Natural Polyphenols: A Potential Therapeutic Approach to Hypoglycemia
- DOI
- 10.2991/efood.k.200302.001How to use a DOI?
- Keywords
- Polyphenols; hypoglycemic; absorption; gut microbiota; microRNAs
- Abstract
Natural polyphenols have been reported much attention in recent years for their hypoglycemic actions and diabetes-related pathologies. Free and simple polyphenols can be digested and absorbed in the small intestine, while complex polyphenols are utilized by intestinal flora in the large intestine. Microbial metabolites of polyphenols may regulate the production of bile acids, thus affecting the metabolism of the host. Polyphenols generally reduce postprandial blood glucose level via inhibition of α-amylase and -glucosidase and regulate glucose metabolism by inhibition of intestinal absorption of glucose and the release of glucose from the liver, stimulation of pancreatic insulin secretion, and enhancement of glucose uptake by muscle cells and adipocytes. The importance of microRNAs as regulators of anti-diabetic effects was recognized. Polyphenols can also regulate the microRNAs as therapeutic targets. This review provides a summary of the mechanisms of polyphenols on lowering blood glucose and the potential hypoglycemic in treating diabetes mellitus.
- Copyright
- © 2020 International Association of Dietetic Nutrition and Safety. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. DIABETES MELLITUS
Diabetes mellitus is one of the well-known metabolic disorders described by high blood glucose and low circulating insulin levels [1]. Long-term diabetes might lead to postprandial hyperglycemia and health issues including cardiovascular complications [2]. The World Health Organization (WHO) reported that the incidence rate of diabetes was more than 200 million people worldwide, which might exceed 400 million by 2030 [3]. Type 2 Diabetes Mellitus (T2DM) is one of the main types of diabetes and contributes to the majority (90–95%) of diabetic patients. Previous studies have demonstrated that reducing the content of postprandial glucose might be a practical therapeutic proposal for T2DM treatment. The excess absorption of postprandial glucose could be postponed via the suppression of carbohydrate-hydrolyzing related enzymes such as α-amylase or -glucosidase in the digestive organs, which could delay the digestion of carbohydrate [4,5]. These enzyme inhibitors led to lower glucose uptake and then consequently inhibit the elevation of postprandial blood sugar. Insulin Resistance (IR) plays an essential role in the pathogenesis of T2DM by motivating glucose transported into the skeletal muscle cells. Although modern medicines are adequate for the treatment of diabetes, unexpected adverse drug reactions limit their clinical application. Thus, searching the natural compounds for facilitating glucose uptake is a vital way to improve insulin resistance.
2. DIETARY POLYPHENOLS
Plants contain various polyphenols, which could interact with mass proteins and inhibit the activities of specific enzymes (Table 1). They also possess various substantial phenolic compounds, including flavonoids, as well as fatty acids, and polysaccharides. Recent studies indicated that plant polyphenols have potential benefits to improve human health by protecting body cells against the damage of free radicals and inhibiting relevant carbohydrate-hydrolyzing enzymes [6]. Inthongkaew et al. [7] have revealed that several plants extracts could down-regulate the activities of α-amylase and -glucosidase and have potential applications in controlling postprandial hyperglycemia. A methanolic extract of Dendrobium formosum (50 μg/mL) was found to have 95% inhibitory activity on both α-glucosidase and pancreatic lipase. Twelve phenolic substances were isolated by chromatographic methods in the extracts of D. formosum (Table 1) [7]. Similarly, the phenolic extracts of Dendrobium tortile also showed strong effects on the inhibition of α-glucosidase [8]. Different processes affected the content of certain phenolic acids and flavonoids [9]. Gong et al. [10] have reported that in corn the germination process increased the polyphenol composition from 12 to 15 species, while the extrusion treatment after germination improved the functional properties of whole-wheat flour. Interestingly, almost all polyphenol extracts showed significantly higher inhibitory activity of α-glucosidase than α-amylase. Ademiluyi et al. [11] have also shown that all the Corchorus species had lower inhibitory activity of α-amylase than that of α-glucosidase. The mass data obtained by Oboh et al. [12] suggested that free polyphenolic extracts, containing chlorogenic acid and isorhamnetin, showed a significantly higher inhibitory property compared with the bound forms. Thus, chlorogenic acid and isorhamnetin might have an essential effect on the suppression of specific enzyme activities [12]. Moreover, a previous study indicated that flavonoids could obviously inhibit the activity of α-amylase more compared with other phenolic compounds [13]. Considering together, phenolic compounds with the active inhibitory activities of α-amylase and -glucosidase might be used as an alternative for controlling the levels of postprandial hyperglycemia and declining the risks of morbidity of T2DM.
