Incidence and economic burden of acute otitis media in children aged up to 5 years in three Middle Eastern countries and Pakistan: A multinational, retrospective, observational study
All principal investigators have contributed equally to the study.
- DOI
- 10.1016/j.jegh.2016.12.004How to use a DOI?
- Keywords
- Acute otitis media; Economic burden; Incidence; Middle east
- Abstract
Epidemiological data on acute otitis media (AOM), an infectious disease frequently affecting children, are lacking in some countries. This study was undertaken to assess the incidence of AOM in children ≤5 years in Saudi Arabia, Oman, Pakistan, and Turkey, as well as the economic burden from a parent/caregiver perspective. Medical records of 4043 children (Saudi Arabia = 1023, Oman = 998, Pakistan = 1022, Turkey = 1000) were retrospectively reviewed and the incidence of AOM episodes calculated from suspected and confirmed cases. Using a standardized Health Economics Questionnaire, parents recorded resource use and expenses incurred per AOM episode [in local currency and converted to US dollars (USD)]. The overall incidence of AOM episodes per 1000 person–years was: Saudi Arabia, 207 [95% confidence interval (CI): 178–238]; Oman, 105 (95% CI: 85–127); Pakistan, 138 (95% CI: 116–163); and Turkey, 99 (95% CI: 79–123). The mean total out-of-pocket healthcare expense incurred by parents/caregivers per episode was: Saudi Arabia USD67.1 [standard deviation (SD) = 93.0], Oman USD16.1 (SD = 16.4), Pakistan USD22.1 (SD = 20.5), and Turkey USD33.6 (SD = 44.9). The incidence of AOM episodes varied across all four countries, probably due to different diagnostic and management practices. Nevertheless, our results confirm that AOM causes a substantial burden to public health, reinforcing the need for cost-effective prevention strategies.
- Copyright
- © 2017 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Acute otitis media (AOM), one of the most common infections in children aged <5 years [1,2], is characterised by the presence of middle-ear effusion together with symptoms including ear discharge (otorrhoea), fever, irritability, and earache (otalgia) [2]. Approximately 80% of children are affected by AOM during their first 5 years of life [3]. The cumulative worldwide AOM incidence rate is 10.85%, that is, 709 million cases per year; out of which, 51% occur in children aged <5 years [1]. Estimates from 2005 suggest that the incidence rates of AOM in South Asia and North Africa (Middle East regions) were 14.52% and 8.67%, respectively [1]. The disease burden is greatest between 6 months and 18 months of age and, if left untreated, may lead to permanent hearing loss [4]. Furthermore, recurrent AOM episodes may lead to chronic forms, such as chronic suppurative otitis media, potentially resulting in severe complications, such as intracranial infection, hearing impairment/loss, and facial paralysis [2,5].
Bacteria and viruses are responsible for 35–55% and 20–30% of AOM cases, respectively [6], and the infection is one of the main reasons for primary care physician (PCP) consultations and antibiotic prescriptions [7]. Indeed, extensive antibiotic use has led to a significant increase in the prevalence of resistant AOM-causing pathogens [8].
AOM has a high economic burden in terms of direct and indirect cost [9,10], but pneumococcal vaccination of children can potentially reduce both the burden of AOM and its associated cost [11,12]. In 2007, the World Health Organization recommended the inclusion of pneumococcal vaccination in national immunization programmes (NIPs) [13]. Pneumococcal conjugate vaccines (PCVs) were subsequently introduced into the NIP of Oman (PCV-7; 2008) [14], Saudi Arabia (PCV-7; 2009) [15], Turkey (PCV-7; 2008) [16], and Pakistan (PHiD-CV; 2012) [17]. At the time of this study, PHiD-CV and PCV-13 were available in Pakistan [18]; PCV-13 was used in Oman [19], Saudi Arabia [20], and Turkey [21]. The vaccination coverage rates during the study period ranged from 99% in Oman, 98% in Saudi Arabia, 97% in Turkey, to 66% in Pakistan [22].
Epidemiological data on the incidence and cost of illness of AOM episodes are important for policy makers to understand both the public health burden and the potential economic impact of the disease. This study was undertaken to estimate the incidence of AOM episodes per person–years (PY) in Children aged ≤5 years in these three Middle Eastern countries and Pakistan, as well as the economic burden from a parent/caregiver perspective.
2. Materials and methods
2.1. Study design and participants
A large multinational, retrospective, observational study (Clinical Study Identifier: BEP115672) was conducted at two centres in Saudi Arabia (December 2012–July 2013), six centres in Oman (October 2012–December 2013), three centres in Pakistan (December 2012–December 2013), and two centres in Turkey (March–August 2013). The participating centres were primary care centres with physicians specialised in paediatrics. They were selected because they had an available ear-nose-throat (ENT) specialist, to identify how diagnosis is made by country and to assess differences between cases diagnosed by a PCP or ENT specialist. Target enrolment was 1000 children per country. In Saudi Arabia a simple randomisation scheme was followed to recruit patients [i.e., all eligible patients’ files were allotted a serial number and stratified by age group (0–2 years and 3–5 years), and those with an odd number file were invited to participate]. In Pakistan, after screening the files, parents/guardians of eligible children were randomly called, although no randomisation scheme was used, and those who met the inclusion criteria and agreed to participate were enrolled. In Turkey and Oman, recruitment was based on convenience sampling, that is, all age-eligible children were included until the target number was reached.
The inclusion of potentially eligible participants was based on their age and the availability of their medical records for the 12 months before enrolment. Male or female children aged 0–5 years at the time of enrolment, registered in medical practices become ineligible at their sixth birthday. Children under the protection of an organisation or foster parents, or living in a care home, were excluded from the study.
2.2. Data collection
Parents/caregivers were invited to participate either during a medical consultation or by telephone.
