Tocotrienols in Bone Protection: Evidence from Preclinical Studies
- DOI
- 10.2991/efood.k.200427.001How to use a DOI?
- Keywords
- Vitamin E; bone strength; animals; cells
- Abstract
Osteoporosis is a degenerative bone disease affecting millions, mostly the aging population. Osteoblasts and osteoclasts maintain the balance in bone resorption and formation. Changes occurring in the differentiation, proliferation and activity of these two cell types result in lower bone mass and microarchitecture deterioration, leading to compromised bone strength. Chronic inflammation and oxidative stress disrupt the balance between osteoblasts and osteoclasts. Tocotrienols, vitamin E isoforms with an unsaturated hydrophobic tridecyl chain, reduce oxidative stress and inflammation by downregulating reactive oxygen species, nuclear factor-κB activation and pro-inflammatory cytokines and upregulating the expression of antioxidant enzyme in bone cells. Consequently, tocotrienols increase bone mineralization, promote osteoblast differentiation, and suppress osteoclast formation and differentiation. In vivo studies using various animal models of osteoporosis show improved biomarkers of bone formation and bone strength with tocotrienol supplementation. Tocotrienol-mediated downregulation of the mevalonate pathway that provides substrates for the biological activities of small guanosine triphosphate-binding proteins may also contribute to the regulation of osteoblasts and osteoclasts. Clinical studies are needed to confirm the bone-protection offered by tocotrienols that are found in abundance in plant foods such as fruits, vegetables, nuts, seeds and oils, which are also known for their antioxidant and anti-inflammatory effects.
- Copyright
- © 2020 International Association of Dietetic Nutrition and Safety. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Osteoporosis is a degenerative bone disease common in the aging population. It is anticipated that by the year 2020, over 200 million individuals worldwide will be affected by the disease [1]. Osteoporosis increases fracture incidence and fracture-related mortality. As the U.S. population over the age of 50 continues to increase, projected medical costs related to osteoporosis are also expected to rise to $25.3 billion by the year 2025 [2]. It is estimated that men comprise 30% of all fractures, though their overall risk for osteoporosis is lower than that for women [2].
Osteoporosis, a disease affecting the skeletal system, is characterized by a decrease in bone mass and breakdown of bone architecture compromising overall structure [2]. Changes in the bone tissue architecture and the weakening of bones result in an elevated risk of fracture and a reduction in functionality [2]. Osteoporosis can be categorized as primary or secondary osteoporosis. Primary osteoporosis generally results from declined hormone levels, specifically estrogen deficiency in females or androgen deficiency in males [3,4]. The aging process can also induce primary osteoporosis in both males and females. In comparison to their male counterparts, females are generally at a higher risk for the development of osteoporosis due to drops in estrogen levels following onset of menopause and/or a smaller initial bone structure [3,4]. Nevertheless, males are prone to osteoporosis later in life due to decreases in testosterone levels [5]. Secondary osteoporosis occurs as a result of another disease, disorder or comorbidity and is often characterized as juvenile, localized, disease-induced, or drug-induced osteoporosis [2].
The bone remodeling process involves bone resorption and formation, and osteoporosis results from an imbalance between the two processes [6]. Osteoblasts, cells responsible for the production of new bone, and osteoclasts, cells that break down old bone, are the two primary types of cells involved in the bone remodeling process [2,6,7]. Changes occurring in the differentiation, proliferation and activity of osteoblasts and osteoclasts can cause lower bone mass and weakening of the microarchitecture of the bone, subsequently reducing the strength of the tissue and increasing fragility [2].
Risk factors for osteoporosis can be characterized as modifiable or non-modifiable. Non-modifiable risk factors include genetics, age, and gender whereas modifiable risk factors may include inactivity, dietary habits, body weight, activity level, smoking, and excessive alcohol intake [6]. Modifiable risk factors - most notably, excessive elevation of oxidative stress stemming from antioxidant insufficiency [8], high levels of Reactive Oxygen Species (ROS) in systemic circulation [9] and an activation of inflammatory pathways - contribute to the development of osteoporosis through a variety of mechanisms.
Excessive oxidative stress impacts actions of the osteoblasts and osteoclasts. Oxidative stress results in apoptosis of osteocytes and osteoblasts, contributing to a lowered Bone Formation Rate (BFR) [9]. Furthermore, oxidative stress increases osteoclast differentiation and consequently bone resorption. Chronic inflammation, characterized by elevated levels of pro-inflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α), Interleukin (IL)-1, and IL-6 and secondary to oxidative stress, has a detrimental impact on the bone remodeling process [6,9,10].
Bioactive compounds with anti-oxidant and anti-inflammatory properties are compounds capable of suppressing or preventing excessive oxidative stress, resulting in lower oxidative stress and reduced effects of ROS/free radicals in the development of bone loss. Among the bioactive compounds, naturally occurring forms of vitamin E, abundant in plant foods such as fruits, vegetables, nuts, seeds and oils, are known for their potent antioxidant and anti-inflammatory effects. Vitamin E is a collective term for two major groups: tocopherols and tocotrienols, each with four distinct isomers (α, β, γ, and δ). The major difference between these two groups lies in the unsaturated hydrophobic tridecyl chain that is present in tocotrienols but absent in tocopherols [11,12]. Within each group, the four isomers are found naturally in varying concentrations. The distribution of tocotrienols in nature is vastly different than that of tocopherols. Tocopherols are widely distributed in the majority of plants, while tocotrienols are most commonly found in annatto seeds (90% δ-tocotrienol and 10% γ-tocotrienol [2]), rice bran, wheat germ, palm fruits, palm oil [2], and the oils of these plants. Vitamin E present in annatto beans consists of approximately 90% δ-tocotrienol and 10% γ-tocotrienol [2]. While prior research has focused on α-tocopherol as the primary bioactive form of vitamin E, recent investigations have shifted to utilizing tocotrienols as an intervention in models of chronic diseases due to their higher potency. In this article, we briefly summarize the state of knowledge regarding the effects of tocotrienols on bone protection and cover the findings from in vitro and in vivo studies.