| Resource | Polyphenols | Structure | Hypoglycemic mechanism | References |
|---|---|---|---|---|
| Penthorum chinense | Pinocembrin-7-O-[4″, 6″-hexahydroxydiphenoyl]-β- |
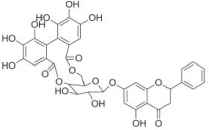 |
Serum levels of glycosylated hemoglolin A1C (HbA1c) ↓, triglyceride (TG) ↓ | Huang et al. [118] |
| Total cholesterol (TC) ↓ | ||||
| Blood glucose ↓ | ||||
| Pinocembrin-7-O-[3-O–galloyl-4″, 6″-hexahydroxydiphenoyl]-β- |
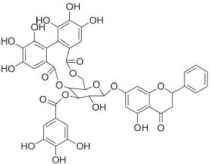 |
Low-density lipoprotein-cholesterol, serum levels of high-density lipoprotein-cholesterol and insulin ↑ | ||
| Oral glucose tolerance test ↑ | ||||
| Thonningianin A | 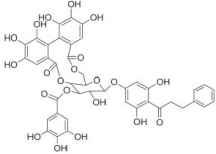 |
|||
| Yellow corn (Zea mays L.) grains | Contain five benzoic acid derivatives, five cinnamic acid derivatives, and seven flavonoids, respectively | / | Inhibited pancreatic α-amylase and intestinal α-glucosidase | Gong et al. [10] |
| Millet grains (barnyard, proso, and foxtail) | Contain three benzoic acid derivatives (gallic, p-hydroxybenzoic and vanillic acids) and five cinnamic acid derivatives (caffeic, chlorogenic, ferulic, sinapic and p-coumaric acids). | / | Inhibited α-amylase and -glucosidase | Pradeep et al. [9] |
| Three flavonols (rutin, kaempferol, and myricetin), one flavone (apigenin) and one flavanone (naringenin) | ||||
| Corchorus olitorius leaves | Caffeic acid | 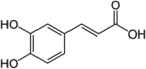 |
Inhibited α-amylase and -glucosidase activity | Oboh et al. [12] |
| Chlorogenic acid |  |
|||
| Isorhamnetin | 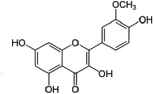 |
|||
| Jute (Corchorus spp.) | Phenols; vanillic acid; caffeic acid; apigenin; kaempferol; luteolin; quercetin; myricetin; rutin; gingerol | / | Inhibited α-amylase and -glucosidase activity | Ademiluyi et al. [11] |
| Senna surattensis | Phenolic acids, flavonol, flavones, flavonones, and flavonoid glycosides | / | Inhibited α-amylase and -glucosidase activity | Thilagam et al. [119] |
| Tropical and subtropical plants | Total phenolic content | / | Inhibited α-glucosidase activity | Indrianingsih et al. [5] |
| Cornus alba (Ca) fruits | Flavonoids (quercetin and kaempferol derivatives), phenolic acids derivatives | Inhibit pancreatic enzymes, α-amylase and pancreatic lipase (PL) activities | Świerczewska et al. [120] | |
| Dendrobium formosum | Confusarin (1); Hircinol (2); Erianthridin (3); Gigantol (4); Nudol (5); Lusianthridin (6); Coelonin (7); Dihydroconiferyl dihydro-p-coumarate (8); Batatasin III (9); 2,5,7-Trihydroxy-4-methoxy-9,10-dihydrophenanthrene (10); Moscatilin (11); 5-Methoxy-7-hydroxy-9,10-dihydro-1,4-phenanthrenequinone (12) |  |
Inhibit α-glucosidase and pancreatic lipase activities | Inthongkaew et al. [7] |
| Dendrobium tortile | 3,4-Dihydroxy-5,4′-dimethoxybibenzyl |  |
Inhibit α-glucosidase activities | Limpanit et al. [8] |
| Eriodictyol | 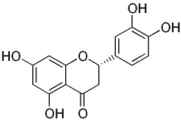 |
|||
| Dendrofalconerol A | 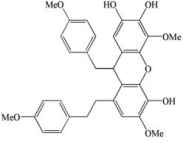 |
|||
| Corchorus olitorius | Caffeic acid | 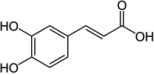 |
Inhibited α-amylase and -glucosidase activity | Oboh et al. [12] |
Hypoglycemic effects of natural polyphenols
3. ABSORPTION OF POLYPHENOLS
Polyphenols exert an essential effect in regulating glucose metabolism. The bioavailability of polyphenols is a limiting factor which could influence their advantageous effects on the human body [14]. Most polyphenols are present in the forms of esters, glycosides, polymers in plants (combined with other phenols and organic acids) [15]. It is generally demonstrated that plant polyphenols are seldom absorbed in the upper alimentary tract due to their molecular complexity and to the properties of the food matrix. Polyphenols are digested by some specific mechanisms; for example, they could be hydrolyzed by Lactase-Phlorizin Hydrolase (LPH) [16]. Besides, the food matrix also affects the release of polyphenols in foods. It was reported that the fiber and fat constituents of cocoa show a protective effect, which increased the absorption of polyphenols [17]. Similarly, cheese and milk with an appropriate fat/protein ratio could protect green tea polyphenols in gastric and intestinal phase as well as promote their antioxidant efficiency. Also, catechin acid and tannic acid combined with endogenous proteins inhibited the degradation of specific polyphenols in the human small intestine [18,19]. However, the function of polyphenols was reduced when dietary fiber was bonded to the surface of polyphenols [20]. Therefore, it is essential to thoroughly detect the effect of food matrices on specific polyphenols and find a valid method to improve the bioavailability of polyphenols [21].