Data were collected from medical records for the 12-month period prior to enrolment, thus ensuring coverage of all seasons. For patients aged <12 months, data were collected from their first month onwards.
The medical histories of the children were retrospectively collected from their medical records. Demographic (e.g., birth date, height, weight, gender, and birth weight) and AOM visit information [medical history, diagnostic procedures (e.g., tympanocentesis, adenoidectomy, and transtympanic aerator tube insertion), AOM treatment (e.g., antibiotics, anti-inflammatories, antihistamines, corticosteroids, and cough medications), treatment failures, recurrent AOM, associated signs and symptoms, vaccination status, and hospitalisation] were recorded. In addition, parents/caregivers were asked to complete a Health Economics Questionnaire (HEQ) with information relating to the direct and indirect cost associated with an AOM episode and the assessed cost was converted from the local currency to US dollars (USD). The variables included out-of pocket expenses: direct medical cost components (e.g., copayment related to treatment, procedures); direct nonmedical cost components (e.g., transportation fees or extraordinary baby-sitting fees); and indirect cost components (e.g., productivity loss of caregiver due to missing work). The cost of private transport was not captured due to a lack of unit cost data. Details on other visits, procedures, and medications for previous episodes in the 12 months before enrolment were also collected. The medical insurance status of the parent/caregiver and the child’s missed attendance of school/day care were confirmed.
2.3. Case definitions
The level of certainty regarding the diagnosis was differentiated; according to whether a PCP or ENT specialist made the diagnosis and how it was made. A clinically suspected AOM (S-AOM) episode was defined as a PCP-diagnosed episode, with or without the visual appearance of the tympanic membrane [i.e. redness, bulging, loss of light reflex, the presence of acute middle-ear effusion (as shown by otoscopy or tympanometry)], and the presence of ≥2 of the following: ear pain, ear discharge, hearing loss, lethargy, irritability, anorexia, vomiting, diarrhoea, fever [temperature ≥38.0 °C (axillary); ≥38.5 °C (rectal)] or analgesic/antipyretic therapy preceding fever.
A clinically confirmed AOM (C-AOM) episode was defined as an ENT specialist-diagnosed episode.
A recurrent AOM (R-AOM) episode was defined as the occurrence of ≥3 new AOM episodes within 6 months, or ≥4 new AOM episodes within 1 year, as confirmed by an ENT specialist, regardless of the aetiology. AOM treatment failure was defined as no improvement in symptoms after 48–72 h of antibiotic therapy.
2.4. Statistical analysis
Analyses were performed on all recruited children who complied with the protocol-defined procedures. Descriptive statistics were used to summarise demographic characteristics by country and centre. The data sets from each country were analysed independently and missing data were excluded from the descriptive analysis.
The sample size estimation was based on a multinational European study that reported an overall incidence of 268 AOM episodes per 1000 PY and incidence rates of 299 per 1000 PY and 212 per 1000 PY in children aged 0–2 years and 3–5 years, respectively [23]. The sample size was estimated for a range of precision levels. For a proportion of ∼28%, with a precision of 4.5%, ∼400 enrolees were required for the 0–2-year age group per country. For a proportion of ∼26%, with a precision of 3.5%, ∼600 enrolees were required for the 3–5-year age group per country. Overall, 1000 children were required in each country. The overall incidence of AOM episodes was calculated according to the different levels of diagnostic certainty (S-AOM and C-AOM) by age (0–2 years and 3–5 years) and pneumococcal vaccination status.
The formula used to calculate the incidence of AOM episodes was:
The exact Poisson confidence limit method was used to calculate 95% confidence intervals (CIs) [24]. These were calculated for incidence, recurrent episodes, treatment failures, clinical management, and health economic factors.
All statistical analyses were performed using the Statistical Analysis Systems (SAS-version 9.2) Drug and Development (SDD) web portal version 3.5 and Microsoft Excel 2007.
2.5. Ethics
This study was conducted in accordance with the principles of Good Clinical Practice, the Declaration of Helsinki, and all applicable regulatory requirements. Parents/caregivers provided written informed consent before their children were enrolled. Ethics committee approvals were obtained from all centres that participated in this study.
3. Results
Enrolment began in October 2012 (Oman), December 2012 (Pakistan and Saudi Arabia), and March 2013 (Turkey) and it took between 5 months (Turkey) and 15 months (Oman) to enrol all patients. Therefore, there was a period of 17 months with overlapping data from all four countries (March 2012 to August 2013).
3.1. Baseline characteristics
A total of 4043 children (Saudi Arabia = 1023; Oman = 998; Pakistan = 1022; Turkey = 1000) were enrolled, with a mean age of 2.6 years [standard deviation (SD): 1.7] in Saudi Arabia; 2.7 years (SD: 1.6) in both Oman and Pakistan; and 1.9 years (SD: 1.8) in Turkey. Over 50% of children (range: 52.9–57.7%) were male. Fifty-eight children were excluded from the final analysis (Fig. 1), and of the remaining 3985 children, 450 experienced 507 AOM episodes. Among children in the According-To-Protocol cohort, 191 AOM episodes (19.5%; 162 suspected; 29 confirmed) were reported in 185 children in Saudi Arabia; 100 episodes (10.1%; 54 suspected; 46 confirmed) occurred in 84 children in Oman; 99 Pakistani children experienced 134 episodes (13.1%; 129 suspected; 5 confirmed); and 82 Turkish children experienced 82 episodes (8.2%; all suspected). Referrals to ENT specialists occurred at the discretion of the PCPs and accounted for <10% of AOM episodes.