2. IN VITRO STUDIES
Bone is a connective tissue that continuously undergoes remodeling through a balance of formation and resorption. A balance between resorption and formation is crucial to maintaining bone health since excessive bone resorption or inadequate bone formation leads to osteoporosis. Reducing bone resorption (osteoclastogenesis) and/or stimulating bone formation (osteoblastogenesis) may restore the balance and prevent bone deterioration [13,14].
Table 1 provides a summary of studies investigating the impacts of tocotrienols in bone health using in vitro models. Osteoblasts and pre-osteoblasts were utilized to study the ability of tocotrienols to promote osteogenesis. γ-Tocotrienol was found to protect osteoblasts from H2O2-induced Malondialdehyde (MDA) elevation and toxicity [14,15] prevent reduction in antioxidant enzymes including Glutathione Peroxidase (GPX), Superoxide Dismutase (SOD), and Catalase (CAT), and reduce capase-3 activity and ssDNA, thereby preventing apoptosis [14,15]. Additionally, γ-tocotrienol has been effective at attenuating inflammation, specifically through TNF-α-induced NF-κB activation and inducing sphingolipid synthesis in bone derived macrophages [16]. Recently, the impact of Annatto-extracted tocotrienol (AnTT) on cell morphology and differentiation was measured in a pre-osteoblastic MC3T3-E1 cell line [17]. AnTT was found to induce a dose-dependent response on the expression of osteblastic differentiation markers such as Osterix (OSX), Alkaline Phosphatase (ALP), and Osteocalcin (OCN) and enhanced mineralization. This study also showed the ability of AnTT to increase collagen formation and calcium deposition in cell culture. AnTT was also found to suppress 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase [18], the rate-limiting enzyme in the mevalonate pathway that provides essential intermediates for the prenylation of small G proteins; consequently AnTT inhibited RhoA activation and increased level of Bone Morphogenetic Protein 2 (BMP-2) protein in MC3T3-E1 cells [17]. The ability of tocotrienols to modulate differentiation of osteoblastic cells was confirmed by Shah and Yeganehjoo [19] who demonstrated the increased expression of bone BMP-2 and subsequent rise in ALP activity potentiating the mineralized matrix. In addition to the role of tocotrienols in osteoblastic differentiation, recent investigations focused on δ-tocotrienol’s inhibitory effect on the t-BHP-induced oxidative stress in pre-osteoblastic MC3T3-E1 cells and osteocyte MLO-Y4 like-cells. δ-Tocotrienol reduced intracellular ROS concentrations and increased the Glutathione to Oxidized Glutathione (GSH:GSSG) ratio, indicating the antioxidant capability of δ-tocotrienol [20].
| Model | Tocotrienol | Mechanistic impact of tocotrienol | References |
|---|---|---|---|
| Pre-osteoblasts | Annatto-TT | ↑ OSX, COL1α1, ALP, and OCN in time dependent manner | Wan Hasan et al. [17] |
| ↑ Mineralization | |||
| ↑ Type 1-collagen levels | |||
| δ-TT | ↑ Calcium deposition | Shah and Yeganehjoo [19] | |
| ↑ BMP-2 expression, ALP activity | |||
| ↓ HMG-CoA reductase expression | |||
| Osteoblasts | γ-TT | Prevented reduction in GPX (1 μM γ-TT), SOD, and CAT activities | Abd Manan et al. [14] |
| ↓ Caspase-3 activity and ssDNA at low doses (1, 10 μM γ-TT) | |||
| ↑ Caspase-3 activity and ssDNA at high dose (100 μM γ-TT) | |||
| At low dose (1 μM), γ-TT protect osteoblasts from H2O2 toxicity | Nizar et al. [15] | ||
| Pre-osteoclasts | α-TT | All α-TT, δ-TT and γ-TT groups: | Brooks et al. [24] |
| δ-TT | • ↓ TRAP+ osteoclast formation | ||
| γ-TT | Relative to control group, in δ-TT and γ-TT groups: | ||
| • ↓ Calcium phosphate resorption in a dose-dependent manner | |||
| Primary bone derived macrophages | γ-TT | ↓ TNF-α-induced NF-κB activation | Wang et al. [16] |
| ↑ Sphingolipid synthesis | |||
| Co-culture of primary bone marrow derived macrophages and osteoblasts | α-TT | ↓ RANKL expression in osteoblasts | Ha et al. [22] |
| ↓ RANKL-induced osteoclast differentiation by suppressing c-Fos expression, ERK and NF-κB activation expression | |||
| ↓ Bone resorbing activity of mature osteoclasts | |||
| Oxidative stress induced in pre-osteoblasts and osteocytes | δ-TT | ↓ ROS | Casati et al. [20] |
| ↑ GSH/GSSG ratio |
↑ Increase; ↓ decrease; TRAP, tartrate-resistant acid phosphatse; TT, tocotrienols.