Polyphenols in foods are subjected to be hydrolyzed by saliva in the mouth and then transferred to the stomach. The polyphenols attached with the solid matrix are released under strong acidic condition and reduced into monomeric units [22]. The anthocyanins of wild blueberry, polyphenols from almond skin, pistachio, as well as phenolics and flavonoids from apples could be well chemically modified and absorbed during gastric phase [23,24]. The partially digested polyphenols with a low degree of polymerization (<5) or low molecular weight might be further degraded during the intestinal phase. Moreover, it was calculated that approximately 48% and 42% of dietary polyphenols are accessible in the small and large intestine, respectively [25]. Similary, a high bioavailability of catechin from white tea and anthocyanins from pomegranate was reported to be observed after intestinal steps [26]. The high absorptivity of polyphenols was mainly attributed to the modification of microbiota and the effect of related enzymes. In the small intestine, some glucose-bound polyphenols (quercitrin 3-O-β-glucoside and quercetin 4′-O-β-diglucoside) were deglucosylated by enterocyte microvilli glycoprotein [27]. Besides, the intestinal microbial enzymes have been demonstrated to be able to cut off the linkage of two benzene rings in monomeric polyphenols and oligomeric polyphenols, as well as promote the dehydroxylation and hydrogenation of polyphenols [28]. During intestinal phase, the methylation and sulfation of polyphenols contribute to the enhanced hydrophilicity which helps polyphenols to be further metabolized by organs and excreted to urine. Most proanthocyanidins are split into phenylvalerolactones and phenolic acids under the process of colon microbes [29]. In addition, the small and large intestine epitheliums make the gut barrier to control the absorption and biotransformation of polyphenols [16]. Once the less complex polyphenols and their metabolites are uptaken from gut to the blood, they would be bound with albumin or other proteins which could lead polyphenols to be effectively transported into metabolic tissues [30]. A portion of polyphenols is transported to the liver by the portal vein and then oxidized by cytochrome P450 enzyme and liver microsome. These polyphenols could also be subjected to form conjugates by the effect of phase I enzyme (hydrolase, reduction) and phase II enzymes (transmethylase, acetyltransferase) [31,32]. After being oxidized and hydrolyzed in hepatocytes, polyphenols are excreted to duodenum with bile acid for the second systematic circulation, and finally, the polyphenols and their metabolites fade away in bile and urine. Resveratrol is stable and easy to be digested in the gut because of the existence of glycosylation [33]. However, there are also some polyphenols like tannins with large molecular weight which are not absorbed by tissues and these tannins are found in their original form in faeces [34,35]. Besides, the colon microflora has different abilities on the degradation of polyphenols, for example, procyanidins are degraded into phenyl valerolactone, phenylpropionic acids, and phenylacetic acids with small molecular weight. A simulated gut experiment was conducted to identify the biotransformation and biological activity of polyphenols in blueberry and potato. This study revealed that the concentrations of flavonoids and anthocyanins in intestine decreased 5.9- and 18-times, the antioxidant activity was significantly reduced, and almost no radical scavenging activity was observed in colon after intestinal usage [23]. The content and the antioxidant activity of total polyphenols of grape pomace in duodenum were significantly higher than that in the colon [36]. Moreover, rutin and quercetin of potato were found in the ascending colons but they did not exist in transverse or descending colons [9].
Increasing evidence has been provided that the plant polyphenols could be a kind of glucose regulator as they enter the intestine. Both Sodium-dependent Glucose Transporter 1 (SGLT1) and Glucose Transporter 2 (GLUT2) play a role in the intestinal glucose uptake. SGLT1 is scattered in the intestinal brush border membrane. However, the conformational change of the SGLT1 complex results in a lower affinity of its binding site and a lower Na+ concentration, so glucose and Na+ are released into the intestinal cells [37]. GLUT2 mainly exists in the basolateral membrane, and a reduced microvillus length was found in GLUT2 deficient mice. When glucose enter into the enterocyte, it will be rapidly delivered to the bloodstream by GLUT2 [38]. Meanwhile, in order to ensure a favouring transition of glucose entering the enterocyte, Na+ would be pumped out by the sodium potassium pump, causing a concentration gradient on both sides of the membrane. Some polyphenols, like quercetin, acting as the substrate of SGLT1, inhibit sodium ions binding to the surface of SGLT [39,40]. Green tea polyphenols could directly prevent the transportation capacity of SGLT1 and GLUT2 [20]. The tight junction plays an important role in the gut barrier which could control the transport of materials into the intestine. Glucose could partially permeate into the blood through the cell gap when the concentration of glucose in the gut lumen is high. Polyphenols could protect the integrity of intestine by regulating the activity of tyrosine kinase. Tumor Necrosis Factor α (TNF-α) could also disintegrate claudin-1 and reduce the expression of claudin-2 through reducing the activity of tyrosine kinase. Besides, polyphenols could inhibit the activity of TNF-α. Both polyphenols and TNF-α could directly suppress the activity of tyrosine kinase to reduce ZO-1 tyrosine phosphorylation [41]. Thus, the gut epithelial tight junction could prevent glucose across the junction to some extent. Polyphenols and glucose-bound quercetin could be hydrolyzed by LPH existing in intestinal brush border. UDP-glucuronosyltransferases could bind with glucose again to reduce the content of glucose in the blood (Figure 1).