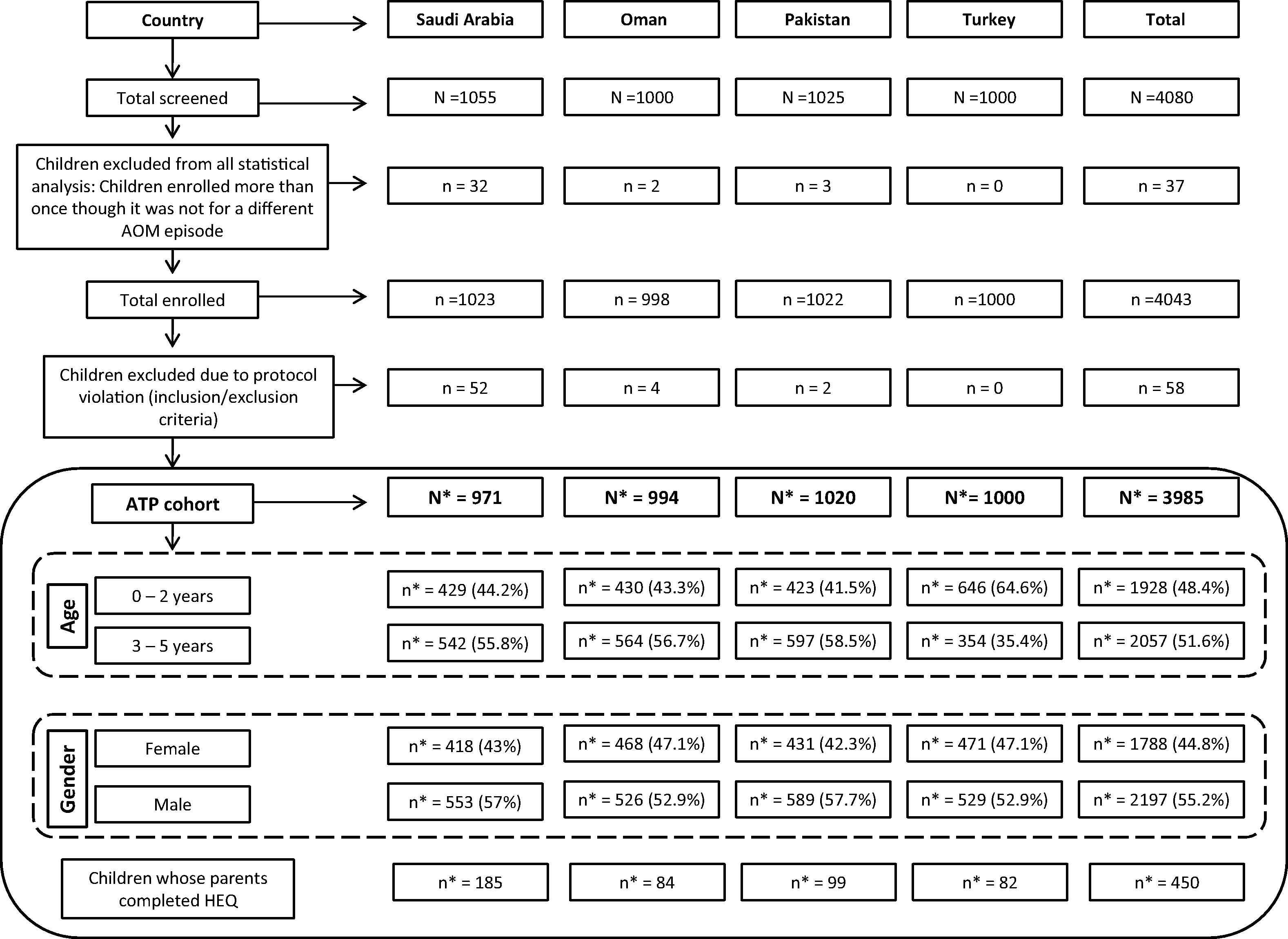
Screening, enrolment, and demographics by country. ATP = According-To-Protocol; HEQ = Health Economics Questionnaire; N = total number of children screened; n = number of children in each category; N* = number of children in the ATP cohort; n* = number of children in each category under ATP cohort.
3.1.1. Pneumococcal vaccination history
Overall, 2750 children [681 (70.1%) Saudi Arabian, 842 (84.7%) Omani, 429 (42.1%) Pakistani, and 798 (79.8%) Turkish] had received ≥1 dose of any PCV before study initiation. Of these, 553 (81.2%; Saudi Arabia), 126 (15%; Oman), 144 (33.6%; Pakistan), and 504 (63.2%; Turkey) had received four PCV doses. Vaccination status was unknown for 250 children; predominantly among those aged 3–5 years in Saudi Arabia and Turkey (Table 1).
| Characteristics | n (%) | Saudi Arabia | Oman | Pakistan | Turkey | Total (n = 3985) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 y (n = 429) | 3–5 y (n = 542) | 0–2 y (n = 430) | 3–5 y (n = 564) | 0–2 y (n = 423) | 3–5 y (n = 597) | 0–2 y (n = 646) | 3–5 y (n = 354) | |||
| Child received ≥ 1 dose of PCV | 388 (90.4) | 293 (54.1) | 425 (98.8) | 417 (73.9) | 207 (48.9) | 222 (37.2) | 550 (85.1) | 248 (70.1) | 2750 (69.0) | |
| Unvaccinated with PCV | 25 (5.8) | 191 (35.2) | 4 (0.9) | 129 (22.9) | 207 (48.9) | 350 (58.6) | 71 (11) | 8 (2.3) | 985 (24.7) | |
| Unknown | 16 (3.7) | 58 (10.7) | 1 (0.2) | 18 (3.2) | 9 (2.1) | 25 (4.2) | 25 (3.9) | 98 (27.7) | 250 (6.3) | |
| No. of doses* | 1 | 9 (2.3) | 0 (0) | 12 (2.8) | 1 (0.2) | 39 (18.8) | 44 (19.8) | 74 (13.5) | 43 (17.3) | 222 (5.6) |
| 2 | 23 (5.9) | 0 (0) | 107 (25.2) | 2 (0.5) | 34 (16.4) | 27 (12.2) | 40 (7.3) | 0 (0) | 233 (5.8) | |
| 3 | 93 (24) | 3 (1) | 196 (46.1) | 398 (95.4) | 81 (39.1) | 59 (26.6) | 137 (24.9) | 0 (0) | 967 (24.3) | |
| 4 | 263 (67.8) | 290 (99) | 110 (25.9) | 16 (3.8) | 53 (25.6) | 91 (41) | 299 (54.4) | 205 (82.7) | 1327 (33.3) | |
| 5 | – | – | – | – | 0 (0) | 1 (0.5) | – | – | 1 (0.03) | |
| NA | 41 | 249 | 5 | 147 | 216 | 375 | 96 | 106 | 1235 (31.0) | |
N = number of children; PCV = pneumococcal conjugate vaccines.