Summary of in vitro studies of tocotrienols on bone health
In terms of osteoclastogenesis, osteoclast formation is known to be induced through protein receptor activator nuclear factor-κB (RANK) bound to its ligand (RANKL), causing downstream signaling to activate osteoclast differentiation and bone resorption activity through osteoclasts. Tocotrienols are widely accepted as being potent antioxidants and having anti-inflammatory actions through acting as a potent ROS scavenger [21]. Ha et al. [22] investigated the effects of α-tocotrienol on osteoclast activation and showed that α-tocotrienol reduced RANKL expression in osteoblasts, likely through modulating Prostaglandin E2 (PGE2) and its downstream targets. In the same study, exogenous RANKL addition failed to reverse the inhibitory effects of α-tocotrienol on osteoclast differentiation in a co-culture system, suggesting α-tocotrienol’s ability to modulate RANK activation [22]. In addition to playing a role in osteogenesis by modulating oxidative stress and inflammation, several tocotrienol isoforms have exhibited the ability to stunt osteoclast activity [23]. Specifically, Brooks et al. [24] demonstrated the ability of γ-tocotrienol to modulate osteoclast formation and activity in human osteoclasts suggesting the anti-resorptive effect of tocotrienol.
Recent evidence presented by Chin et al. [25] suggested the role of AnTT in exerting joint protective effects following induction of osteoarthritis, a condition driven by the inflammatory process within the joint. This evidence suggested the ability of AnTT to increase the bone formation activity of existing osteoblasts without impacting proliferation of osteoblasts or osteoclasts. Similar evidence was presented in an in vitro model where AnTT treatment of pre-osteoblastic MC3T3-E1 cells resulted in upregulation of osteoblastic differentiation markers such as OSX, ALP and OCN [17]. These results suggest the dual ability of AnTT to support the bone formation activity of osteoblasts and the proliferation of bone forming cells.
Taken together, these results indicate the ability of tocotrienols to promote osteoclastogenesis [17] and modulate oxidative stress [19], potential mechanisms through which tocotrienols impact bone health.
3. IN VIVO STUDIES
Table 2 summarizes the in vivo studies on how tocotrienols impact bone health. Primary osteoporosis often results from hormonal changes in older adults. In females, hormonal changes following onset of menopause are a major contributor to the development of osteoporosis. A declining concentration of circulating estrogen contributes to the loss of bone mass and functional integrity of the bone structure as estrogen aids in regulating bone homeostasis [26]. The Ovariectomized (OVX) female rodent model is commonly used to study the benefit of tocotrienols in bone health [27–32]. The traditionally used OVX model, bilateral ovariectomy (i.e., surgical removal of ovaries), results in estrogen deficiency that mimics estrogen status in postmenopausal women [2]. Osteoprotective effects of tocotrienol have been studied and confirmed in the OVX model of bone loss induced by estrogen deficiency. Several tocotrienol types have shown a protective effect in regard to bone structural parameters in OVX animals. In OVX mice receiving γ-tocotrienol treatment, Deng et al. [28] demonstrated the ability of γ-tocotrienol to prevent the reduction in structural parameters including Trabecular Number (Tb.N), Trabecular Thickness (Tb.Th) and Bone Volume/Trabecular Volume (BV/TV). Several studies have shown that γ-tocotrienol also improves static and dynamic bone parameters including osteoclast and osteoblast numbers as well as bone formation and Mineral Apposition Rate (MAR) [28,33,34]. Osteoprotective effects of tocotrienols similar to those demonstrated by Deng et al. have been shown in other tocotrienols including δ-tocotrienol [31,35], Palm Tocotrienol (PTT) extract [15,27,29,30], and AnTT [36,37]. Tocotrienol supplementation has been evidenced to increase Osteoblast Surface (Ob.S) [38], Os.S/bone surface [38], Osteoid Surface/Bone Surface (OS/BS) [38–40], Osteoid Volume/Bone Volume (OV/BV) [38], Tb.Th [28], Tb.N [36,37], and BFR [28,36,37] all of which are indicators of tocotrienols’ ability to improve bone formation and impact osteoblast activity. Additionally, tocotrienols have been shown to suppress bone resorption by reducing Trabecular Separation (Tb.Sp), Eroded Surface (ES/BS) and Osteoclast Surface (Oc.S), indicating diminished osteoclast activity in OVX models.