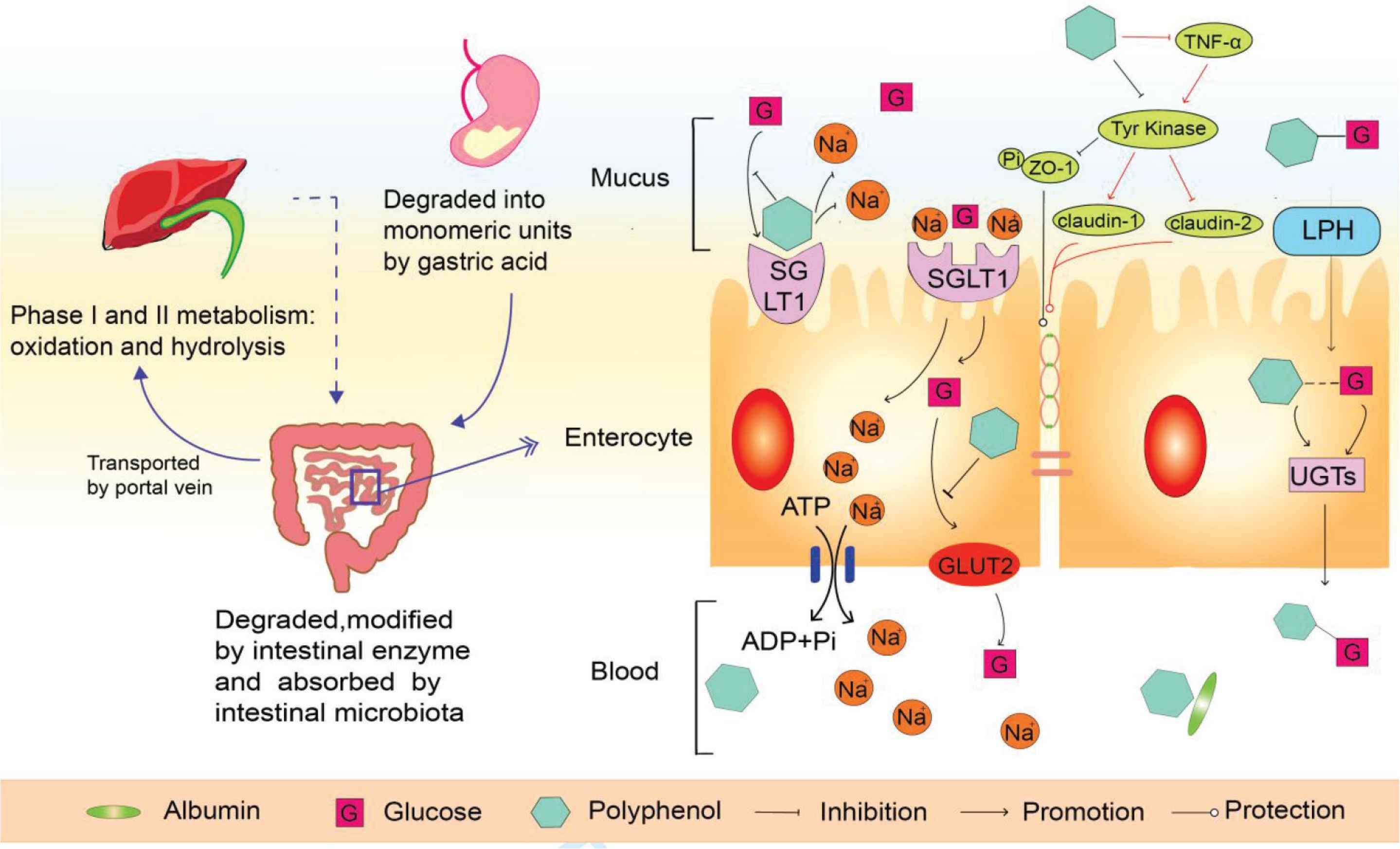
The absorption of polyphenols and their mechanisms on inhibiting glucose absorption.