n (%) = number (percentage) of children in a given category.
% (for Number of doses) = n/Number of children who received at least one dose of a pneumococcal vaccine × 100.
NA = Not applicable (i.e., children without any pneumococcal vaccination or with unknown status).
Pneumococcal vaccination history by age and number of doses received (ATP cohort).
3.2. Incidence of AOM episodes and clinical management of AOM by country
3.2.1. Saudi Arabia
The overall incidence of AOM episodes was 207 (95% CI: 178–238) per 1000 PY, comprising 175 suspected (95% CI: 149–205) and 31 confirmed (95% CI: 21–45) episodes per 1000 PY. The incidence was 309 (95% CI: 256–370) per 1000 PY in children aged 0–2 years and 135 (95% CI: 106–169) per 1000 PY in those aged 3–5 years (Fig. 2).
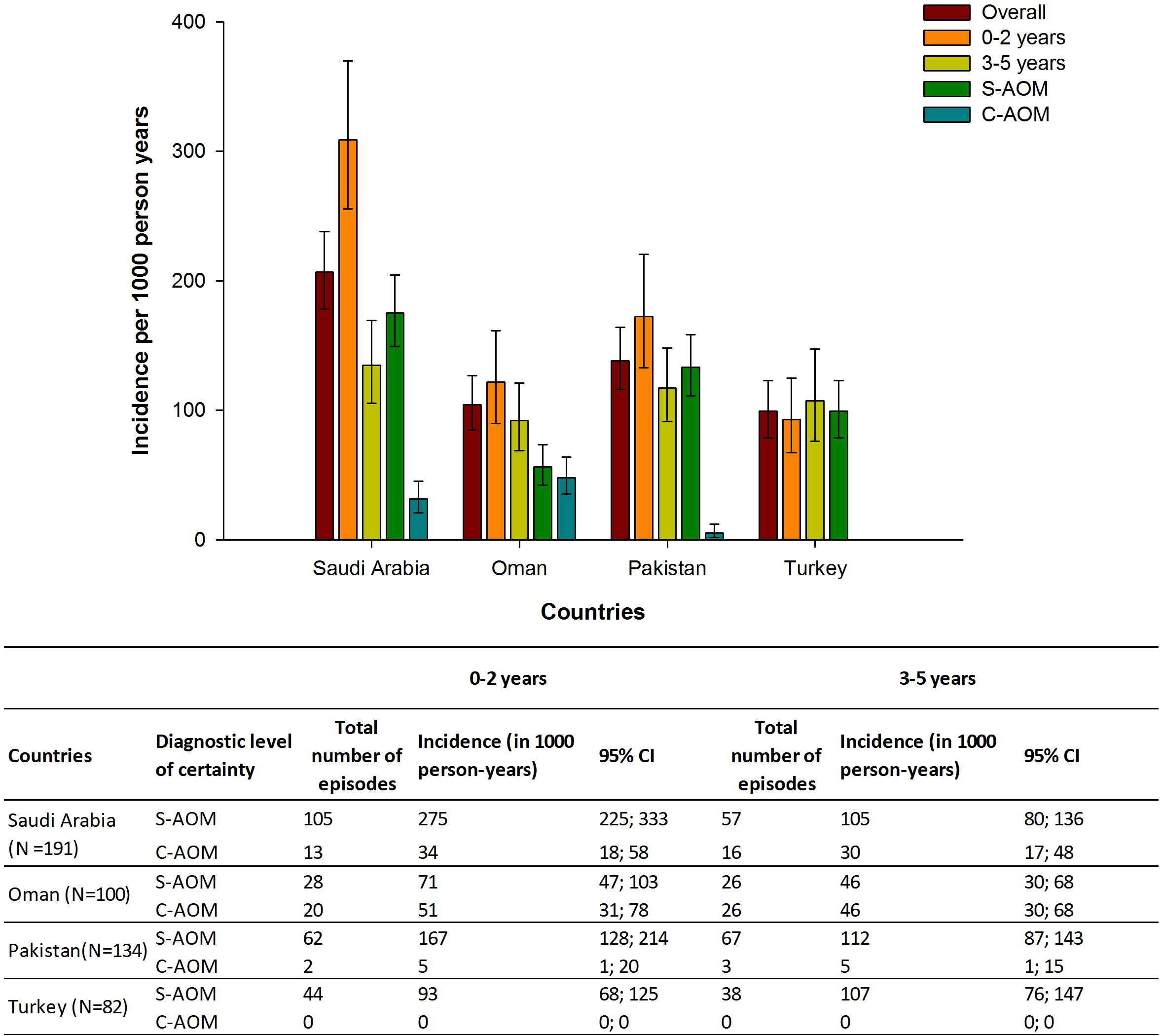
Incidence of clinical AOM episodes between October 2011 and December 2013 in children aged ≤5 years, by diagnostic level of certainty and age. S-AOM: clinically suspected AOM; C-AOM = clinically confirmed AOM; 95% CI: Exact Poisson 95% confidence limits; N = Number of positive AOM episodes. Note: there were no C-AOM episodes in Turkey.