| Model | Tocotrienol | Effect of tocotrienol | Mechanism of action | References |
|---|---|---|---|---|
| Female models of osteoporosis | ||||
| OVX animal model | Palm derived vitamin E and tocotrienols | ↑ Bone Ca content and BMD | ↑ GPX, SOD, ALP activity | Soelaiman et al. [27], Muhammad et al. [29,30] |
| ↑ MAR, BFR | ↓ TRAP | |||
| ↑ BV/TV, Tb.N, Oc.S | ↓ MDA levels | |||
| ↓ sLS/BS, dLS/BS | ↓ Serum osteocalcin, IL-1, IL-6 levels | |||
| ↓ Tb.Sp | ||||
| ↔ Tb.Th, Ob.S | ||||
| δ-tocotrienol | ↑ Bone formation and bone strength | ↑ Serum osteocalcin | Abdul-Majeed et al. [31,35] | |
| ↑ Ob.S/BS, OS/BS, OV/BV | ↓ Serum CTX-1 | |||
| ↓ ES/BS, Oc.S/BS | ||||
| Tocotrienol rich fraction | ↑ dLS/BS, BFR, MAR | ↔ Serum osteocalcin and CTX-1 | Aktifanus et al. [32] | |
| ↓ sLS/BS | ||||
| ↔ MS/BS | ||||
| Annatto tocotrienols + LOV | ↑ dLS/BS, MS/BS, MAR, BFR | ↑ BMP-2 expression | Chin et al. [37] | |
| ↓ sLS/BS | ||||
| γ-Tocotrienol | ↑ Femur BMD and bone matrix | ↑ Serum osteocalcin | Deng et al. [28] | |
| ↑ BV/TV, Tb.Th, Tb.N of femur and spine | ↓ Serum CTX-1 | |||
| ↑ N.Ob/B.Pm, MAR, BFR of proximal tibia | Reversal of tocotrienol benefits through exogenous mevalonate | |||
| ↓ Tb.Th of femur and spine | ||||
| ↓ N.Oc/B.Pm of proximal tibial bone | ||||
| OVX + fracture | Annatto tocotrienols + LOV | ↑ BV, BV/TV, mBMD at callus | Ibrahim et al. [39,40] | |
| ↑ N.Ob, OS/BS, dLS/BS, OV/BV, MS/BS, MAR, BFR/BS | ||||
| ↑ Bone strength (load and stress) | ||||
| ↓ N.Oc, ES/BS, sLS/BS | ||||
| ↓ TV at callus | ||||
| Total tocotrienol | ↑ Force implied to a unit area | Mohamad et al. [47] | ||
| ↔ Maximum load, strain, Young’s modulus | ||||
| Male models of osteoporosis | ||||
| Normal animal model | δ-Tocotrienol and γ-tocotrienol | ↑ BV/TV, Tb.Th, Tb.N | Shuid et al. [33], Mehat et al. [34] | |
| ↑ N.Ob, OS/BS, dLS/BS, OV/BV, MS/BS, MAR, BFR/BS | ||||
| ↑ Load, displacement, stress, stiffness, strain, elastic modulus at femur | ||||
| ↓ Tb.Sp | ||||
| ↓ N.Oc, ES/BS, sLS/BS | ||||
| Palm tocotrienol | ↓ Femur TBARS | Maniam et al. [57] | ||
| ↑ Femur GPX | ||||
| ↔ Femur SOD | ||||
| ORX animal model | Annatto tocotrienols | ↑ BV/TV and Tb.N of distal femur | ↑ ALP and COL-1α-1 mRNA expression | Chin et al. [36], Chin and Ima-Nirwana [42] |
| ↓ Tb.Sp of tibia and femur | ↓ RANKL and PPARG mRNA expression | |||
| ↔ Biomechanical strength (load, stress, strain, extension at femur) | ||||
| ADX | ||||
| ADX animal model | Palm vitamin E and tocotrienol | ↑ Femoral BV/TV, Ob.S/BS | ↑ Gene expression of osterix (sp7) | Suhana et al. [49] |
| ↓ Oc.S/BS | ↓ Gene expression of osteocalciun, Co1α1 | |||
| ↔ Whole body BMD and femur length | ||||
| ↔ Ca content of femur and LV-5 | ||||
| γ-Tocotrienol | ↑ Ca content of LV-4 | |||
| ↓ Body fat | ||||
| GnRH agonist | Annatto tocotrienol | ↑ Bone volume, Tb.Th, N.Ob, Ct.Th | Mohamad et al. [44,45] | |
| ↑ Femoral biomechanical strength | ||||
| ↑ Bone calcium content | ||||
| ↓ sLS/BS | ||||
| Free radical-induced bone damage | ||||
| Free radical-induced damage animal model | Palm tocotrienol | ↑ N.Ob, Tb.Th, Tb.BFR/BS | ↓ Serum IL-6 and osteocalcin | Ahmad et al. [48], Nizar et al. [15] |
| ↓ N.Oc, ES/BS, OV/BV, OS/BS | ↓ Urine DPD | |||
| ↔ BV/TV, Tb.N, Tb.Sp, N.Oc | ||||
| Nicotine-induced bone damage | ||||
| Sprague-Dawley male rats after nicotine | Palm tocotrienol and γ-tocotrienol | ↑ BV/TV, Tb.Th, Tb.N, MAR, BFR/BS | ↓ Serum IL-1, IL-6 level | Norazlina et al. [60,61], Hermizi et al. [8] |
| ↑ Femur calcium content | ↓ Serum PYD level | |||
| ↓ Oc.S/BS, ES/BS, dLS/BS | ↔ Serum OPG and RANKL levels | |||
| ↔ Bone remodeling index, bone calcium content at femur and LV-4 | ||||
| Obesity, insulin resistance, and metabolic syndrome | ||||
| High-fat diet-induced obesity, type 2 diabetes and osteoporosis | Annatto tocotrienol | ↑ Femoral strength, strain and Young’s modulus of elasticity | ↑ Insulin sensitivity and secretion | Wong et al. [53] |
| ↓ Serum leptin | ||||
| ↑ Tb.N, SMI, Ct.Th | ↑ Insulin tolerance and glucose homeostasis | Shen et al. [52] | ||
| ↑ Serum P1NP | ↓ Serum inflammatory cytokines (i.e., IL-2, MCP-1, IFN-γ) | |||
| ↓ Tb.Sp, Conn.Dn | ||||
| ↓ Serum CTX | ||||
| ↓ sRANKL, FGF-23 | Wong et al. [51] | |||
| Osteoarthritis | ||||
| Osteoarthritis induced by MIA | Annatto tocotrienol | ↑ ES/BS, Oc.S/BS | ↑ BFR of osteoblasts | Chin et al. [25] |
| ↓ COMP | ↔ OPG | |||
| ↔ Osteocalcin and CTX-1 | ||||