4. INHIBITION CARBOHYDRATE-HYDROLYZING ENZYMES
Type 2 diabetes mellitus is associated with non-enzymatic glycosylation of diverse biomacromolecules, production of reactive oxygen, and changes of endogenous antioxidants [42]. Although complex polysaccharides cannot be directly absorbed by the intestinal epithelium, they can be hydrolyzed into free glucose by α-amylase and -glucosidase when they enter the stomach. Thus, the level of postprandial blood glucose could be controlled by restraining the activity of these enzymes (Figure 2). The polyphenolic extracts of soybean can regulate postprandial blood glucose level by inhibiting α-amylase and -glucosidase [43]. It was also reported that these phenolic extracts regulated the glucose level through suppressing the digestion and absorption of carbohydrates [43]. Glucose homeostasis plays a vital role in human health. Conversely, glucose metabolism disorders might develop the morbidity of T2DM, cardiovascular disease and result in a high risk of mortality. The previous studies showed that polyphenols could regulate glucose homeostasis and improve T2DM [44]. Polyphenols affect glucose metabolism by inhibiting the absorption of glucose, increasing the secretion of insulin, and restraining the release of glucose in the liver. Rienks et al. [45] have revealed that the daily administration of flavonoids is negatively correlated with T2DM. Kaempferol, a common flavonol, is present in herbal and edible plants. Similarly, kaempferol supplementation distinctly ameliorated hyperglycemia and the level of blood sugar [46]. Also, kaempferol could control the expression of pancreatic Insulin Receptor Substrate-1 (IRS-1), IRS-2 and forkhead box protein-1 (FoxO1), leading to the proliferation of pancreatic β-cell [47]. It was reported that α-amylase and -glucosidase were crucial enzymes for the modification of postprandial blood glucose. Polyphenols extracted from berries including rowanberries, raspberries, blueberries, and strawberries can restrain both enzymes. Spinola et al. [48] have concluded that polyphenols extracted from Cherimoya annona leaves (ellagitannins) could inhibit the effect of glucose degradation related enzyme in an in vitro experiment. Resveratrol improved atherosclerosis and reduced oxidative stress in T2DM patients after 45 days of oral therapy. Also, 3-month resveratrol administration reduced the level of fasting blood glucose, significantly improved IR [49], inhibited α-glucosidase activity, and then prevented the rise of postprandial blood sugar levels [50]. Catechins of green tea affect the inhibition of α-glucosidase and could regulate glucose homeostasis [51]. Growing evidence showed that supplementation of Epigallocatechin-3-Gallate (EGCG) could protect patients from cardiovascular diseases. A previous study reported that EGCG played a role in binding plasma proteins which were usually absorbed in the digestive system and suppressed the modifications of Low Density Lipoprotein under hyperglycemic conditions [52].

Polyphenols and their inhibition of carbohydrate-hydrolyzing enzymes.
5. EFFECT OF POLYPHENOLS ON GUT MICROBIOTA RELATED TO DIABETES
The gut microbiota is regarded as the “separate organ” of human health due to its high number of bacteria compared to other organs in the body [53]. Studies showed that there were 1014 microorganisms, mainly bacteria, commonly found in the gastrointestinal tract. Based on 16S rRNA gene high throughput sequencing of intestinal flora samples obtained from diverse individuals, the major bacteria were divided into four different phyla: Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria from high to low [54,55]. Results indicated that intestinal flora was closely related to the incidence of obesity, T2DM, and other metabolic diseases [56]. The microbial ecosystem is fluctuant, which is related to various factors including environmental dietary and host health. Diet is associated with the alteration of gut microbiota composition and determines the function of the microbial community [57]. Obesity increases the ratio of Firmicutes to Bacteroidetes, which could be reversed by having a low-fat diet [58]. Dietary polyphenols [59], fibers [60], other nutrients, and intestinal flora form a complicated ecosystem.
Polyphenol-rich foods regulated glycolipid metabolism by decreasing the postprandial glycaemic and fasting glucose levels, as well as ameliorating insulin sensitivity and secretion [61]. As mentioned above, different polyphenols in simple or complex structure could be digested and absorbed in various digestive organs [62]. They are broken down into small bioactive components and generate the prebiotic effects by the intestinal microorganisms. Bacteria adjust the removal of glucoside at oxygen and carbon position, the deglucuronidation and fermentation of flavonoids, and the hydrolysis of ester and amide. Thus, aglycones could be dehydroxylated, demethoxylated, and demethylated in the aromatic moieties, instead of hydrogenation, α- and β-oxidation, and ring rupture of substituted aliphatic groups. Ultimately, these simple phenolics are converted to microbial metabolites, such as Short-chain Fatty Acids (SCFAs), lactate, Lipopolysaccharide (LPS), CO2 and H2, and ethanol through gut microbiota [63].