The incidence of AOM episodes (suspected and confirmed) was 267 (95% CI: 228–310) per 1000 PY in vaccinated and 71 (95% CI: 40–118) per 1000 PY in unvaccinated children (Table 2). The incidence in 0–2-year and 3–5-year age groups was higher among vaccinated compared to unvaccinated children (Fig. 3).
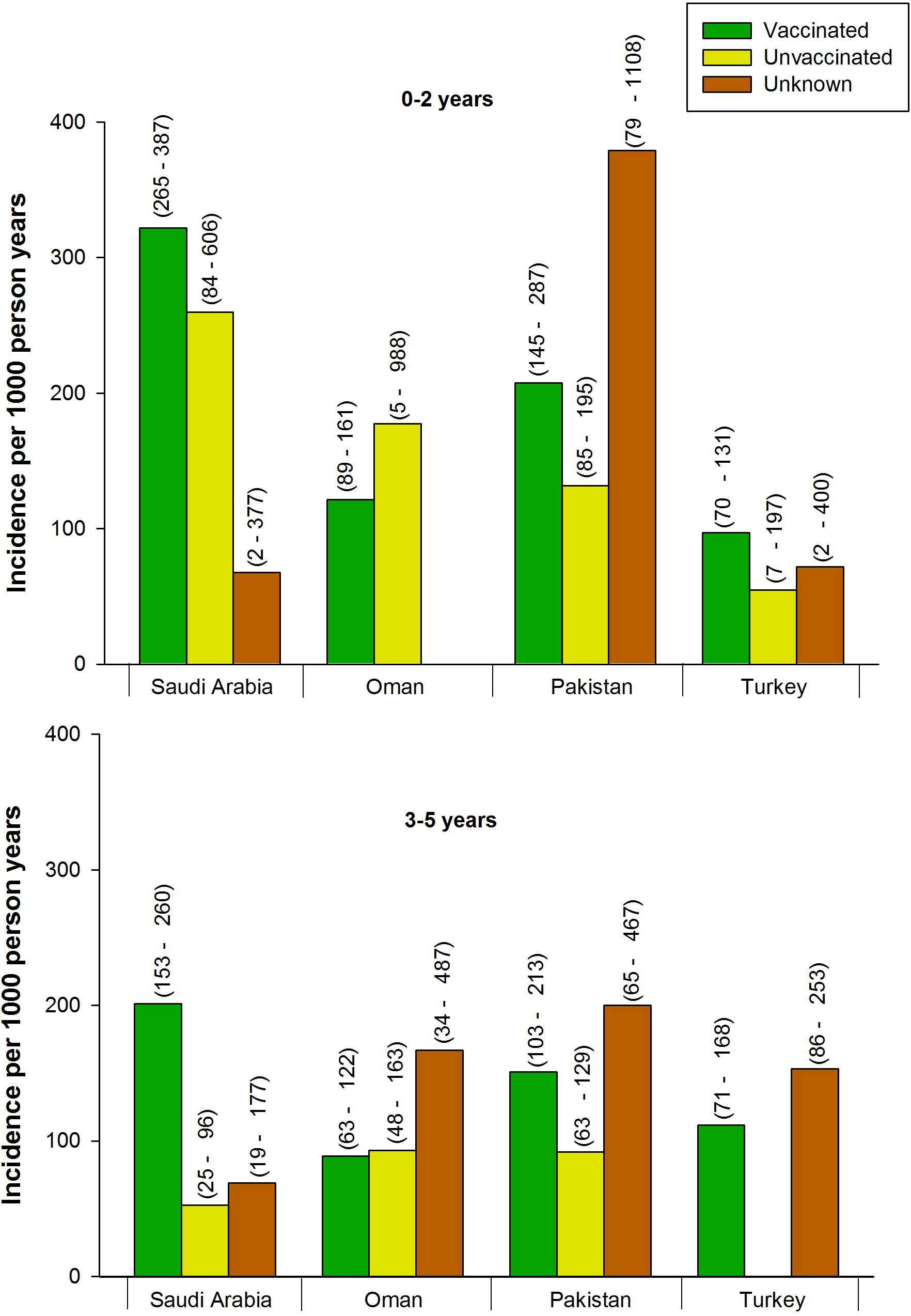
Incidence of AOM episodes observed in children aged 0–2 years and 3–5 years according to pneumococcal vaccination status. In Oman, the number of AOM episodes in the 0–2-year age group among children with unknown vaccination status (n = 1) was zero; in Turkey, the number of AOM episodes among unvaccinated children in the 3–5-year age group (n = 8) was zero. 95% confidence intervals are shown in parentheses. AOM = acute otitis media.
| Countries | Diagnostic level of certainty | Vaccinateda | Unvaccinatedb | Unknown | |||
|---|---|---|---|---|---|---|---|
| n (incidence in 1000 PY) | 95% CIc | n (incidence in 1000 PY) | 95% CIc | n (incidence in 1000 PY) | 95% CIc | ||
| Saudi Arabia (N = 191) | Suspected AOM | 144 (225) | 189–264 | 13 (62) | 33–106 | 5 (69) | 22–160 |
| Confirmed AOM | 27 (42) | 28–61 | 2 (10) | 1–34 | 0 (0) | 0–51 | |
| Oman (N = 100) | Suspected AOM | 49 (61) | 45–81 | 3 (22) | 5–65 | 2 (105) | 13–380 |
| Confirmed AOM | 35 (44) | 30–61 | 10 (74) | 36–137 | 1 (53) | 1–293 | |
| Pakistan (N = 134) | Suspected AOM | 67 (172) | 133–217 | 56 (103) | 78–134 | 6 (182) | 67–397 |
| Confirmed AOM | 1 (3) | 0–14 | 2 (4) | 1–13 | 2 (61) | 7–220 | |
| Turkey (N = 82) | Suspected AOM | 64 (95) | 73–122 | 2 (45) | 5–162 | 16 (143) | 82–232 |
| Confirmed AOM | – | – | – | – | – | – | |
AOM = acute otitis media; CI = confidence interval; PY = person–years.