ADX, adrenalectomized; mBMD, volumetric BMD of the mineralized tissue; BMP-2, bone morphogenetic proteins-2; BSP, bone sialoprotein; Ca, calcium; Col-1α-1, type 1 collagen; Col-2α-1, type II collagen; COMP, cartilage oligomeric matrix protein; Conn. Dn., connectivity density; CTX-1, carboxyterminal cross-linking telopeptide type 1 collagen; DEX, dexamethasone; DOC, deoxycorticoxterone; DPD, deoxypyridinoline; ERT, estrogen replacement therapy; GnRH, gonadotropin-releasing hormone; HCHF, high-carbohydrate high-fat diet; IFN-γ, interferon gamma; LOV, lovastatin; dLS, double-labeled surface; LV-4, lumbar vertebra-4; MCP-1, monocyte chemoattractant protein-1; MetS, metabolic syndrome; MS, mineralizing surface; NC, nicotine; N.Ob, number of osteoblast; N.Oc, number of osteoclast; ObS/BS, osteoblast surface/bone surface; Oc.S, osteoclast surface; OV/OS, osteoid volume/osteoid surface; PPARG, peroxisome proliferator-activated receptor gamma, transcript variant 2; PTH, parathyroid; PVE, palm vitamin E; PYD, pyridinoline; RUNX-2, runt-related transcription factor 2; TBARS, thiobarbituric acid-reactive substances; TGF-β2, transforming growth factor beta 2; TGF-β3, transforming growth factor beta 3; αTP, α-tocopherol; TRAP, tartrate resistant acid phosphatase; TRF, tocotrienol rich fraction; α-TT, alpha-tocotrienol; β-TT, beta-tocotrienol; δ-TT, delta-tocotrienol, γ-TT, gamma-tocotrienol; VED, vitamin E deficient; ↑ increase; ↓ decrease; ↔ no change.
Summary of animal studies of tocotrienols on bone health
While primary osteoporosis in women commonly develops after the onset of menopause, osteoporosis in men typically develops after 70 years of age and at this advanced age is known to be associated with testosterone deficiency [5,41]. Animal models of testosterone deficiency in males is induced by an orchiectomy to investigate the effects of compounds on the male skeletal system [13,42]. The commonly used Orchiectomized (ORX) model has been utilized to evaluate the impact of AnTT on testosterone deficiency-induced osteoporosis [42,43]. Following 8 weeks of AnTT supplementation, the ORX animals exhibited increased BV/TV and Tb.N in the distal femur [42]. Additionally, animals displayed increased Ob.S, OS/BS, and OV/BV [43]. Similar results have been shown in rats with buserelin-treated, testosterone-deficient rats where AnTT increased bone calcium content, showing this tocotrienol composition as a possible anti-osteoporotic agent for men with testosterone deficiency [44]. In this same study, Sprague-Dawley rats treated with oral AnTT had significantly higher bone volume, trabecular thickness and osteoblast number [45]. AnTT may work to modulate trabecular bone loss by increasing the mineralizing surface and osteoblast number.
Osteoporosis in both men and women arises with little to no symptoms and frequently the diagnosis may not be made until fracture occurs [46]. It is well established that tocotrienols mitigate bone loss in OVX and ORX models of osteoporosis. Recent evidence also supports the ability of tocotrienols to aid in healing fractures. Studies performed by Ibrahim et al. and Mohamed et al. utilized tibial and femoral fracture models in OVX rats to address the ability of tocotrienols to aid in bone healing [39,40,47]. In these studies, supplemental total tocotrienol and AnTT improved the functional load without changing the Young’s modulus [39,40,47].
Several models of inflammation and oxidation-induced osteoporosis have been utilized to evaluate the effects of tocotrienol on these causes of osteoporosis. In a Ferric Nitrilotriacetate (FeNTA) induced oxidative stress condition, palm oil tocotrienols were able to suppress IL-1 and IL-6 levels. Additionally, PTT protected against FeNTA-induced reduction of BV/TV and Tb.Th. Similar results have been seen in studies of oxidative stress induced by nicotine administration where tocotrienols showed the ability to reduce and reverse effects of nicotine. Tocotrienols have been evidenced to increase BV/TV, MAR, and BFR/BS and to decrease sLS/BS and Oc.S/BS [8]. Additionally, tocotrienols reduced the concentrations of IL-1 and IL-6 cytokine secreted in response to nicotine exposure.
Cigarette smoking is an important modifiable risk factor for osteoporosis. Several previously reviewed studies have focused on the role of nicotine and associated production of free radicals. Free radicals and oxidants contribute to bone degradation, cause a disruption in the balance between the building and breakdown of bone [37] and lower Bone Mineral Density (BMD); these effects have been shown to be reversed by tocotrienols [48]. In investigating the ability of tocotrienols to mitigate the free radical effects, Ahmad et al. [48] showed that PTT attenuated the iron deposition induced by FeNTA, affecting bone’s ability to maintain remodeling homeostasis and reducing the levels of serum IL-1, IL-6, and osteocalcin and urine deoxypyridinoline. Suhana et al. [49] demonstrated the impact of dexamethasone-induced oxidative stress on osteoblast and osteocyte apoptosis, leading to a reduction in osteoblast related gene expression. Treatment with PTT was found to inhibit lipid peroxidation as evidenced by decreased MDA [49]. Additionally, PTT induced SOD enzyme activity. These effects are postulated to have reversed the damaging effects of oxidative stress induced by glucocorticoids. Based on these results several mechanisms through which tocotrienols attenuate free radical damage and oxidative damage are proposed, and further research is needed to determine their primary functional mechanism.