Intestinal flora also affects the IR and T2DM by improving the gut epithelial barrier and mucosal immunity [55,64]. Intestinal epithelial barrier limits the entry of intestinal contents including inflammatory mediators, chyme, and bacteria and also modulates the immune response. Odenwald et al. [65] found that intestinal flora or its metabolites led to the increase of Interleukin (IL) and TNF in HFD-induced mice. They increase transcription and activity of claudin-2 which affects myosin light-chain kinase and IL-13 and then elevate the importation of LPS [66,67]. This process caused an increase of chronic inflammation in the liver, spleen, pancreas, and other tissues and results in various metabolic diseases [68]. Moreover, gut microbiota could directly enter intestinal barrier and metastasize to the pancreas, stimulate NOD-like receptor 2 (NOD2), and then lead to T2DM [69]. The intestinal mucosal plays an essential role in protecting intestinal ecological balance by goblet cells, Intestinal Epithelial Cells (IEC), macrophages, and neutrophils. The goblet cells and IEC generate Anti-microbial Peptides (AMPs) and mucin to defense against pathogens penetrating the gut [70]. IEC could also secrete anti-inflammatory mediators including IL-25, IL-33, and Transforming Growth Factor-β (TGF-β ), which could regulate micro-associated molecular patterns by linking to Toll-like Receptors 5 (TLR5) or NOD2. Innate lymphoid cell (ILC) inhibits the body’s low-level chronic inflammation by the secretion of IL-22, IL-17A, and IL-17F [71]. A high-fat diet could reduce the diversity and alter the distribution balance of gut microbiota and thus decline the production of mucin and other antimicrobial factors [72]. Creely et al. [73] have revealed that obese mice and T2DM human elevated the contents of LPS in the blood. Besides, intestinal microorganism-derived LPS increases the inflammation of body tissues in mice and subsequently leads to T2DM [74]. The polyphenols have prebiotic effects on the intestinal epithelial barrier function [75]. Naringenin, the primary polyphenol of citrus, improves intestinal barrier integrity of Caco-2 cells and ameliorates tight junction barrier of dextran sulfate sodium-induced mice [76]. In addition, quercetin reinforces the levels of claudin-4, ZO-2, claudin-1, and occludin, as well as increases the transepithelial electrical resistance of Caco-2 intestinal cells [77], which are attributed to the enhanced protein levels of CDX2, an essential transcriptional factor administrating intestinal epithelial barrier [78]. This review summarized possible molecular mechanisms and mediators binding polyphenols to their potential beneficial effects on tight junction proteins and intestinal barrier integrity considering previous researches performed so far. Polyphenols inhibit Nuclear Factor-κB (NF-κB) signaling and modulate the mitogen-activated protein kinases, phosphoinositide-3-kinases/protein kinase B (PKB/Akt), or AMP-activated Protein Kinase (AMPK) signaling [41].
Short-chain fatty acids as the fermentation products of intestinal microbiota exert a vital function in maintaining gut homeostasis [79]. SCFAs administration improved diet-induced obesity and T2DM [80], motivated the formation of gut epithelial tight junction, and ameliorated intestinal barrier function [81,82]. SCFAs are also crucial energy sources and improve the conversion rate in intestinal epithelial cells [83,84] and recovered the frequency and number of CD4+ T-cell in germ-free mice [85]. Researches showed that grape polyphenols or purple sweet potato anthocyanins improved their probiotics and SCFAs by gut microbiota [36,86].
Short-chain fatty acids (mainly acetate, propionate, and butyrate) regulate glycolipid metabolism by binding with SCFA receptors such as the G-protein coupled Receptors (GPR41 and GPR43) [87]. GPR43 could be stimulated by acetate and propionate, while GPR41 is primarily motivated by butyrate and propionate. GPR41 and GPR43 are linked with Gi/o and Gq-proteins after SCFAs bound them, respectively, both of which increase the level of cytosolic Ca2+ and regulate Erk1/2 and Akt signaling [88]. Gi/o signaling restrains adenylyl cyclase to decline the production of cAMP, while Gq stimulates the expression of protein kinase-C [89]. Also, Erk1/2 and Akt signaling improve epithelial cell renewal and anti-inflammatory effects and calcium-mediated signaling promotes epithelial barrier function [90]. Butyrate is the inhibitor of histone deacetylase inhibitor, which reduces the penetration of immune cells thereby inhibiting inflammation [91]. SCFAs could exert their beneficial functions by motivating the secretion of intestinal satiety hormone for the extensive existence of SCFA receptors in endometrial epithelial cells [92,93]. Propionate treatment could activate glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) secretion in rodent animals [93]. Olli et al. [94] found that dietary fibers increased satiety and reduced the onset of hunger, which was associated with the enhanced content of SCFAs. Besides, the wheat bran administration enhanced the level of SCFAs and plasma GLP-1 in hyperinsulinemic humans [95]. Fermentable inulin treatment could improve the concentrations of PYY and GLP-1 in the colon and inhibit obesity in HFD-induced mice by increasing SCFA production [96]. To sum up, intestinal microbiota exerts a vital effect in maintaining a healthy diet metabolism and influencing the secretion of satiety hormone as well as improving intestinal epithelial barrier function.