N = Number of positive AOM episodes.
n = number of positive AOM episodes in each category.
Children who had ≥1 dose of pneumococcal conjugate vaccine.
Children who had no PCV or did not have their history of pneumococcal vaccination.
Exact Poisson 95% CI.
Incidence of AOM episodes by pneumococcal vaccination status.
Antibiotics were prescribed for 181 of the 191 AOM episodes (94.8%); other nonantibiotic treatments were prescribed in 140 episodes (73.3%). Procedures such as tympanocentesis, adenectomy, and transtympanic aerator tube insertion were performed in 3.7% episodes [1 (0.6%) suspected; 6 (20.7%) confirmed].
3.2.2. Oman
The overall incidence of AOM episodes was 104 (95% CI: 85–127) per 1000 PY, comprising 56 suspected (95% CI: 42–74) and 48 confirmed (95% CI: 35–64) episodes per 1000 PY. The incidence was 122 (95% CI: 90–161) per 1000 PY in children aged 0–2 years and 92 (95% CI: 69–121) per 1000 PY in those aged 3–5 years (Fig. 2).
The incidence of AOM was 104 (95% CI: 83–129) per 1000 PY in vaccinated and 97 (95% CI: 51–165) per 1000 PY in unvaccinated children (Table 2). The incidence of episodes was higher in unvaccinated as compared to vaccinated children in the 0–2-year age group, but the incidences were similar in vaccinated and unvaccinated children aged 3–5 years (Fig. 3).
Of the 100 AOM episodes, antibiotics were prescribed for 82 (82%) and other nonantibiotic treatments for 96 (96%). Other procedures were performed for only one episode.
3.2.3. Pakistan
The overall incidence of AOM episodes was 138 (95% CI: 116–164) per 1000 PY, comprising 133 suspected (95% CI: 111–158) and five confirmed (95% CI: 2–12) episodes per 1000 PY. The incidence was 173 (95% CI: 133–220) per 1000 PY in children aged 0–2 years and 117 (95% CI: 91–148) per 1000 PY in those aged 3–5 years (Fig. 2).
The incidence of AOM episodes was 174 (95% CI: 135–220) per 1000 PY in vaccinated and 107 (95% CI: 81–138) per 1000 PY in unvaccinated children (Table 2). The incidence of AOM episodes in 0–2-year and 3–5-year age groups was higher among vaccinated compared to unvaccinated children (Fig. 3).
Of the 134 AOM episodes, antibiotics were prescribed for 117 (87.3%) and nonantibiotic treatments for 118 (88.1%) episodes. Other procedures were performed in 4.5% episodes [3.9% (n = 5) suspected; 20% (n = 1) confirmed].
3.2.4. Turkey
The overall incidence of AOM was 99 suspected episodes (95% CI: 79–123) per 1000 PY; there were no confirmed episodes. The incidence was 93 (95% CI: 68–125) per 1000 PY in children aged 0–2 years and 107 (95% CI: 76–147) per 1000 PY in those aged 3–5 years (Fig. 2).
The incidence of AOM episodes was 95 (95% CI: 73–122) per 1000 PY in vaccinated and 45 (95% CI: 5–162) per 1000 PY in unvaccinated children (Table 2). In the 0–2-year age group the incidence was higher among vaccinated children compared to unvaccinated children (Fig. 3). There were no episodes of AOM among unvaccinated children in the 3–5-year age group.
Antibiotics were prescribed in three of the 82 AOM episodes (3.7%; all suspected). No clinical procedures were performed.
3.3. Signs, symptoms, and complications of AOM
At least one sign or symptom was recorded during the study period for 80% (405/507) of AOM episodes in all four countries. The most common were ear pain [46.4% (235/507)] and fever [50.3% (255/507)], although fever was reported less frequently in Turkey 10.9% (9/82) than other countries. Tympanic membrane redness was frequently reported in Pakistan [70% (91/134)], but only rarely reported in Turkey [7.1% (1/82)]. Complications occurred in 3.6% (18/507) of episodes in Saudi Arabia, Oman, and Pakistan; the most common was tympanic membrane perforation [3.4% (17/507)]. No complications were reported in Turkey.
3.4. Proportion of recurrent episodes and treatment failures
Recurrent episodes required confirmation by an ENT specialist in this study. No recurrent episodes were reported among C-AOM cases in Saudi Arabia, Oman, and Turkey, but 1% (95% CI: 0.0–5.5) of Pakistani children had R-AOM episodes. Therefore among C-AOM cases, 20% [1/5 [95% CI: 1–81)] were recurrent in Pakistan.
The overall proportion of AOM treatment failures reported in the four countries ranged between 4.5% and 8.5% (Table 3).
| AOM | Countries, % (95% CI)a | |||
|---|---|---|---|---|
| Saudi Arabia n = 191 |
Oman n = 100 |
Pakistan n = 134 |
Turkey n = 82 |
|
| S-AOM | 7.4 (3.9–12.6) | 5.6 (1.2–15.4) | 3.9 (1.3–8.8) | 8.5 (3.5–16.8) |
| C-AOM | 13.8 (3.9–31.7) | 4.3 (0.5–14.8) | 20.0 (0.5–71.6) | – |
| Overall | 8.4 (4.9–13.2) | 5 (1.6–11.3) | 4.5 (1.7–9.5) | 8.5 (3.5–16.8) |
AOM = acute otitis media; C-AOM = clinically confirmed AOM; S-AOM = clinically suspected AOM; N = Number of positive AOM episodes; n = number of positive AOM episodes in each category.