Recent research has focused on the role of metabolic syndrome, obesity, and type 2 diabetes in osteoporosis and the underlying mechanisms. Body weight and associated comorbidities serve as an additional modifiable risk factor for osteoporosis. Long-term hyperglycemia is known to be associated with chronic inflammation with increased inflammatory cytokines and oxidative stress, both of which are known to negatively impact bone cells [50]. A rat model for high-fat diet-induced metabolic syndrome and osteoporosis was used in a recent study to evaluate the effects of AnTT on osteocyte-derived peptides and determine the mechanistic impact of tocotrienol. AnTT supplementation downregulated the activity of osteocyte-related peptides, such as sRANKL, Osteoprotegerin (OPG) and Fibroblast Growth Factor (FGF)-23 [51]. OPG is a decoy substrate for RANK. The binding of OPG to osteoclasts prevents their differentiation into mature osteoclasts, thereby decreasing bone resorption activity. FGF-23 is a product of osteocytes and is known to inhibit bone mineralization. Normalization of these osteocyte-derived peptides serves as an indicator of tocotrienols ability to protect bone cells from imbalances associated with Metabolic Syndrome (MetS).
Shen et al. [52] recently investigated the osteo-protective effects of AnTT in a model of high-fat diet-induced type 2 diabetes. Results indicated the detrimental effects of a high-fat diet on bone parameters, including cortical thickness and bone volume at trabecular bone; these effects were correlated with liver mRNA expression of inflammatory cytokines, indicating an association of bone deterioration with an inflammatory state. Supplementation with AnTT reversed the effects on bone parameters, indicating a potential inflammation-dependent mechanism of action. Additionally, AnTT supplementation enhanced bone formation indicated by increased serum N-Terminal propeptide of type I procollagen (P1NP) and decreased bone resorption. The benefits of tocotrienol in MetS have also been noted in rats fed a high-carbohydrate, high-fat diet. Tocotrienols were noted to lower leptin levels, improve insulin sensitivity and modulate static and dynamic bone parameters. In Shen’s study, it was noted that insulin secretion was increased along with improved blood glucose levels following supplementation of tocotrienol. Additionally, hormone changes and more specifically, decreased serum leptin concentrations, were noted following tocotrienol supplementation.
Related research by Wong et al. [53] showed evidence for the role of tocotrienols in modulating leptin levels in animals with MetS. A high-carbohydrate, high-fat diet-induced MetS that resulted in insulin resistance altered hormone levels and reduced several dynamic and static bone parameters such as Ob.S/BS, Oc.S/BS, ES/BS, OS/BS and OV/BV. While total bone mineral density was unchanged following supplementation with palm vitamin E, improvements in parameters including Ob.S/BS, OS/BS and decreased ES/BS and sLS/BS were noted, indicating the benefit of tocotrienols supplementation in MetS as a osteo-protective measure. Wong et al. [51] further demonstrated oral supplementation of annatto and PTT reduces the protein expression levels of sRANKL, FGF-23, sclerostin, and Dickkopf-related protein-1 in the animals fed with high-carbohydrate and high-fat diet, suggestion tocotrienol exerts potential skeletal promoting benefit by modulating the levels of osteocytes-derived bone-related peptides.
These animal studies, along with previous studies that showed a relationship between leptin deficiency and high bone mass, indicate a possible osteo-protective mechanism of leptin [54,55]. In addition, insulin has been shown to decrease OPG/RANKL ratio, which leads to the increased bone resorption activity in the osteoclast [56]. While several studies have shown the impact of AnTT on increasing insulin sensitivity and reducing blood glucose levels, no impact on insulin levels was observed. However, it is postulated that the effect exerted by AnTT is on insulin sensitivity, thereby improving glucose homeostasis [21]. In addition, the known inflammatory state of obesity and type 2 diabetes may serve as a point of intervention for tocotrienols in impacting bone health.
In summary, the animal studies have demonstrated the benefits of tocotrienol supplementation to overall bone health. Benefits include mitigation of bone loss and microstructural deterioration and improvements in structural and functional capacities of bone. The mechanism through which these effects take place remains unknown and should be the focus of future work.
4. POTENTIAL MECHANISMS OF ACTION FOR TOCOTRIENOLS IN BONE PROTECTION
Figure 1 summarized the postulated mechanisms of action by which tocotrienols exert their bone-protective effect. Two main impacts of tocotrienol may contribute to improved bone strength and reduced risk of fracture: antioxidant/anti-inflammation and suppression of the mevalonate pathway. Tocotrienols are best known for their potent antioxidant and anti-inflammatory effects. These effects have lent their use as a possible treatment in oxidation-induced osteoporosis. The effects of tocotrienols on general bone health was studied by Maniam et al. [57] utilizing normal male rats. Osteoporotic bones often have concomitant lipid accumulation that can undergo oxidation, disturbing the control of biomineralization and resulting in an increase in bone osteolysis and soft tissue calcification [57]. Lipid oxidation often promotes osteoclast recruitment and differentiation. In addition, osteoblast differentiation is generally inhibited, leading to induction of bone resorption [57]. Maniam et al. [57] investigated the impact of vitamin E isoforms on the levels of lipid peroxidation and antioxidant enzyme following treatment. Results showed rats treated with 100 mg/kg BW of PTT at 6 days/week for 4 months had lowered levels of lipid peroxidation, evidenced by a decrease of thiobarbitruric-acid-reactive substance [57]. Additionally, PTT treatment increased the levels of GPX, preventing additional oxidation and bone decomposition [57].