Moreover, microbial metabolites of polyphenols might regulate the production of bile acids, which affect the host metabolism. Bile acids are synthesized in hepatocytes and transported to the intestine to improve the absorption of dietary lipid [97]. They are signaling molecules that control glucose homeostasis in the serum. Bile acids could increase the secretion of incretin hormone GLP-1 through combining with nuclear Farnesoid X Receptor (FXR) and Takeda GPR5 in gut L cell [98]. Shapiro et al. [99] reported that bile acids reduced gluconeogenesis and increased the production of glycogen in the liver, promoted the consumption energy in brown adipose tissue, motivated the secretion of insulin of β-cells in the pancreas, and inhibited inflammation in immune cells. Previous studies have proved that FXR signaling played a negative-feedback effect in controlling the synthesis of bile acids in ileum or liver [100]. The gut microbiota regulates primary bile acids of host-derived by deconjugation, dehydrogenation, and dehydroxylation in distal small intestine and colon, which could improve the diversity of the bile acid pool. Meanwhile, bile acids can also influence the composition of gut microbiota [101].
At present, the metabolic mechanism between polyphenolic nutrients and host–microbe is not precise, which causes a difficulty to explore the causal relationship and molecular mechanisms involved. Metabolomics data has revealed that some polyphenolic metabolites are related to intestinal microbial activity including SCFAs, indole and phenyl derivatives, and sulphated and glucuronidated species [102]. These metabolites involved a series of metabolic pathways leading to potential prebiotic effects in human health. The underlying mechanisms of polyphenolics and their possible prebiotic benefits remained to be further elucidated.
6. POLYPHENOLS AND microRNAs
MicroRNAs (miRNAs) are non-coding RNAs with approximately 19–25 nucleotides in length exist in various creatures [103]. Recent evidence has revealed that miRNA plays a substantial effect in regulating diverse biological processes by replacing the base-interacting sequence of original mRNA (Figure 3) [104]. miRNAs could work on multiple mRNAs and their effects are different. Therefore, the changed level of miRNA leads to the alterations in the expression of corresponding mRNAs. Let-7 is one of the key miRNAs that is associated with human aging [105]. High expression of Let-7 miRNA increased the IR in the mice, which raises the risk of obesity or diabetes [106]. In another experiment, the inhibition of Let-7, significantly improved the insulin-sensitivity and decreased the level of postprandial blood glucose. It could be speculated that the suppression of the expression of Let-7 is useful for treating patients with obesity and diabetes [107]. Numerous studies showed that plant polyphenols could target the miRNAs to affect the biological functions including high fat diet-induced obesity, IR, T2MD, and some cardiovascular diseases [108]. Among these polyphenols, curcumin and (−)-EGCG are widely used and their regulation on miRNA was extensively investigated [109]. Tian et al. [110] have demonstrated that miR-17-5p presented a significant upward trend in high energy diet-fed mouse white adipose tissue. Curcumin could obviously decrease miR-17-5p content and increase the expression of Tcf7l2 [110]. It also declined the relative protein expression level of ILF2 which indicated that curcumin could be a novel strategy for the treatment of pancreatic cancer [111]. Moreover, other studies reported that analogue C66 extracted from curcumin inhibited diabetic-induced nephropathy by regulating the expression of miR-21 in the Nrf2-knockout mice experiment [112].