Exact Poisson 95% confidence intervals.
Proportion of suspected, confirmed, and overall AOM episodes with treatment failures.
3.5. Economic impact of AOM (based on HEQs)
HEQs were completed by the parents of the 450 children with a history of AOM in the four countries (Table 4). The number of AOM episodes that led to visits to any healthcare professional, other than the study doctors, varied between countries [Saudi Arabia: 6.5% (12/185); Oman: 6% (5/84); Pakistan: 7.1% (7/99); Turkey: 25.6% (21/82)]. Medications taken for AOM episodes unrecorded in the medical charts were fewer in Saudi Arabia [5.4% (10/185)], Oman [1.2% (1/84)], and Pakistan [3% (3/99)] than in Turkey [41.5% (34/82)]. Procedures unrecorded in the medical charts were performed in 1.1% episodes (n = 2) in Saudi Arabia, 3% (n = 3) in Pakistan, 1.2% (n = 1) in Turkey, and none in Oman.
| Country (HEQ respondents) | No. (%) of children covered by medical insurance | No. (%) of AOM cases with out-of-pocket expenses | Out-of-pocket expensesa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Public/government funding | Private insurance | Self payment/no insurance | Visit to doctor | Diagnostic exam | Hospital stay | Medication | Babysitting fees | Public transport | |||
| Of sick child | Of sibling | ||||||||||
| Saudi Arabia (n = 185) | 180 (97.3) | 2 (1.1) | 3 (1.6) | 71 (38.4) | 0.00 | 0.00 | 0.00 | 10.70 | 101.90 | 142.20 | 46.60 |
| Oman (n = 84) | 55 (65.5) | 5 (6.0) | 24 (28.6) | 19 (22.6) | 6.00 | 0.00 | 20.80 | 26.00 | 0.00 | 0.00 | 0.00 |
| Pakistan (n = 99) | 0 (0.0) | 26 (26.3) | 73 (73.7) | 89 (89.9) | 13.90 | 35.40 | 0.00 | 7.80 | 0.00 | 0.00 | 3.10 |
| Turkey (n = 82) | 82 (100.0) | 0 (0.0) | 0 (0.0) | 19 (23.2) | 0.00 | 14.70 | 0.00 | 28.40 | 52.50 | 0.00 | 0.00 |
AOM = acute otitis media; HEQ = Health Economics Questionnaire; USD = US dollars.
Mean values in USD (2013). Exchange rates (USD 2013): Saudi Riyal: 3.75, Omani Rial: 0.3845, Pakistani Rupee: 101.6288992, Turkish Lira: 1.903768242.
Medical insurance coverage and out-of-pocket expenses by country.
Day care or school absences corresponded to 4.9% AOM episodes (median 5 days missed) in Saudi Arabia, 7.1% (median 1.5 days missed) in Oman, and 20.2% (median 2 days missed) in Pakistan. In Turkey, no children missed day care/school due to AOM episodes. The parent/caregiver missed work for 14.1% of the episodes (median 16 h missed) in Saudi Arabia; 28.6% (median 3 h missed) in Oman; 2% (median 10 h missed) in Pakistan; and 3.7% (median 120 h missed) in Turkey.
Table 4 illustrates the medical insurance status of the enrolled children. A total of 44% of the parents/caregivers incurred out-of-pocket expenses. The mean cost of the total expenses per episode varied between countries: USD67.1 (SD: 93.0) in Saudi Arabia; USD16.1 (SD: 16.4) in Oman; USD22.1 (SD: 20.5) in Pakistan; and USD33.6 (SD: 44.9) in Turkey.
4. Discussion
The results of this multinational analysis showed a variable incidence of suspected and confirmed AOM episodes in children aged 0–5 years in three Middle Eastern countries and Pakistan. The incidence was the lowest in Turkey, 99 (95% CI: 79–123) per 1000 PY, and highest in Saudi Arabia, 207 (95% CI: 178–238) per 1000 PY. These incidences were comparable to other countries (range: 150–256 per 1000 PY) [1,23,25]. These regional differences may be attributable to region-specific seasonal variability, but also due to other factors like characteristics of NIP, standard of care, and otitis media case ascertainment.
Apart from Oman, the incidence of C-AOM episodes was lower than that of S-AOM episodes. This might be explained by the low referral to ENT specialists and illustrates the differences in case ascertainment.
Except for Turkey, AOM disease burden was higher in children aged 0–2 years than 3–5 years in the studied countries; in agreement with the incidence rates reported in Europe [23].
In all countries, the overall incidence of AOM was higher in vaccinated children compared to unvaccinated children (Fig. 3). These findings were not statistically significant due to small sample sizes for unvaccinated children (i.e., unvaccinated versus vaccinated children were 14 vs. 166 in Saudi Arabia, 11 vs. 71 in Oman, and, 2 vs. 64 in Turkey), and due to overlapping confidence intervals (except for Saudi Arabia). Therefore, their clinical significance should be treated with caution. In Pakistan, there was a comparable sample size for vaccinated (n = 44) and unvaccinated (n = 51) children, and no significant difference was found between these groups regarding incidence of AOM. Other than statistical considerations, the differences could be explained by several factors including case ascertainment, lack of aetiological information, ascertainment of vaccination status from parent recall, the fact that not all serotypes are covered by the available vaccines, or by the lower relative vaccine uptake in the older age group, which is associated with naturally declining AOM incidence. Further analysis of AOM incidence by vaccination status and age group confirmed this difference in all countries except for Oman, where the incidence of AOM in vaccinated 0–2-year-old children was lower than in the unvaccinated children of the same age (Fig. 3). Among the differences observed in Oman were the lower proportion of unvaccinated children in the 0–2-year-old group, and higher proportion of C-AOM cases, which might be indicative of higher ENT referral rate. Indeed, the percentage of vaccinated children in the 0–2-year and 3–5-year age groups was highest in Oman (98.8% and 73.9%, respectively) and lowest in Pakistan (48.9% and 37.2%, respectively).