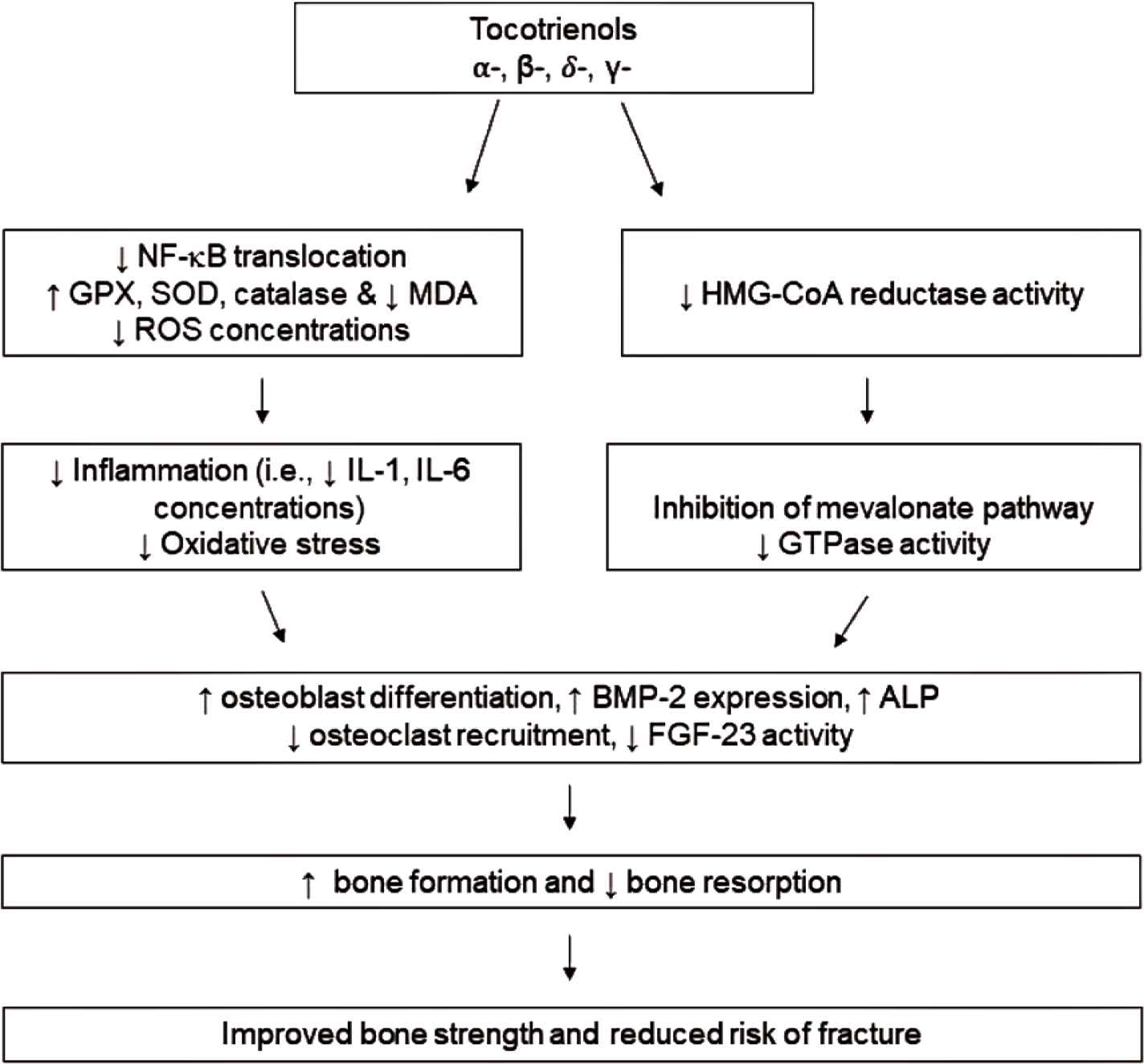
The proposed mechanism by which tocotrienols exhibit osteo-protective activity. Tocotrienols (α-, β-, δ-, γ-) suppress NF-κB activation and increase action of endogenous antioxidants, thereby reducing inflammation and oxidative stress. Additionally, tocotrienols potentially attenuate the flux of the mevalonate pathway and subsequent GTPase activity. Together, these actions lead to the ability of tocotrienols to increase osteoblast differentiation while suppressing osteoclast recruitment, resulting in enhanced bone formation and preventing bone resorption to reduce risk of fracture.