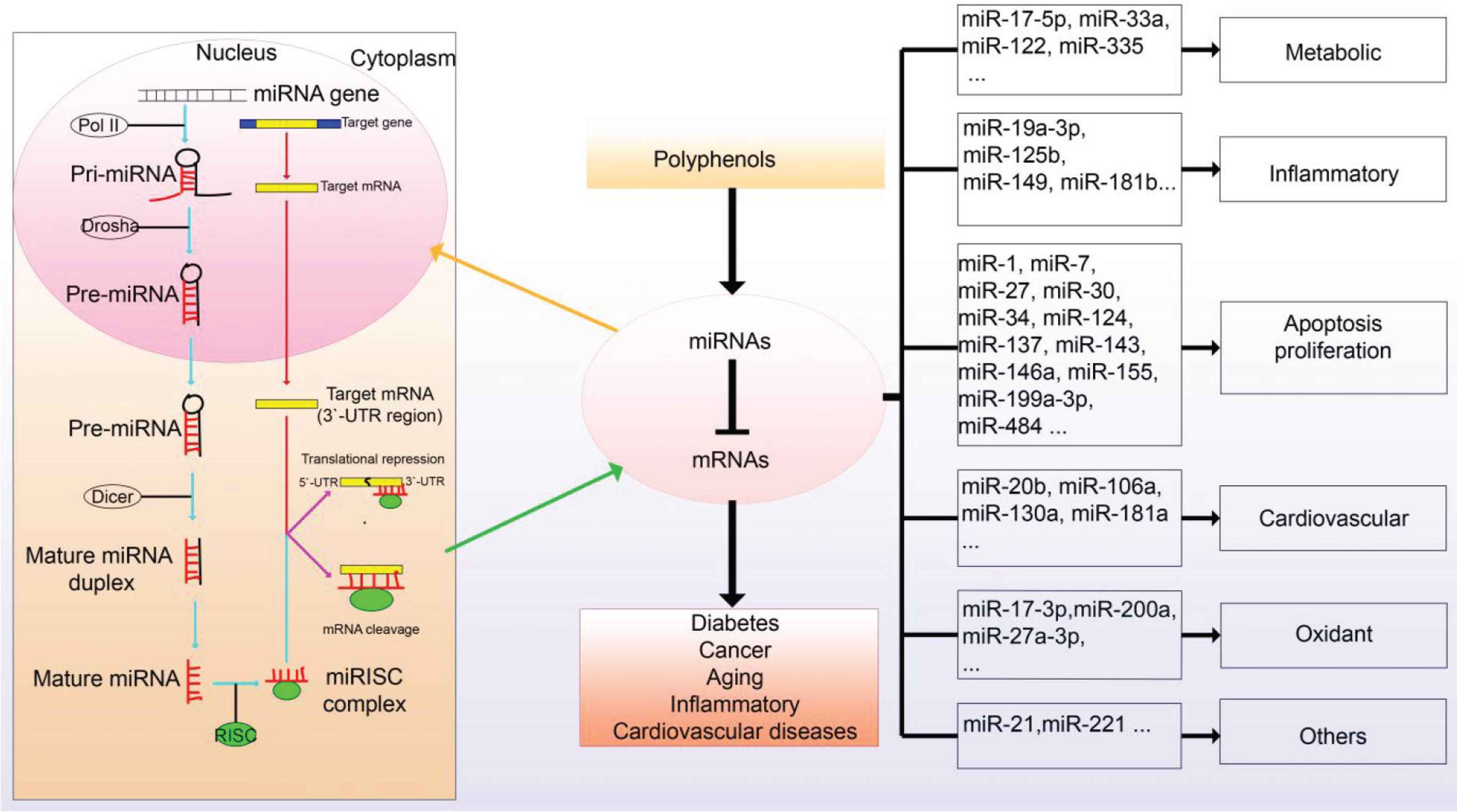
Polyphenols and microRNAs.
Epigallocatechin-3-Gallate, as a kind of polyphenols abundant in green tea, can suppress the growth of the osteosarcoma cell. miRNAs analysis showed that the relative level of miR-1 was enhanced after EGCG treatment in the MG-63 and U-2OS cells [113,114]. It also promoted energy consumption, reduced body weight, and ameliorated glucose tolerance and IR [115]. Baselga-Escudero et al. [116] have revealed that EGCG could regulate both miR-33 and miR-122, which were related to T2MD and metabolic syndromes. EGCG inhibited osteopontin-dependent hepatic fibrosis through up-regulating the content of miR-221 [117]. Moreover, EGCG reduced the expression of cyclooxygenase-2 (COX-2) or prostaglandin E-2 (PGE-2) by the activation of hsa-miR-199a-3p [114]. Polyphenols possess a variety of pharmacological effects on cancer, inflammation, T2DM, aging, and cardiovascular diseases by regulating the levels of miRNAs. To sum up, polyphenols can regulate the levels of various miRNAs, which will guide the application of polyphenols or their active compounds on different diseases.
7. CONCLUSION
Natural polyphenols attracted the attention of researchers due to their numerous positive health effects including their hypoglycemic effects. This vital function can promote the applications of natural polyphenols in food, cosmetic, and pharmaceutical industries. Their excellent performance is related with their chemical structures, and the types and contents of natural polyphenols may differ in different plants. However, the structure-activity relationships of polyphenols still remain largely unclear, and the opportunities and challenges coexist. It is rational to declare that natural polyphenols appear to possess excellent growth potential in modern medicine and healthy foods.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
CZ contributed in study conceptualization, writing (original draft), visualization and funding acquisition. XW contributed in writing (original draft) the manuscript. SZ and HC contributed in writing (review and editing) the manuscript.
ACKNOWLEDGMENTS
The project was financially supported by
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Chao Zhao AU - Xuzhi Wan AU - Sheng Zhou AU - Hui Cao PY - 2020 DA - 2020/03/10 TI - Natural Polyphenols: A Potential Therapeutic Approach to Hypoglycemia JO - eFood SP - 107 EP - 118 VL - 1 IS - 2 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.200302.001 DO - 10.2991/efood.k.200302.001 ID - Zhao2020 ER -