Regarding the clinical symptoms, we found fever [50.3%; (255/507)] and ear pain [46.4%; (235/507)] to be the most common symptoms across all four countries, in consonance with the European countries, where ear pain is one of the most common symptoms of AOM [48.4% (666/1376)] [23].
Antibiotics were used to treat AOM in all countries. However, in Turkey antibiotics were prescribed much less frequently (3.7% episodes) than in the other countries (range: 82.0–94.8%). Interestingly, the percentages of treatment failures were low, but similar across the four countries (4.5–8.5%). Despite guidelines recommending their reduction [26], antibiotics remain widely prescribed for the clinical management of AOM in many countries [2]. With antibiotic resistance becoming an increasing public health concern, alternative approaches, such as ‘watch-and-wait’ in Canada, or prevention through vaccination, should be considered [27,28].
Apart from Pakistani children, most of the enrolled children had medical insurance, or were covered by state-owned healthcare provision; the direct costs incurred by parents/caregivers were therefore mostly covered. Indirect costs relating to taking time off work to provide care while a child is absent from school or day care, varied in the four countries, due predominantly to cultural differences.
The strengths of this study were the diversity of the population, the large sample size, and the use of a standardised methodology to evaluate the public health burden of AOM, and to generate both epidemiological and health economic evidence for these four countries.
The low number of C-AOM cases was one of the study limitations, as referrals to ENT specialists rarely occurred in most countries. In addition, the definition of recurrent cases in this study required confirmation by an ENT specialist, however, given the lower number of ENT-referred cases, the estimate of recurrent cases may have been underestimated. Furthermore, a retrospective review of medical charts for identifying study participants may have potentially underestimated the incidence, as some children may not have visited their medical practice. Additionally, since the inconsistent strategy for recruitment of patients carried the risk of selection bias, the true magnitude of the estimated AOM incidence cannot be assessed in our study. Finally, it was also not possible to estimate the potential impact of vaccination on AOM incidence due to the low number of unvaccinated children in this study. Regarding the economic burden, only costs from the parent perspective were estimated. As parents completed a HEQ for every AOM episode experienced by their child over the past 12 months, the details collected could be influenced by the individuals’ ability for recall.
The lessons learnt during the present study might facilitate the design of future studies in individual countries. For instance, a prospective study would eliminate reliance on recall, a standardised case definition to accurately diagnose AOM would allow better comparisons across countries, and, consistently applying an appropriate randomisation scheme and collecting data on variables that could represent sources of bias is likely to shed light on the true burden of AOM in these countries. Further studies exploring public financing of AOM treatment (type and extent of financial coverage, and unit cost/reimbursement of healthcare resources) are, however, needed to determine the economic burden of AOM from the public payer perspective. Additional indirect cost components, including productivity loss and private transportation, will help to estimate the economic burden of AOM from a societal perspective.
5. Conclusion
This study is believed to be the first to assess the public health burden of AOM in three Middle Eastern countries and Pakistan using a standardised methodology, and highlights the need for cost-effective prevention strategies.
Conflicts of interest
GM, ISA, and SB declare grants and personal fees from the GSK group of companies during the study. MC declares project support from the GSK group of companies, Pfizer, Sanofi Pasteur, and Merck Sharp & Dohme. KM, KG, JES, and RD are employees of the GSK group of companies. JES, KM, and RD also hold stock options/restricted shares from the sponsoring company. AAA and MAT have no conflicts of interest to declare.
Funding source
GlaxoSmithKline Biologicals SA funded this study/research and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Acknowledgements
The authors thank all the investigators involved in this trial and the participating parents and children. The authors also thank the whole monitoring team, Karin Hallez (GSK) for operational coordination in the Emerging Market region and the GSK local teams of the four countries for their logistic support. In addition, Debasish Saha, Ivo Vojtek, and Carla Talarico (employees of GSK) are acknowledged for critical review of the manuscript and expert input. The authors would also like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Amandine Radziejwoski (Business & Decision Life Sciences), Manjula K (GSK) and Angeles Ceregido (XPE Pharma & Science on behalf of GSK) coordinated the manuscript development and provided editorial support. The authors also thank Kavi Littlewood (Littlewood Writing Solutions, on behalf of GSK), Prachee Panda (former employee of GSK) and Varshini S (GSK) for providing medical writing support and Julia Donnelly (freelance medical writer on behalf of GSK) for language editing.
References
Cite this article
TY - JOUR AU - Ghulam Mustafa AU - Amal Y. Al Aidaroos AU - Idris S. Al Abaidani AU - Kinga Meszaros AU - Kusuma Gopala AU - Mehmet Ceyhan AU - Mohamad Al-Tannir AU - Rodrigo DeAntonio AU - Shyam Bawikar AU - Johannes E. Schmidt PY - 2017 DA - 2017/02/08 TI - Incidence and economic burden of acute otitis media in children aged up to 5 years in three Middle Eastern countries and Pakistan: A multinational, retrospective, observational study JO - Journal of Epidemiology and Global Health SP - 123 EP - 130 VL - 7 IS - 2 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2016.12.004 DO - 10.1016/j.jegh.2016.12.004 ID - Mustafa2017 ER -