Ample evidence shows tocotrienols benefit bone health through the regulation of the mevalonate pathway [58,59], in addition to the regulation of NF-κB [60,61]. Deng et al. [28] suggested the involvement of tocotrienol in the downregulation of HMG-CoA reductase, the rate-limiting enzyme in the mevalonate pathway. Mevalonate deprivation has been shown to prevent activation of small Guanosine Triphosphate-binding Proteins (GTPases), proteins that regulate the differentiation of osteoclasts and osteoblasts, the expression of RANKL, and the activation of NF-κB. The involvement of the mevalonate pathway was also addressed in OVX rats by Chin et al. [37] who showed the synergistic effects of AnTT and lovastatin in improving bone formation and mineralization activity. Furthermore, several studies have compared δ-tocotrienol with lovastatin, a HMG-CoA reductase inhibitor. While δ-tocotrienol did not exhibit as powerful of an effect on bone parameters, there did appear to be a synergistic effect of the two agents on bone strength parameters [35,40] with an increased Ob.S/BS [35] and decreased Oc.S/BS [35]. Additionally, an impact on gene expression of BMP-2, an integral protein that bridges the mevalonate pathway with osteoblastic differentiation [37,39], and other genetic markers of bone growth morphology was observed [39]. Recent evidence presented by Shah and Yeganehjoo [19] demonstrated that tocotrienol increased BMP-2 expression in pre-osteoblastic MC3T3-E1 cells and increased ALP activity. Additionally, expression of HMG-CoA reductase was downregulated following tocotrienol treatment. The tocotrienol effect was eliminated with additional of exogenous mevalonate. Further research is needed to assess the role of the mevalonate pathway in tocotrienol-mediated bone protection. Importantly, α-tocopherol does not share the ability of tocotrienols in modulating the mevalonate pathway [62].
5. CONCLUSION AND FUTURE DIRECTION
The in vitro and in vivo studies summarized in this review support the bone-protective activity of tocotrienols, vitamin E isoforms found in unique plant sources. At concentrations evaluated, tocotrienols reduce oxidative stress and inflammation, upregulate antioxidant enzyme expression, increase bone mineralization, and promote osteoblast differentiation while suppressing osteoclast formation and differentiation. In vivo studies show improved biomarkers of bone formation and bone strength. Impact on inflammatory cytokines, ROS, RANK/RANKL, and the mevalonate pathway have been suggested as possible mechanisms of action for tocotrienols in bone protection. Extended human trials with tocotrienols may further evaluate the bone-protective activity of tocotrienols and elucidate the underlying mechanisms. Emerging evidence suggests that the gut microbiome plays an important role in bone health via modulating nutrient absorption, the permeability of intestinal mucosal barrier, immune system functionality, gut–brain axis, and excretion of functional metabolites [63–65]. It would be worthy to investigate the potential impacts of tocotrienols in gut microbiome composition and functionality along with bone parameters in animals to advance our understanding of its effects on bone health, in part, through the modification of gut microbiota. Tocotrienols have potential as a novel approach in the prevention and treatment of osteoporosis, the degenerative bone disease often associated with aging.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
CS and HM conceptualized the manuscript. All authors were involved in the literature search and analysis and the writing of the original draft. MM, HM and CS contributed to the editing and formatting, and CS coordinated the overall writing.
ACKNOWLEDGMENTS
This work was supported by the FAMRI foundation YFEL141014 to XJ. All of the authors listed on the manuscript reviewed and approved of the work presented here.
ABBREVIATIONS
- ALP,
alkaline phosphatase;
- AnTT,
annatto-extracted tocotrienol;
- BFR,
bone formation rate;
- BMD,
bone mineral density;
- BMP-2,
bone morphogenetic protein 2;
- BV/TV,
bone volume/trabecular volume;
- BW,
body weight;
- CAT,
catalase;
- FeNTA,
ferric nitrilotriacetate;
- FGF,
fibroblast growth factor;
- GPX,
glutathione peroxidase;
- GSH:GSSG,
glutathione to oxidized glutathione ratio;
- GTPases,
guanosine tri-phosphate-binding proteins;
- HMG-CoA,
3-hydroxy-3-methyglutaryl coenzyme A;
- IL,
interleukin;
- MAR,
mineral apposition rate;
- MDA,
malondialdehyde;
- MetS,
metabolic syndrome;
- NF-κB,
nuclear factor-κB;
- OCN,
osteocalcin;
- Ob.S,
osteoblast surface;
- Oc.S,
osteoclast surface;
- OPG,
osteoprotegerin;
- ORX,
orchiectomized;
- OS/BS,
osteoid surface/bone surface;
- OSX,
osterix;
- OV/BV,
osteoid volume/bone volume;
- OVX,
ovariectomized;
- P1NP,
N-terminal propeptide of type I procollagen;
- PTT,
palm tocotrienol;
- PGE2,
prostaglandin E2;
- RANK,
nuclear factor-κB;
- RANKL,
nuclear factor-κB ligand;
- ROS,
reactive oxygen species;
- sLS/BS,
single-labeled surface/bone surface;
- SOD,
superoxide dismutase;
- Tb.N,
trabecular number;
- Tb.Sp,
trabecular separation;
- Tb.Th,
trabecular thickness;
- TNF-α,
tumor necrosis factor-α.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Maureen L. Meister AU - Huanbiao Mo AU - Xiangming Ji AU - Chwan-Li Shen PY - 2020 DA - 2020/04/30 TI - Tocotrienols in Bone Protection: Evidence from Preclinical Studies JO - eFood SP - 217 EP - 225 VL - 1 IS - 3 SN - 2666-3066 UR - https://doi.org/10.2991/efood.k.200427.001 DO - 10.2991/efood.k.200427.001 ID - Meister2020 ER -
