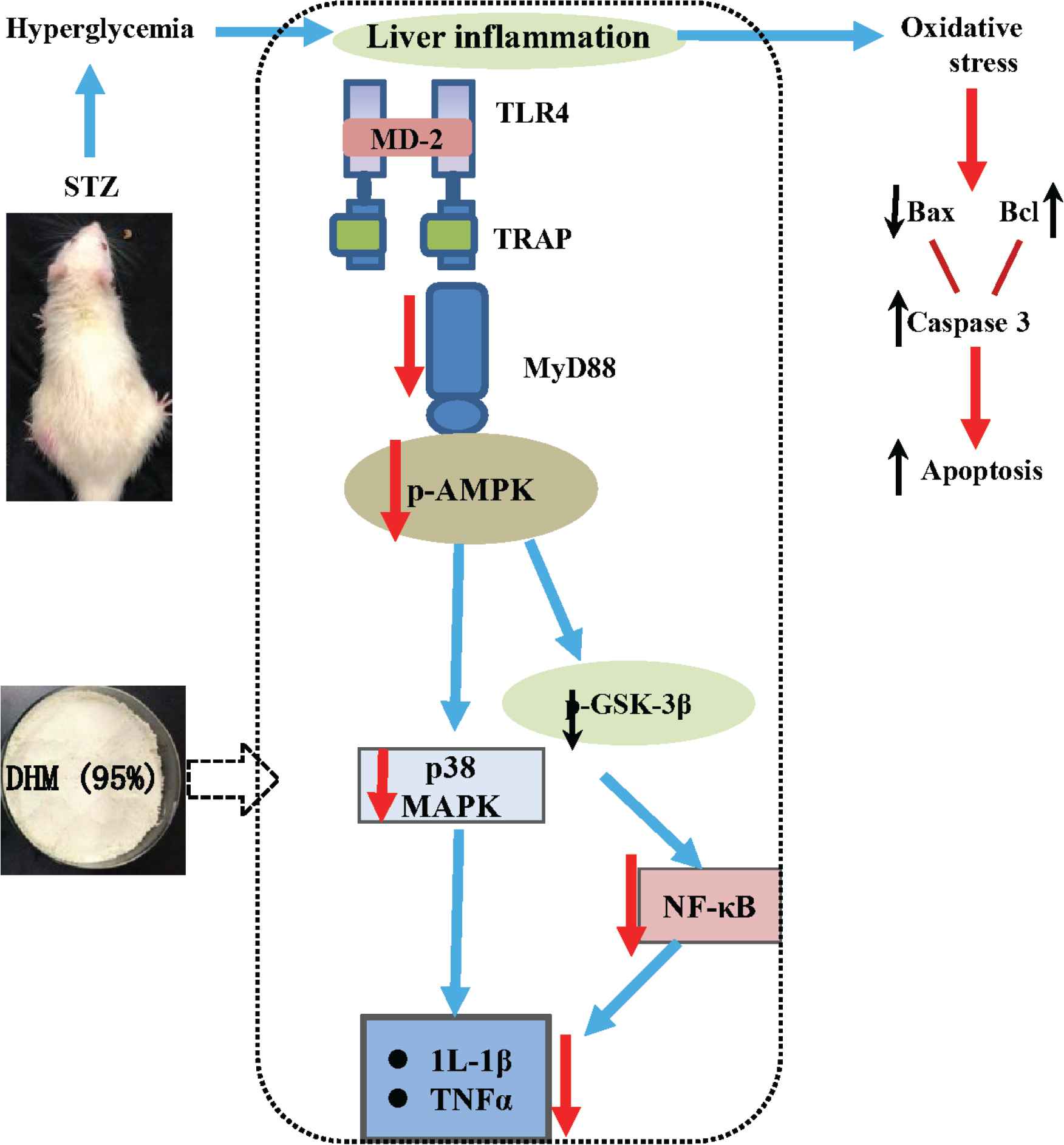
Proposed underlying mechanism of hepatoprotective effects in diabetic rats. DHM, dihydromyricetin; IL, interleukin; TLR, toll-like receptor; TNF, tumor necrosis factor.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
LC, MY, XF and XL performed the experiments. HT and JX analyzed the action mechanism. LC conceived and designed the experiments. SW and HT revised the manuscript. RA, AS, BS, SD and PP reviewed and edited the manuscript.
ACKNOWLEDGMENTS
This work is supported by the
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Lei Chen AU - Maojun Yao AU - Xiaoyun Fan AU - Xiujun Lin AU - Randolph Arroo AU - Aline Silva AU - Bunleu Sungthong AU - Simona Dragan AU - Paolo Paoli AU - Shaoyun Wang AU - Hui Teng AU - Jianbo Xiao PY - 2020 DA - 2020/02/19 TI - Dihydromyricetin Attenuates Streptozotocin-induced Liver Injury and Inflammation in Rats via Regulation of NF-κB and AMPK Signaling Pathway JO - eFood SP - 188 EP - 195 VL - 1 IS - 2 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.200207.001 DO - 10.2991/efood.k.200207.001 ID - Chen2020 ER -
